Abstract
The specific mechanism underlying the effect of changes in brain iron content on the development of RBD secondary to PD remains unclear. 27 PD patients with secondary RBD (PD-RBD+), 24 PD patients without RBD (PD-RBD-), and 32 healthy controls (HCs) were included. Paramagnetic susceptibility was isolated using APART-QSM. The QSM and paramagnetic susceptibility (χpara) values of the whole brain and subcortical nuclei were compared among groups. The diagnostic performance was evaluated using receiver operating characteristic curve analysis. Compared with PD-RBD-, PD-RBD+ patients presented a lower χpara value in the left middle temporal gyrus. The QSM and χpara values of bilateral substantia nigra pars compacta (SNpc) were increased in both groups. The diagnostic performance of the combined index of χpara values was superior to that of the QSM value or the single index. In conclusion, paramagnetic susceptibility isolated from QSM is helpful for iron quantification and exploring the mechanism of PD-RBD+.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder accompanied by a range of non-motor symptoms, primarily sleep disturbances, cognitive impairment, and mood disorders1. Among these manifestations, rapid eye movement (REM) sleep behavior disorder (RBD) is the most distinctive sleep disturbance in PD patients and can develop either before or after the onset of motor symptoms. RBD is characterized by the dysregulation of muscle tone during REM sleep, which is often accompanied by violent or aggressive behaviors associated with dreaming2. Research has indicated that the prevalence of RBD among PD patients increased from 25%–52% over a 6 year follow-up period3. The presence of RBD in PD patients is related to long-term motor progression and cognitive decline, which seriously affect the quality of life and long-term outcomes of patients4,5.
The pathogenic mechanisms underlying PD, particularly in individuals presenting with secondary RBD, remain an area of active investigation. An imbalance in iron homeostasis is recognized as a key pathogenic mechanism contributing to PD. Iron overload can result in its pathological deposition within the brain, facilitating α-synuclein aggregation amid oxidative stress responses and neuroinflammatory processes6. Moreover, metabolic toxins generated through interactions between iron and dopamine may exacerbate dopaminergic neuron loss within the substantia nigra (SN); this loss ultimately triggers both the onset and progression of PD7. One study revealed that PD patients with RBD had significantly greater iron levels in their cerebrospinal fluid than those in PD patients without RBD and that there was a significant correlation between iron levels and RBD symptom severity8. These findings suggest that iron imbalance may be related to the occurrence of RBD in patients with PD. The quantification of iron levels is essential for studying the pathological mechanisms in PD patients with secondary RBD.
Currently, R2* mapping and quantitative susceptibility mapping (QSM) have been extensively utilized as in vivo magnetic resonance imaging (MRI) biomarkers for the quantification of iron levels9,10,11. These mapping techniques capture local magnetic field alterations induced by components with magnetic susceptibility in the brain12. Research indicates that QSM is more sensitive than R2* mapping for the quantification of iron13,14. However, only one study on QSM, which compared the iron contents of nigrosome-1 between groups and found no significant differences, stratified PD patients on the basis of RBD15. There is a notable scarcity of comprehensive quantitative imaging studies focusing on brain iron content in PD patients with secondary RBD.
Notably, traditional QSM methods yield only voxel-averaged susceptibility values, which do not account for interference from diamagnetic components, such as calcifications and myelin, when determining the paramagnetic iron content16,17. Recently, several studies have developed various methodologies aimed at distinguishing the contributions of paramagnetic and diamagnetic magnetization components. Shin et al.18 introduced the χ-separation method, in which magnetic susceptibility maps are successfully separated by leveraging both frequency shift and reversible transverse relaxation. Subsequent research has expanded on this foundation by incorporating deep learning algorithms to effectively mitigate fringe artifacts and generate high-quality susceptibility maps19. Emmerich et al.20 and Dimov et al.21 also performed transformations and improvements with similar separation methods. Chen et al.22 proposed the DECOMPOSE-QSM model for magnetic susceptibility source separation using multi-echo gradient echo (ME-GRE) data under the assumption of a static dephasing mechanism. Kan et al.23 developed an innovative method that incorporates adaptive relaxometric constant estimation for improved susceptibility source separation.
However, most existing methods rely on space-invariant amplitude attenuation kernels and static dephasing mechanisms, which are insufficient to accurately describe susceptibility values in complex physiological microenvironments. The recently introduced APART-QSM24 approach addresses these limitations by employing a more comprehensive complex signal model and an alternative fitting scheme, thereby increasing separation accuracy. The isolated paramagnetic susceptibility value, χpara, has demonstrated superior efficacy in the quantification of brain iron levels in both ex vivo macaque brain experiments and in vivo human brain imaging studies24,25.
