Abstract
The study goal was to use a very large study cohort to establish normative data for the revised UPSIT (UPSIT-R) and to compare the resultant percentiles to those of the original UPSIT. A second study was performed to compare the performance of these two tests in a cohort of persons with and without Parkinson’s disease (PD). UPSIT-R percentiles were derived by age and sex in 16,972 volunteers. Non-parametric statistics were employed to compare the results of those with and without PD. UPSIT-R performance declined with increasing age; deficits were more pronounced in men than women. The magnitude of the difference between the original and revised test percentile scores differed by age and sex. Olfactory deficits in PD were confirmed on the UPSIT-R. This study provides normative data clinically useful for assessing the relative degree of dysfunction in persons 60 years of age and older using the UPSIT-R. Trial Registration Information: ClinicalTrials.gov NCT05065060.
Similar content being viewed by others
Introduction
Olfactory dysfunction has been recognized as a marker of neurological disease, specifically with evidence from individuals with Parkinson disease (PD), Alzheimer disease, and myasthenia gravis1,2,3,4,5.
Historically, much research designed to understand the prognostic value of olfactory function for neurodegenerative diseases has utilized The University of Pennsylvania Smell Identification Test (UPSIT), a 40-item test that was originally developed in 19846.
Previous studies have described the use of threshold values based on UPSIT score to assign olfactory diagnoses ranging from normosmia (UPSIT ≥ 34 in males; ≥35 in females) to total anosmia (≤18 for males and females)7,8. The limitations of a classification approach based on absolute UPSIT scores have been previously discussed9. We recently published updated age- and sex-specific UPSIT percentiles for adults 50 years of age and older based on a large combined sample from the Parkinson Associated Risk Syndrome (PARS) and Parkinson’s Progression Markers Initiative (PPMI) cohorts9. While those percentiles remain valuable for interpretation of data from studies using the original form of the UPSIT6, the instrument was revised in 2020 to modify some of the odorants and response distractors. Thus, as use of the revised form of the UPSIT becomes more widespread, it will be useful to understand the performance differences between the two versions of the test and to have age- and sex-specific percentile values for the revised UPSIT available that can inform data interpretation.
The research presented here was designed to explore differences between the original UPSIT from 1984 and the revised version (UPSIT-R) released in 2020. It is of importance to understand how the UPSIT-R compares in performance to the original UPSIT so that existing and future datasets can be appropriately interpreted and compared.
Results
Bridging study: within-subject comparison of performance between original and revised UPSIT
The bridging study cohort included n = 89 HC and n = 48 PD participants recruited from IU and Penn. Demographics of this population are shown in Table 1. All PD patients were recruited from Penn and most (89%) of the HC participants were recruited from IU. The median age was 67.0 and 69.5 years in the HC and PD groups, respectively.
As predicted, olfactory function was lower in the PD compared to the HC participants (Table 2). The median (IQR) UPSIT raw score for the original test was 17 (14–21.5) among PD and 34 (31–36) among HC participants. Similarly, the median (IQR) UPSIT score for the revised test was 19 (15–27) among PD and 36 (33–37) among HC participants.
Higher scores on the revised UPSIT were observed in 102 (74%) of participants; 23 (17%) had lower scores on the revised UPSIT; 12 (9%) scored the same on both versions. Wilcoxon signed rank tests indicated significantly higher raw scores on the revised UPSIT than the original UPSIT, both among HCs (P < 0.0001) and those with PD (P < 0.0001); median (IQR) differences were 2 (0–3) and 2 (1–5), respectively (Table 2). The magnitude of the difference in the average raw scores between the revised and original UPSIT significantly differed between control and PD participants (P = 0.0446). Scores were not impacted by test order.
Percentile scores were higher with the revised UPSIT compared to the original in both study groups; the median (IQR) percentile difference was 13.5 (1.5–27) among HC, and only 2 (0–6) in the PD cohort (Table 2). The magnitude of the difference in the percentile scores between the revised and original UPSIT significantly differed between HC and PD participants (P = 0.0019).
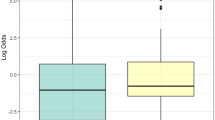
A Bland-Altman plot is used in Fig. 1 to visualize the level of agreement between the original and revised versions of the UPSIT. A systematic difference is observed in which scores on the revised UPSIT are generally higher (mean: 2) than on the original UPSIT. The figure also visualizes the finding that, on average, HC participants had higher UPSIT scores than PD participants. See also supplementary Fig. S1.
Cohort study to derive updated percentiles: between-subject comparison of percentile scores
Original UPSIT raw scores among 4246 females (mean [SD] age: 67.8 [6.1]) and 3679 males (69.7 [6.7]) aged ≥60 years from the previously published PARS/PPMI combined cohort9 were compared to the raw scores of the revised UPSIT among two newly enrolled cohorts. The new cohorts included 16,972 total individuals: 2485 females (mean [SD] age: 68.0 [5.6]) and 1908 males (69.6 [6.2]) recruited online (PPMI Online); and 9269 females (68.0 [5.9]) and 3310 males (69.9 [6.5]) aged ≥60 who completed the revised UPSIT through ST Direct. Demographics of this study population are shown in Table 3. The ST Direct population (n = 12,579) was much larger than the PPMI Online (n = 4393) population; demographic characteristics were found to be evenly matched in the two populations (i.e., age, distribution by age category, race, and ethnicity). The proportion with a family history of PD differed between the groups as a function of the recruitment methods; 20% of the ST Direct population and 48% of the PPMI Online population reported a family history of PD.
The PPMI Online and ST Direct populations were similar in age, race and ethnicity as the PPMI and PARS cohorts previously reported9. In the PARS/PPMI combined cohort, 34% reported a first-degree relative with PD, as compared to 20% and 48% in the ST Direct and PPMI Online cohorts, respectively. Although not diagnosed with PD, some prodromal features were reported by study participants. Specifically, 12% of female and 10% of male PPMI Online participants self-reported regular (at least weekly) use of laxatives; 23% of female and 18% of male PPMI Online participants endorsed constipation (i.e., <1 bowel movement per day). Symptoms of REM sleep behavior disorder (RBD) were self-reported by 10% of female and 22% of male participants in the combined PPMI Online/ST Direct cohort, with 2% and 6% of females and males, respectively, having received a diagnosis of RBD.
Additional data for these demographic parameters including the prodromal features by the separate cohorts are provided in the supplementary materials for this article (Table S1).
Revised UPSIT percentiles
Percentile scores for the revised UPSIT derived from the newly enrolled PPMI Online/ST Direct cohort are presented in Table 4 for females and Table 5 for males, broken down into five age categories (i.e., 60–64, 65–69, 70–74, 75–79, and ≥80 years).
A comparison of the percentiles for the original (PARS/PPMI cohorts) and revised (PPMI Online/ST Direct cohorts) UPSIT is provided in Table 6. Across all age and sex categories, UPSIT mean raw scores were higher with the revised compared to the original UPSIT, but the magnitude of the difference varied by age and sex. The smallest mean differences (range 0.6–1.2) were observed among the three oldest (70–74, 75–79, ≥80) male categories and the oldest (≥80) female category. The largest mean differences (range: 1.9–2.1) were evident among the three youngest (60–64, 65–69, 70–74) female categories and the youngest (60–64) male category. When comparing differences in the raw scores that defined key percentile cutoff values (i.e., ≤25th, ≤20th, ≤15th, and ≤10th percentile) for the original and revised UPSIT percentiles, the greatest differences were observed in females; several of the male subgroups had no change or a worsening (i.e., a lower raw score defining the cutoff) for a given percentile with the revised UPSIT. Overall, no meaningful impact of PD family history on scores or percentiles was observed (Supplemental Table S4).
Discussion
As assessment tools evolve over time, so too must our understanding of the data provided by these tools. The measurement of olfactory function is an important prognostic marker of neurodegeneration, and thus it is important for neurologists or any researcher who investigates olfaction to ensure accurate interpretation of such data. The UPSIT has been a mainstay in the evaluation of olfaction, and, after nearly 4 decades of use, has been updated to remain current.