PD patients with secondary RBD constitute a critical subgroup and a strong marker of the hypothesized brain-first subtype proposed in recent years26. We hypothesize that QSM can specifically the specific distribution pattern of iron in such patients and its possible role in the occurrence and development of RBD. Consequently, we explored differences in iron levels between PD patients with secondary RBD (PD-RBD+) and those without RBD (PD-RBD-). We employ the APART-QSM method to isolate paramagnetic susceptibility values for precise quantification of brain iron content. We assessed the diagnostic efficacy of these susceptibility values for PD with RBD and investigates the associations between iron levels and clinical disease features.
Results
Demographic and clinical characteristics of the participants
The demographic and clinical characteristics of the participants are presented in Table 1. No significant differences in sex, age, years of education, PSQI score, or HAMD score were observed among the three groups. No significant differences in H-Y stage, or MDS-UPDRS-III score were observed between the PD-RBD- and PD-RBD+ groups. PD-RBD+ patients had significantly higher LEDD (p = 0.007) compared with PD-RBD- patients. Compared with the HCs, the PD-RBD+ patients had lower MMSE scores (p = 0.008) and greater HAMA scores (p = 0.009). The RBDSQ score was significantly greater in the PD-RBD+ group than in both the PD-RBD- group (p < 0.001) and the HC group (p < 0.001).
Comparison of magnetic susceptibility values based on whole-brain analysis
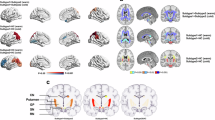
Compared with the PD-RBD- group, the PD-RBD+ group presented greater QSM values in the left rectus gyrus and lower QSM values in the right superior frontal gyrus dorsolateral regions (p < 0.05, cluster-level FWE-corrected; Table 2, Fig. 1a). Compared with the HCs, the PD-RBD+ patients presented greater QSM values in the bilateral SNpc, right precentral gyrus, and left middle cingulate cortex and lower QSM values in the right postcentral gyrus. Additionally, the PD-RBD- patients had a lower QSM value in the left inferior cerebellum (p < 0.05, cluster-level FWE-corrected; Table 2, Fig. 1a).
The results were visualized in the MNI coordinate system. a comparison of QSM. b. comparison of χpara. Warm colors indicate regions where the susceptibility values of the PD-RBD+ group are greater than those of the PD-RBD- group, as well as regions where the susceptibility values of the PD-RBD+ group and PD-RBD- group are greater than those of the HC group. The cool colors indicate the opposite. QSM quantitative susceptibility mapping, χpara paramagnetic susceptibility mapping, PD-RBD+ Parkinson’s disease with secondary rapid eye movement sleep behavior disorder, PD-RBD- Parkinson’s disease without rapid eye movement sleep behavior disorder.
Compared with the PD-RBD- group, the PD-RBD+ group presented a lower χpara value in the left middle temporal gyrus (p < 0.05, cluster-level FWE corrected; Table 2, Fig. 1b). Compared with the HC group, the PD-RBD+ group presented greater χpara values in the bilateral SNpc (p < 0.05, cluster-level FWE-corrected; Table 2, Fig. 1b), exhibiting a similar pattern to that observed for the QSM value. Compared with the HCs, the PD-RBD- patients presented greater χpara values in the left inferior temporal gyrus and left middle temporal gyrus and lower χpara value in the left cerebellar lobule IX (p < 0.05, cluster-level FWE-corrected; Table 2, Fig. 1b).
Comparison of magnetic susceptibility values based on regions of interest
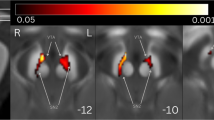
At the ROI level, both the QSM and χpara values indicated that compared with the HC group, patients in the PD-RBD- and PD-RBD+ groups exhibited significantly greater magnetic susceptibility in the bilateral SNpc (p < 0.05, post hoc test with Bonferroni correction; Table 3, Fig. 2). However, there were no significant differences between the two patient groups.
a, b QSM values of the region of interest (ROIs); c, d χpara values of the ROIs. Post hoc Bonferroni correction indicated significant differences at *p < 0.05. All elements of the violin plots: (i) centre line: median, 50th percentile; (ii) lower inner line: 25th percentile; (iii) upper inner line: 75th percentile; (iv) width of the violin: kernel density estimation representing data density. SNpc_R right substantia nigra pars compacta, SNpc_L left substantia nigra pars compacta, QSM quantitative susceptibility mapping (value), χpara paramagnetic susceptibility mapping (value), ppb parts per billion, PD-RBD- Parkinson’s disease without rapid eye movement sleep behavior disorder, PD-RBD+ Parkinson’s disease with secondary rapid eye movement sleep behavior disorder.