This investigation compares participant performance on the original UPSIT from 1984 to the revised version from 2020 and provides three main insights. First, better performance (i.e., higher scores indicating greater olfactory function) was observed with the revised test compared to the original test. This is an expected finding, consistent with the intention of the revision to modernize the test through updated odorants and distractor responses. Second, this report provides age- and sex-specific percentile look-up tables for the revised UPSIT, which complement the tables previously published for the original version of the test9. Third, of particular relevance to investigators of neurodegeneration, the deficit of olfactory function in PD participants compared to HC participants persists with the revised version of the UPSIT as it has been consistently reported with the original version.
While the main finding of this study is that the revised UPSIT yields slightly higher raw scores, upon closer examination of differences between the original and revised versions of the UPSIT, some nuanced observations can be made. The magnitude of the differences varied by age and sex, with smaller mean differences observed with increasing age, and the larger mean differences observed in females compared to males. The within-subject design of the bridging study allows for the most direct comparison of test performance with the two versions of the UPSIT. On average there is approximately a 2-point improvement in raw scores on the revised UPSIT compared to the original (controls mean difference: 1.7, PD mean difference: 2.5). However, again this finding is nuanced; what appear to be rather consistent differences in mean scores translates into a greater observed difference in percentile scores (controls percentile mean difference: 14.7, PD percentile mean difference 7.9).
Taken together, these observations highlight the importance of referring to the revised percentile tables provided herein when scoring the revised UPSIT in clinical or research settings. Our findings suggest that it would not be accurate to refer to the percentile look-up tables previously reported for the original version when using the revised test. While raw scores on the revised UPSIT are generally 2–3 points higher than on the original, there are subtle differences based on age and sex that make the granular data provided by our large cohort the best reference for deriving percentiles for the revised UPSIT in cohorts such as ours. The results of the within-subjects bridging study (i.e., median +2-point difference between original and revised UPSIT scores) are consistent with a comparison of the original and revised UPSIT using the large cohorts to generate the percentiles (see comparisons in Table 6), leveraging our original work on percentile score published in 20239, giving further support for our estimates for age- and sex-specific percentiles.
As with the previous report, this work is strengthened by the large cohort size. Data from a total of 9396 individuals were analyzed in the prior study of the original UPSIT9; an even larger total cohort of 16,972 was analyzed here from PPMI Online and ST Direct for the purposes of generating percentile scores for the revised UPSIT. Because these percentile look-up tables are age- and sex-specific, it is important to have a very robust sample size so that each individual cell (age group by sex) is of sufficient size to reliably estimate corresponding percentiles. Although we do have a direct comparison of original and revised UPSIT scores within the large cohort, the recruitment methods used in this study and our prior work9 are similar enough to expect that the results would not be dramatically different. The concordant results in the smaller within-subject component of this report support this expectation.
Despite the robust sample size, this study is limited by a lack of racial and ethnic diversity; the cohort study participants self-reported as 97% White and 98% non-Hispanic. It was anticipated that the large sample size combined with the broad recruitment strategies (particularly for ST Direct) would provide a more diverse cohort as compared to previous efforts. However, this was not the case. Although the sample may be inadequate to draw conclusions based on race/ethnicity, the reader is referred to the supplementary materials for this article to review the demographic features and UPSIT raw and percentile scores by self-reported race (Table S2) and ethnicity (Table S3) for this study. The bridging study did introduce perhaps a somewhat more diverse cohort based on purposeful sampling, and 17% (n = 9) of the PD participants were self-reported persons of color. Nevertheless, it is clear that the findings reported here are most generalizable to White, non-Hispanic populations. Known differences in odorant recognition and prevalence of olfactory dysfunction by race and ethnicity have been documented10,11,12,13,14,15, thereby making it a priority to investigate olfactory function in much more diverse cohorts.
Several limitations pertain to the within-subjects design employed in the bridging study. There is an uneven distribution of participants by site and by disease status. Specifically, 100% of the PD participants were recruited at Penn and a large majority of the HC participants (89%) were recruited at IU. Thus, differences in UPSIT performance attributed to disease status (HC vs. PD) could be driven by unrecognized site differences. However, we did not observe substantial differences between the results for normosmic subjects recruited at Penn compared to those recruited through IU. In addition, in IU participants, all completed the original test first and the revised test second. However, average performance improved on the revised test, suggesting that a change in olfactory function during the inter-test interval was not likely to account for the observed effect.