Discriminative ability of magnetic susceptibility values
The discriminative ability of the QSM and χpara values of the bilateral SN is shown in Fig. 3. When the PD-RBD+ group was compared with the HC group, the QSM value had an accuracy, sensitivity, and specificity of 0.757, 0.522, and 0.839, respectively, for the right SNpc; 0.759, 0.652, and 0.742, respectively for the left SNpc; and 0.757, 0.565, and 0.871, respectively, for the joint indicator of the bilateral SNpc (Fig. 3a). In contrast, the χpara value exhibited an accuracy, sensitivity, and specificity of 0.809, 0.913, and 0.613, respectively, for the right SNpc; 0.796, 0.870, and 0.645, respectively, for the left SNpc; and 0.812, 0.870, and 0.645, respectively, for the joint indicator (Fig. 3b). The χpara values of the right SNpc and bilateral joint indicator demonstrated superior diagnostic performance compared to the QSM values (p = 0.017, p = 0.02).
The diagnostic performance of the magnetic susceptibility value was quantified by the area under the curve (AUC). a, b QSM and χpara values for the PD-RBD+ and HC groups. c, d QSM and χpara values for the PD-RBD- and HC groups. PD-RBD+ Parkinson’s disease with secondary rapid eye movement sleep behavior disorder, PD-RBD- Parkinson’s disease without rapid eye movement sleep behavior disorder, QSM quantitative susceptibility mapping (value), χpara paramagnetic susceptibility mapping (value), SNpc_R right substantia nigra pars compacta, SNpc_L left substantia nigra pars compacta, Multi-Index joint indicator of bilateral substantia nigra pars compacta, AUC area under the curve.
For the PD-RBD- group compared with the HC group, the QSM value had an accuracy, sensitivity, and specificity of 0.739, 0.762, and 0.645, respectively, for the right SNpc; 0.747, 0.810, and 0.613, respectively, for the left SNpc; and 0.763, 0.810, and 0.645, respectively, for the joint indicator (Fig. 3c). Furthermore, the χpara value had an accuracy, sensitivity, and specificity of 0.760, 0.857, and 0.581, respectively, for the right SNpc; 0.747, 0.667, and 0.774, respectively, for the left SNpc; and 0.791, 0.810, and 0.742, respectively, for the joint indicator (Fig. 3d).
Correlations between magnetic susceptibility values and clinical features
At the regional level, the χpara value exhibited better diagnostic performance than the QSM value, suggesting that the χpara value reflects the iron content more accurately. Therefore, we conducted a correlation analysis between the χpara values and clinical features. After controlling for sex, age, years of education, and LEDD, the χpara values of the right SNpc (r = 0.426, p = 0.03; Fig. 4a) and left SNpc (r = 0.509, p = 0.008; Fig. 4b) in PD-RBD+ patients were positively correlated with HAMA scores. The χpara value of the left SNpc in PD-RBD- patients was positively correlated with the PSQI score (r = 0.486, p = 0.019; Fig. 4c).
a, b the paramagnetic susceptibility values of the bilateral substantia nigra pars compacta in PD-RBD+ patients were positively correlated with HAMA scores. c The paramagnetic susceptibility value of the left substantia nigra pars compacta in PD-RBD- patients was positively correlated with the PSQI score. *p < 0.05, **p < 0.01; HAMA Hamilton Anxiety Scale, PSQI Pittsburgh Sleep Quality Index, SNpc_R right substantia nigra pars compacta, SNpc_L left substantia nigra pars compacta.
Discussion
To our knowledge, this study is the first to isolate the paramagnetic susceptibility values from the QSM to quantify iron deposition in patients with PD accompanied by RBD. The separation method, APART-QSM, aids in quantifying the iron content more precisely to determine the mechanism of the RBD in PD. We measured the differences in the QSM and χpara values and compared the diagnostic ability of these values for quantifying the iron content in patients. This study reports several important findings: (1) The susceptibility values in PD patients without RBD and those with secondary RBD mainly differed in the middle temporal gyrus. (2) The QSM and χpara values of the bilateral SNpc were significantly higher in both PD-RBD- and PD-RBD+ groups, but they were similar between the two patient groups. (3) The magnetic susceptibility values in the SNpc had good diagnostic accuracy in PD patients with RBD, and the diagnostic capability of the χpara value was better than that of the QSM value.
In our whole-brain analysis, many of the regions with differences in χpara values did not coincide with regions with differences in QSM values. This finding suggested that the QSM measurements were influenced by the inverse magnetic component, leading to frequent false positives and false negatives in the comparison results. Consequently, QSM may not fully capture the subtle changes in iron content associated with disease status. We found that the χpara value of the bilateral SNpc in PD-RBD+ patients was significantly greater than that in HCs, which was verified in our subsequent ROI analysis. In addition, our findings revealed alterations in the iron content in the middle temporal gyrus of PD-RBD+ patients. The temporal lobe, which is part of the paralimbic system, plays a crucial role in human memory consolidation, complex cognitive processing, and emotional regulation27. The abnormalities detected in this study may be central to the pathogenesis of PD with secondary RBD, since dysfunctions in the pathways involved in these regions can lead to diminished inhibition of imagery and behaviors, resulting in vivid dreams, violent behavior, and negative emotions in RBD patients28.
Research has demonstrated that in PD patients, the presence of RBD is associated with extensive cortical thinning, volume reduction, shape contraction, and increased mean cortical dispersion, predominantly in the temporal, frontal, parietal, and occipital regions29,30,31. Our study expands upon these previous findings by confirming that early stage PD patients, particularly those with RBD, exhibit alterations in iron metabolism in the temporal gyrus, not just structural changes. In contrast, the χpara value of the left middle temporal gyrus was greater in PD-RBD- patients than in PD-RBD+ patients, and the χpara value of the left cerebellar lobule IX was lower in PD-RBD- patients than in HCs. These findings were unexpectedly. A prospective cohort autopsy study revealed that the presence of RBD was associated with more extensive α-synuclein pathology within the brains of PD patients32. We thus hypothesized that iron deposition would progressively increase as RBD develops secondary to PD. However, this hypothesis is inconsistent with our experimental findings. Given the paucity of longitudinal studies on magnetic susceptibility changes in PD patients with RBD, iron deposition may not reach an irreversible level in the early stages of the disease. Even if RBD progresses, local iron deposition may be dynamically reversed because of unknown factors, such as medications, diet, and brain metabolic regulation, which could contribute to dynamic changes in iron levels during the course of the disease33. Further data and clinical studies are necessary to elucidate this complex mechanism.
Notably, whole-brain analyses may obscure true effects due to stringent multiple comparison corrections, particularly in small nuclei such as the SN34,35,36. Consequently, our hypothesis-driven ROI analysis revealed significant intergroup differences. Both the QSM and χpara values of the bilateral SNpc were significantly greater in both PD subgroups than in the HC group, which is consistent with the results of the whole-brain analysis. This finding is consistent with a recent meta-analysis of 17 studies (11 QSM, 6 R2*), confirming pathologically increased susceptibility in the bilateral SNpc in PD patients11. This increased susceptibility is attributed primarily to iron accumulation in dopaminergic neurons and microglia37,38. Interestingly, we observed that magnetic susceptibility values in the SNpc were similar between the PD-RBD- and PD-RBD+ groups, but associations with clinical features suggest that increased iron content in the SNpc is correlated with severity of symptoms. Our results suggest that changes in the iron content of the SNpc may not be causative of secondary RBD in PD patients, at least in the early stages of the disease. As the PD progresses and RBD develops, the iron content of the SNpc may gradually increase, affecting the severity of RBD symptoms. However, this speculation requires verification, especially through longitudinal studies.
Despite our focus on χpara for more accurate quantification of iron levels, no significant changes were observed in nuclei other than the SN. This finding consistent with previous literatures39,40, which indicate that increased susceptibility is primarily localized to the SN. Magnetic susceptibility is known to increase within the SNpc during the early stage of PD and to extend into the SNpr as the disease progresses to the later stage41. In addition to the SN, alterations in magnetic susceptibility associated with PD have been reported in several subcortical structures, including the red nucleus, dentate nucleus, subthalamic nucleus, striatum, and thalamus11. However, variations in iron levels across these nuclei have shown less consistency; some studies report increased levels, whereas others report decreased levels or no differences11. Factors such as variability among patient samples and differences in QSM techniques, processing methods, and ROI segmentation methods may contribute to this inconsistency.
In our study design, we clearly delineated the occurrence of RBD after the onset of PD to ensure that both patient groups were classified as the brain-first subtype and exhibited a comparable disease stage. This approach improves the rigor of our findings by minimizing confounding effects from iron deposition influenced by PD progression and pathology related to precursor RBD (body-first subtype). However, owing to our emphasis on early stage manifestations of the disease in this study, we did not observe significant differences across other nuclei or broader cortical regions. Additionally, PD-RBD- may develop RBD in the future, and the long-term association with clinical symptoms remains unclear. Therefore, longitudinal studies across different disease stages are warranted to further elucidate the relationship between iron deposition changes and clinical progression.