In summary, our results provide a reliable and definitive basis for converting raw scores on the revised UPSIT into percentiles that are appropriate for screening older populations for neurodegenerative disorders such as PD, and more broadly, neuronal synuclein disease16. The case has previously been made for the utility of percentiles over threshold values for the assessment of olfactory function9. Therefore, it is hoped that this report, and specifically the inclusion of percentile look-up tables for the revised UPSIT (2020), helps support ongoing and future investigations of the role of olfactory function to aid interpretation of findings, particularly among persons with neurodegenerative diseases. In PD, this work is critical to the mission of PPMI to identify markers of disease progression and, in particular, enable a deeper investigation of the important role of olfactory function in that pursuit.
Methods
Bridging study participants and design
Participants included healthy controls (HC) and people diagnosed with PD who completed both versions of the UPSIT at either Indiana University (IU) or the University of Pennsylvania (Penn). The bridging study used a within-subjects design to compare performance on the original and revised versions of the UPSIT.
At Penn, PD and HC participants between 50 and 89 years of age were recruited during routine clinical or research visits at the Parkinson’s Disease and Movement Disorders Center. PD participants had a known diagnosis of idiopathic PD, while control participants had no known neurodegenerative disease. Exclusion criteria were as follows: atypical Parkinsonian syndrome, history of prior head trauma or traumatic brain injury, underlying sinus or nasal dysfunction, current upper respiratory tract infection, use of inhaled tobacco products within the preceding 6 months, or an inability to give consent as judged by the treating physician.
At IU, HC participants were recruited from a registry of individuals who had previously agreed to future contact for new PD research. Eligible subjects were ≥60 years of age, able to provide written informed consent, and able and willing to comply with online study procedures. Interested participants were provided a link to review and sign the informed consent online and complete the screening process. Signature of the informed consent was delayed until all questions by the participant were addressed by study staff.
Participants from both sites completed a standardized questionnaire to collect the following demographic and clinical information: age, sex, race/ethnicity, history of sinus or nasal dysfunction, prior tobacco use, and subjective hyposmia/anosmia and dysgeusia/ageusia. All participants were requested to complete both the original and revised versions of the UPSIT. Penn participants completed one version of the UPSIT in the office and the second version at home. IU participants completed both versions of the UPSIT at home. Participants were provided with stamped, self-addressed envelopes and instructed to return tests by mail within two weeks of their initial test date. At Penn, the order of UPSIT administration was determined by a random number generator, so that half of the participants completed the original version in the office and the revised version at home and vice versa. At IU, all participants were administered the original UPSIT first, followed by the revised UPSIT. UPSIT raw scores were calculated for both test versions and converted to age/sex-adjusted percentile scores based on normative data derived from the original UPSIT. Each participant was assigned an ID number, and de-identified scores were stored in a password-protected database.
Cohort study participants and design
Two newly enrolled populations from PPMI Online and Smell Test (ST) Direct were utilized to generate new age- and sex-specific percentile scores for the revised version of the UPSIT. Raw scores and percentiles on the original UPSIT from the combined PARS and PPMI cohort previously reported9 were compared to those for the revised UPSIT collected from PPMI Online and ST Direct cohorts.
PPMI is a longitudinal, observational, multi-center natural history study of PD. PPMI Online refers to a large cohort of individuals recruited online, from which subgroups are further selected based on qualifying criteria to participate in various investigations of the progression of clinical features, imaging outcomes, or biologic and genetic markers possibly related to PD. For more information, see study link. In the study reported here, only data from participants in PPMI Online aged 60 years or older who did not have a PD diagnosis were included. Recruitment strategies included invitation to participate through other ongoing PPMI studies, Fox Insight, independently managed PD studies, digital and social marketing recruitment efforts to the PD community, and events/activities conducted by The Michael J. Fox Foundation or other representatives of PPMI. Participants completed a screening questionnaire and consented online using Evidation Health’s eConsent process prior to performing other study activities. Participants completed online questionnaires to collect demographics and vital statistics, and medical and family history. An UPSIT (revised version) was provided in person or mailed to eligible participants to complete. Participants were instructed to submit test responses via a web-based portal. Enrollment into PPMI Online is ongoing with a long-term goal of recruiting 500,000 participants globally. PPMI Online is registered on clinicaltrials.gov as NCT0506506017.