We observed that the magnetic susceptibility values in the SNpc demonstrated strong diagnostic accuracy in distinguishing patients with PD (PD-RBD+ and PD-RBD-) from HCs. Generally, the χpara value outperformed the QSM value in diagnostic performance, indicating that separating paramagnetic susceptibility values significantly improves diagnostic accuracy. The χpara value of SNpc had high accuracy in distinguishing patients from HCs, and the accuracy of the combined model of the χpara values in the bilateral SNpc is slightly greater than the performance of the single index. Further analysis revealed a significant correlation between the χpara values of the SNpc and clinical symptoms, especially emotional and sleep symptoms. These findings suggest that the χpara value of the SNpc can serve as a biomarker of early PD and reflect disease progression and symptom severity. Future studies should explore the potential applications of the χpara value in different PD stages, such as prodromal diagnosis and monitoring of disease progression.
Our study has several limitations. First, as a cross-sectional study focusing on early stage patients, our findings on the evolution of iron accumulation in PD patients with secondary RBD should be considered preliminary. Longitudinal studies are needed in the future to better understand the causal relationship between iron accumulation and RBD, as well as the temporal and spatial patterns involved. Second, due to our stringent enrollment criteria, participants who underwent vPSG were required to stay overnight in the neurology department for comprehensive sleep monitoring. This rigorous process led to reduced patient cooperation, resulting in a smaller sample size. Consequently, this limitation affects the statistical power needed to detect subtle differences between the two patient groups. Therefore, establishing a larger cohort is essential to improve the detection of group differences and to evaluate diagnostic efficacy more accurately.
Collectively, our findings underscore the efficacy of paramagnetic susceptibility value isolated from QSM in improving the quantification of iron content and the diagnostic performance of this value for neurodegenerative diseases. In addition, these results expand upon previous findings that iron imbalance in the middle temporal gyrus may play a crucial role in the pathogenesis of PD with secondary RBD, and we preliminarily speculate that alterations in iron content within the SNpc, at least during the early stages of PD, may not be a reliable surrogate marker that can be used to monitor secondary RBD in PD patients. Rather, iron accumulation may progressively increase alongside disease progression and potentially influence the severity of clinical symptoms related to RBD. These insights are crucial for guiding future research aimed at integrating more sophisticated imaging modalities, as well as longitudinal studies involving PD patients with RBD to elucidate the underlying mechanisms driving disease development.
Methods
Participant enrollment
From June 2022 to December 2024, PD patients from Beijing Friendship Hospital, Capital Medical University, and local healthy volunteers were recruited for this study. The study was approved by the Ethics Committee of Beijing Friendship Hospital, Capital Medical University (2022-P2-172-01), and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants provided written informed consent. The inclusion criteria were as follows: (1) Participants were aged 40–80 years. (2) Participants were right-handed. (3) PD patients were diagnosed by movement disorder experts according to the UK Parkinson′s Disease Society Brain Bank Clinical Diagnostic Criteria42 and classified as having early stage PD with Hoehn and Yahr (H-Y) stage <3. PD-RBD- (30 cases) and PD-RBD+ (35 cases) patients were evaluated by the gold-standard method of video polysomnography (vPSG)43 and the RBD screening questionnaire (RBDSQ)44, and the subjective RBD-related symptoms were confirmed to have occurred at least 6 months after the onset of PD according to inquiries with the patients and their families. (4) Healthy controls (HCs, 32 cases) were confirmed not to have potential RBD and other sleep disorders, including: obstructive sleep apnea, periodic limb movement disorder, restless legs syndrome, and other significant sleep disturbances through vPSG and the RBDSQ before enrollment. The exclusion criteria were as follows: (1) history of neurological diseases; (2) history of mental disorders; (3) contraindications for MRI; (4) obvious head movement during MRI; and (5) MRI showed obvious head lesions. According to the above criteria, 6 PD-RBD- and 8 PD-RBD+ patients were excluded. A total of 24 PD-RBD- patients, 27 PD-RBD+ patients, and 32 HCs were included in the study and were matched for sex, age, and education level. The detailed information of the study participants is shown in Fig. 5.
PD Parkinson’s disease, HC healthy control, vPSG video polysomnography, RBDSQ Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire, H-Y Hoehn and Yahr, PD-RBD- Parkinson’s disease without rapid eye movement sleep behavior disorder, PD-RBD+ Parkinson’s disease with secondary rapid eye movement sleep behavior disorder.
On the day of the MRI examination, experienced neurologists conducted clinical evaluations of the participants. Data on sex, age, and educational level were recorded. Sleep-related conditions were evaluated using the RBDSQ and the Pittsburgh Sleep Quality Index (PSQI). Emotional and cognitive states were assessed using the Hamilton Depression Scale (HAMD), Hamilton Anxiety Scale (HAMA), and Mini-Mental State Examination (MMSE). Additionally, for all patients, motor function was evaluated using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III, H-Y stage was performed, and the levodopa equivalent daily dose (LEDD) was calculated. In general, high scores on these clinical scales, except the MMSE, indicate severe clinical symptoms.