ST Direct seeks to identify individuals in the general population with an impaired sense of smell. This protocol engaged individuals from the general population aged 60 and older without a PD diagnosis living in the United States, Canada, England and the Netherlands. Participants were led to a web portal via QR code or URL where they were screened for eligibility. Contact information was collected, and consent was completed online. An UPSIT (revised version) was mailed to eligible participants to complete. Participants were instructed to submit test responses via a web-based portal.
Paid media and advertisements, collaborations with The Michael J. Fox Foundation and other organizations (including Quest Diagnostics, 23 and Me, Smell and Taste Association of North America), and active senior living outreach were some of the more successful recruitment efforts utilized as part of a multi-faceted approach. The study utilized a centralized, remote screening approach that de-emphasized direct site team outreach and made it easier for individual and community organization partners to refer participants. Enrollment into ST Direct is ongoing with a long-term goal of recruiting 500,000 participants.
Standard protocol approvals, registrations, and patient consents
All study participants provided written informed consent to participate in the associated studies. Bridging Study IRB approvals provided by IU IRB (protocols #1906568726 and 10630) and Penn IRB (protocol #843055); PPMI Online (protocol #20211908), ST Direct (protocol #12886). All research was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines after approval of the local ethics committees of the participating sites.
Statistical analysis
For the bridging study, descriptive statistics were calculated for the overall sample and by study group (HC vs. PD). Wilcoxon signed-rank tests were used to assess within-individual differences in UPSIT performance by version; Wilcoxon rank-sum tests were used to compare differences by study group and block order. To compare the revised and original UPSIT, the average UPSIT raw score and the difference in UPSIT percentiles were plotted using the “BlandAltmanLeh” package in R18,19.
Within the cohort study, descriptive statistics were calculated separately by sex and by population (PPMI Online, ST Direct, and combined). UPSIT percentiles were derived separately by sex and age category (60–64, 65–69, 70–74, 75–59, and ≥80 years) using the default method (based on an empirical distribution function with averaging) in the UNIVARIATE procedure in SAS v9.4 (SAS Institute Inc., Cary, NC; sas.com; RRID:SCR_008567). Percentiles corresponding to each UPSIT raw score were rounded to the nearest integer. If a raw score corresponded to multiple percentile values, the median percentile was used. If a raw score fell between two percentiles, the upper and lower bordering percentiles were averaged and rounded up to the nearest integer. All scores that corresponded to a percentile less than 1 were rounded to the 1st percentile.
Data availability
This analysis used data openly available from PPMI; data used in the preparation of this manuscript were obtained on July 3, 2023 from the Parkinson’s Progression Markers Initiative (PPMI) database (ppmi-info.org/access-data-specimens/download-data), RRID: SCR 006431. For up-to-date information on the study, visit www.ppmi-info.org. This analysis was conducted by the PPMI Statistics Core and used actual dates of activity for participants, a restricted data element not available to public users of PPMI data.
Code availability
Statistical analysis codes used to perform the analyses in this article are shared on Zenodo [10.5281/zenodo.11644053].
References
Ross, G. W. et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 63, 167–173 (2008).
Siderowf, A. et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov. Disord. 27, 406–412 (2012).
Devanand, D. P. et al. Olfactory deficits predict cognitive decline and Alzheimer's dementia in an urban community. Neurology 84, 182–189 (2015).
Woodward, M. R. et al. Validation of olfactory deficit as a biomarker of Alzheimer's disease. Neurol. Clin. Pract. 7, 5–14 (2017).
Leon-Sarmiento, F. E., Bayona, E. A., Bayona-Prieto, J., Osman, A. & Doty, R. L. Profound olfactory dysfunction in myasthenia gravis. PLoS One 7, e45544 (2012).
Doty, R. L., Shaman, P. & Dann, M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol. Behav. 32, 489–502 (1984).
Doty, R. L. Psychophysical testing of smell and taste function. Handb. Clin. Neurol. 164, 229–246 (2019).
Doty, R. L. The Smell Identification Test Administration Manual. third ed. Sensonics (1995).
Brumm, M. C. et al. Updated percentiles for the University of Pennsylvania smell identification test in adults 50 years of age and older. Neurology 100, e1691–e1701 (2023).
Yücepur, C. et al. University of Pennsylvania smell identification test: application to Turkish population. Kulak Burun. Bogaz. Ihtis Derg. 22, 77–80 (2012).
Fornazieri, M. A. et al. A new cultural adaptation of the University of Pennsylvania Smell Identification Test. Clinics 68, 65–68 (2013).
Rodríguez-Violante, M. et al. Comparing the accuracy of different smell identification tests in Parkinson’s disease: relevance of cultural aspects. Clin. Neurol. Neurosurg. 123, 9–14 (2014).
Pinto, J. M., Schumm, L. P., Wroblewski, K. E., Kern, D. W. & McClintock, M. K. Racial disparities in olfactory loss among older adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 69, 323–329 (2014).
Liu, G., Zong, G., Doty, R. L. & Sun, Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open 6, e013246 (2016).
Muirhead, N. B. & Saleh, E. H. Is the University of Pennsylvania Smell Identification Test (UPSIT) valid for the UK population? Otorhinolaryngologist 6, 99–103 (2013).
Simuni, T. et al. A biological definition of neuronal alpha-synuclein disease: towards an integrated staging system for research. Lancet Neurol. 23, 178–190 (2024).
Marek, K. The Parkinson’s Progression Markers Initiative (PPMI) Clinical - Establishing a Deeply Phenotyped PD Cohort AM 3.2. protocolsio. 2024.
R CoreTeam. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023) https://www.R-project.org/.
Lehnert, B. BlandAltmanLeh: Plots (Slightly Extended) Bland-Altman Plots: R Package Version 0.3.1; 2015 [Available from: https://CRAN.R-project.org/package=BlandAltmanLeh.
Acknowledgements
The PARS study was funded by the Department of Defense award number W81XWH-06-067. PPMI—a public–private partnership—is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including 4D Pharma, Abbvie, AcureX, Allergan, Amathus Therapeutics, Aligning Science Across Parkinson’s, AskBio, Avid Radiopharmaceuticals, BIAL, BioArctic, Biogen, Biohaven, BioLegend, BlueRock Therapeutics, Bristol-Myers Squibb, Calico Labs, Capsida Biotherapeutics, Celgene, Cerevel Therapeutics, Coave Therapeutics, DaCapo Brainscience, Denali, Edmond J. Safra Foundation, Eli Lilly, Gain Therapeutics, GE HealthCare, Genentech, GSK, Golub Capital, Handl Therapeutics, Insitro, Jazz Pharmaceuticals, Johnson & Johnson Innovative Medicine, Lundbeck, Merck, Meso Scale Discovery, Mission Therapeutics, Neurocrine Biosciences, Neuron23, Neuropore, Pfizer, Piramal, Prevail Therapeutics, Roche, Sanofi, Servier, Sun Pharma Advanced Research Company, Takeda, Teva, UCB, Vanqua Bio, Verily, Voyager Therapeutics, the Weston Family Foundation and Yumanity Therapeutics.The authors sincerely appreciate the numerous and valuable contributions of the large number of study participants in PPMI and ST Direct, as well as the study teams.
Author information
Authors and Affiliations
Consortia
Contributions
Study concept or design: K.M., A.S. Data collection: W.A., L.E.H., M.T., A.S. Data analysis or interpretation: K.A.P., W.A., C.G., R.K., M.C.B., C.S.C., L.E.H., M.T., R.L.D., K.M., A.S. Primary drafting of manuscript: K.A.P. All authors participated in the review, revision, and editing of the manuscript prior to finalization. All authors read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
R.L.D. is president and major shareholder in Sensonics International, the manufacturer and distributor of the olfactory test employed in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pierz, K.A., Aamodt, W., Gochanour, C. et al. Percentile scores for the revised University of Pennsylvania Smell Identification Test for 16,972 individuals 60 years of age and older. npj Parkinsons Dis. 11, 280 (2025). https://doi.org/10.1038/s41531-025-01095-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01095-9