MRI acquisition
All the participants underwent image acquisition with a 3.0-T MAGNETOM Prisma MRI system (Siemens Healthineers, Erlangen, Germany). The QSM data were obtained using a multi-echo gradient echo (ME-GRE) sequence with the following parameters: repetition time (TR) = 50 ms, first echo time (TE) = 6.8 ms, TE interval = 4.1 ms, number of echoes = 10, flip angle = 15°, and voxel size = 0.8 × 0.8 × 2.0 mm³. Additionally, abnormalities in the brain were detected using fluid-attenuated inversion recovery (FLAIR) sequences with the following parameters: TR = 8500 ms, TE = 87 ms, inversion time (TI) = 2440 ms, flip angle = 150°, and voxel size = 0.4 × 0.4 × 6.0 mm³.
Susceptibility separation and registration
Post-processing was performed using the following steps: The GRE magnitude images were first used to extract brain tissue and generate a binary brain mask for subsequent processing using the brain extraction tool (BET) in FSL45. The GRE phase images were processed using Laplacian-based phase unwrapping46. The background field was removed, and the tissue phase was extracted from the unwrapped phase using the V-SHARP method47,48. Next, STAR-QSM49 was used to generate the susceptibility maps. The recently proposed APART-QSM method was subsequently used to reconstruct the paramagnetic and diamagnetic susceptibility maps24. The QSM, paramagnetic, and diamagnetic maps of each participant were co-registered to Montreal Neurological Institute (MNI) space using Advanced Normalization Tools (ANTs)50. The resulting magnetic susceptibility maps were used for subsequent voxel-based whole-brain analyses.
Segmentation of regions of interest
The atlases were applied using ITK-SNAP software. The T1w-QSM hybrid atlas and deep brain nuclei (DBN) labels were obtained from GitHub (https://github.com/SMILE-Lab-ShanghaiTech/MuSus-100_Atlas). The pedunculopontine nucleus (PPN) was manually delineated on each axial slice in the HR hybrid atlas using ITK-SNAP software, and the corresponding PPN labels were subsequently generated. The DBN and PPN labels were utilized as binary masks and applied to the QSM, paramagnetic, and diamagnetic maps, all of which were coregistered to MNI space. This process enables the extraction of mean values from the DBN and PPN regions for each individual brain dataset. In summary, the regions of interest (ROIs) included the caudate, putamen, external globus pallidus, ventral pallidum, internal globus pallidus, nucleus accumbens, substantia nigra pars compacta (SNpc), substantia nigra pars reticulata (SNpr), red nucleus, subthalamic nucleus, and PPN. The QSM and χpara values were calculated to evaluate the iron content for later comparative analysis. The processing workflow is shown in Fig. 6.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 10.1.2 and SPSS 27.0 software. The sex distribution was assessed with the Pearson chi-square test. The single-sample Shapiro-Wilk test was used to determine the normality of the distributions of demographics, clinical characteristics, and magnetic susceptibility values of the ROIs. Analyses of variance (ANOVAs), Kruskal-Wallis tests, and Mann-Whitney U tests were used to determine differences among the three participant groups depending on data distribution, and post hoc tests with Bonferroni correction were performed if necessary. Sex, age, years of education, and LEDD were used as covariables to examine the differences among groups, and familywise error (FWE) correction was performed. The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was used to evaluate the diagnostic power of the magnetic susceptibility values. The DeLong test was used to compare the diagnostic value of QSM and χpara. Finally, partial correlation analysis with sex, age, years of education, and LEDD as covariables was performed to determine the relationships between χpara and clinical features. p < 0.05 was considered statistically significant.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available owing to privacy and ethical considerations. However, they can be obtained from the corresponding author upon reasonable request.
Code availability
The code for the APART-QSM method used in this study is available at (https://github.com/AMRI-Lab/APART-QSM). The FSL software package (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki) and ANTs toolkit (http://stnava.github.io/ANTs/) were utilized for image processing. Region of interest segmentation was performed using ITK-SNAP (http://www.itksnap.org). The T1w-QSM hybrid atlas and deep brain nuclei labels were obtained from the publicly available repository on GitHub (https://github.com/SMILE-Lab-ShanghaiTech/MuSus-100_Atlas). Custom scripts for statistical analysis are available from the corresponding authors upon request, subject to institutional data sharing agreements.
References
Armstrong, M. J. & Okun, M. S. Diagnosis and treatment of Parkinson disease: a review. JAMA 323, 548–560 (2020).
Iranzo, A., Cochen De Cock, V., Fantini, M. L., Pérez-Carbonell, L. & Trotti, L. M. Sleep and sleep disorders in people with Parkinson’s disease. Lancet Neurol. 23, 925–937 (2024).
Zimansky, L. et al. Incidence and progression of rapid eye movement behavior disorder in early parkinson’s disease. Mov. Disord. Clin. Pract. 8, 534–540 (2021).
Martinez-Nunez, A. E. et al. Clinically probable RBD is an early predictor of malignant non-motor Parkinson’s disease phenotypes. NPJ Parkinson’s Dis. 11, 25 (2025).
Pagano, G. et al. REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology 91, e894–e905 (2018).
Zhou, Z. D. & Tan, E. K. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol. Neurodegener.12, 75 (2017).
Hare, D. J. & Double, K. L. Iron and dopamine: a toxic couple. Brain J. Neurol. 139, 1026–1035 (2016).
Hu, Y. et al. Investigation on abnormal iron metabolism and related inflammation in parkinson disease patients with probable RBD. PloS one 10, e0138997 (2015).
Treit, S. et al. R2* and quantitative susceptibility mapping in deep gray matter of 498 healthy controls from 5−90 years. Hum. Brain Mapp. 42, 4597–4610 (2021).
Lee, H. et al. Differential effect of iron and myelin on susceptibility MRI in the substantia nigra. Radiology 301, 682–691 (2021).
Jin, J. et al. Iron deposition in subcortical nuclei of Parkinson’s disease: a meta-analysis of quantitative iron-sensitive magnetic resonance imaging studies. Chin. Med. J. 138, 678-692, (2025).
Hametner, S. et al. The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation - a biochemical and histological validation study. NeuroImage 179, 117–133 (2018).
Langkammer, C. et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? a post mortem validation study. NeuroImage 62, 1593–1599 (2012).
Barbosa, J. H. et al. Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2. Magn. Reson. Imaging 33, 559–565 (2015).
Lancione, M. et al. Evaluation of iron overload in nigrosome 1 via quantitative susceptibility mapping as a progression biomarker in prodromal stages of synucleinopathies. NeuroImage 260, 119454 (2022).
Luo, Y. et al. Differentiating calcifications from cerebral microbleeds using quantitative susceptibility mapping. Eur. Radiol 35, 2043–2052 (2025).
Cohen, Z., Lau, L., Ahmed, M., Jack, C. R. & Liu, C. Quantitative susceptibility mapping in the brain reflects spatial expression of genes involved in iron homeostasis and myelination. Hum. Brain Mapp. 45, e26688 (2024).
Shin, H. G. et al. χ-separation: Magnetic susceptibility source separation toward iron and myelin mapping in the brain. NeuroImage 240, 118371 (2021).
Kim, M. et al. χ-sepnet: deep neural network for magnetic susceptibility source separation. Hum. Brain Mapp. 46, e70136 (2025).
Emmerich, J., Bachert, P., Ladd, M. E. & Straub, S. On the separation of susceptibility sources in quantitative susceptibility mapping: theory and phantom validation with an in vivo application to multiple sclerosis lesions of different age. J. Magn. Reson.330, 107033 (2021).
Dimov, A. V. et al. Susceptibility source separation from gradient echo data using magnitude decay modeling. J. Neuroimag. Off. J. Am. Soc. Neuroimag. 32, 852–859 (2022).
Chen, J., Gong, N. J., Chaim, K. T., Otaduy, M. C. G. & Liu, C. Decompose quantitative susceptibility mapping (QSM) to sub-voxel diamagnetic and paramagnetic components based on gradient-echo MRI data. NeuroImage 242, 118477 (2021).
Kan, H. et al. Quantitative susceptibility mapping for susceptibility source separation with adaptive relaxometric constant estimation (QSM-ARCS) from solely gradient-echo data. NeuroImage 296, 120676 (2024).
Li, Z. et al. APART-QSM: An improved sub-voxel quantitative susceptibility mapping for susceptibility source separation using an iterative data fitting method. NeuroImage 274, 120148 (2023).
Zhou, X. et al. Alterations in brain iron and myelination in children with ASD: a susceptibility source separation imaging study. NeuroImage 310, 121128 (2025).
Borghammer, P. & Van Den Berge, N. Brain-first versus gut-first Parkinson’s disease: a hypothesis. J. Parkinson’s Dis. 9, S281–s295 (2019).
Kucewicz, M. T., Cimbalnik, J., Garcia-Salinas, J. S., Brazdil, M. & Worrell, G. A. High frequency oscillations in human memory and cognition: a neurophysiological substrate of engrams?. Brain J. Neurol. 147, 2966–2982 (2024).
Liu, J. et al. Altered connectivity in the cognitive control-related prefrontal cortex in Parkinson’s disease with rapid eye movement sleep behavior disorder. Brain Imaging Behav. 17, 702–714 (2023).
Rahayel, S. et al. Brain atrophy in Parkinson’s disease with polysomnography-confirmed REM sleep behavior disorder.Sleep 42, zsz062 (2019).
Kim, H. J. et al. Brain atrophy of secondary REM-sleep behavior disorder in neurodegenerative disease. J. Alzheimer’s Dis.: JAD 52, 1101–1109 (2016).
Pardo, J. et al. Cortical macro- and microstructural changes in Parkinson’s disease with probable rapid eye movement sleep behavior disorder. Mov. Disord. Off. J. Mov. Disord. Soc. 39, 814–824 (2024).
Postuma, R. B. et al. REM sleep behavior disorder and neuropathology in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 30, 1413–1417 (2015).
Chen, L. et al. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct. Target. Ther. 10, 31 (2025).
Poldrack, R. A. et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 18, 115–126 (2017).
Botvinik-Nezer, R. et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582, 84–88 (2020).
Bennett, C. M. & Miller, M. B. How reliable are the results from functional magnetic resonance imaging?. Ann. N. Y. Acad. Sci. 1191, 133–155 (2010).
Yu, H. et al. Metabolic reprogramming and polarization of microglia in Parkinson’s disease: Role of inflammasome and iron. Ageing Res. Rev. 90, 102032 (2023).
Song, N. & Xie, J. Iron, Dopamine, and α-Synuclein interactions in at-risk dopaminergic neurons in parkinson’s disease. Neurosci. Bull. 34, 382–384 (2018).
Ghassaban, K. et al. Regional high iron in the substantia nigra differentiates Parkinson’s disease patients from healthy controls. Front. Aging Neurosci. 11, 106 (2019).
Yan, Y. et al. Correlation of brain iron deposition and freezing of gait in Parkinson’s disease: a cross-sectional study. Quant. imaging Med. Surg. 13, 7961–7972 (2023).
Chen, Q. et al. Iron deposition in Parkinson’s disease by quantitative susceptibility mapping. BMC Neurosci. 20, 23 (2019).
Hughes, A. J., Daniel, S. E., Ben-Shlomo, Y. & Lees, A. J. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain J. Neurol. 125, 861–870 (2002).
Cesari, M. et al. Video-polysomnography procedures for diagnosis of rapid eye movement sleep behavior disorder (RBD) and the identification of its prodromal stages: guidelines from the international RBD study group. Sleep 45, zsab257(2022).
Stiasny-Kolster, K. et al. The REM sleep behavior disorder screening questionnaire-a new diagnostic instrument. Mov. Disord. : Off. J. Mov. Disord. Soc. 22, 2386–2393 (2007).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, S208–S219 (2004).
Schofield, M. A. & Zhu, Y. Fast and robust phase unwrapping algorithm for electron holography. Microsc. Microanal.8, 532–533 (2002).
Li, W., Avram, A. V., Wu, B., Xiao, X. & Liu, C. Integrated Laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR Biomed. 27, 219–227 (2014).
Wu, B., Li, W., Guidon, A. & Liu, C. Whole brain susceptibility mapping using compressed sensing. Magn. Reson. Med. 67, 137–147 (2012).
Wei, H. et al. Streaking artifact reduction for quantitative susceptibility mapping of sources with large dynamic range. NMR Biomed.28, 1294–1303 (2015).
Klein, A. et al. Evaluation of volume-based and surface-based brain image registration methods. NeuroImage 51, 214–220 (2010).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82202097, 62471296), Capital's Funds for Health Improvement and Research (No.2022-1-1111), Beijing Scholars Program (No. [2015] 160), the National Key Research and Development Program of China (2024YFC2421100), Tongzhou District Health Development Program (KJ2024CX023) and Tongzhou Medical Research and Technology Transfer Special Project (WS2025051).
Author information
Authors and Affiliations
Contributions
L.D., Z.W. and X.W. designed and conducted the study. L.D. and R.A. conducted the study and collected the data. L.D. and W.Z. contributed to the data analysis and drafted the manuscript. Z.W., H.W. and W.W. provided advice on the data analysis and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dong, L., Zhou, W., An, R. et al. Paramagnetic susceptibility mapping better quantifies brain iron content in Parkinson’s disease with RBD. npj Parkinsons Dis. 11, 192 (2025). https://doi.org/10.1038/s41531-025-01043-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-01043-7