Abstract
Motor and cognitive symptoms are key features of Parkinson’s disease (PD), which typically appear over time as the disease progresses. The thalamus, a central hub within basal ganglia-thalamocortical circuits, plays a crucial role in motor, sensory, and cognitive functions. This study examined thalamic nuclei volume and lateralization in individuals with PD and healthy controls, and explored their associations with cognitive and motor measures using partial least squares analysis. We found that cognitive alterations were linked to volume changes in specific nuclei of the anterior, ventral, medial, and posterior thalamic nuclear groups. Notably, the anteroventral and ventral posterolateral nuclei showed strong associations with the Montreal Cognitive Assessment used to assess mild cognitive impairment. Motor impairment was associated with volume lateralization asymmetries in the centromedian, mediodorsal medial, pulvinar anterior, and pulvinar medial nuclei. These findings underscore the multifaceted role of the thalamus in the motor and cognitive manifestations of this neurodegenerative disorder.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease characterized by the degeneration of dopaminergic neurons in the substantia nigra generating motor symptoms, such as tremors, rigidity, and bradykinesia1. However, this neurodegeneration extends to other regions in the brain and, although PD is typically defined by motor symptoms, it also tends to give rise to significant non-motor symptoms as PD progresses2. These non-motor symptoms encompass cognitive dysfunctions, which can potentially lead to long-term disability, mild cognitive impairment (MCI), and dementia3. Clinical research has focused on the basal ganglia-thalamo-cortical circuits and has shown that the thalamus plays a critical role4. Indeed, in PD the parallel motor, associative and limbic circuitries are all affected by dysfunctional dopaminergic modulation, and all of these circuitries go through thalamic regions. The thalamus includes several highly specialized nuclei involved in both motor and cognitive functions (see Fig. 1)5,6.
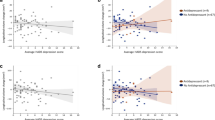
A Volume-PLS 1st latent component saliences showing the contribution to covariance of clinical, motor and cognitive tests. B Saliences for the thalamic nuclei volumes. The saliences shown are the stable ones that contribute significantly to the covariance. C Axial anatomical sections from a representative subject showing the thalamic nuclei with significant contributions to the covariance, using the same color-code used in (B). D Scatterplots showing statistically significant associations, after regressing out thalamic volume and age from each nuclei volume and age from the clinical and cognitive measures. Only statistically significant regressions are shown.
A substantial body of literature on PD has focused on the role of the first-order thalamic nuclei. Such studies have shown that the motor cortex receives innervation from the ventral lateral (VL) thalamus4,7, which has been found sometimes to be affected in PD patients with motor deficits8. For instance, deep brain stimulation of the posterior VL (VLp) has proven effective in specifically treating resting tremors in PD9. An additional crucial nucleus in the sensorimotor cortex along the VL pathway is the ventral posterolateral nucleus (VPL)10, which is also implicated in common motor manifestations in PD11.
Germane to research focused on first-order motor-related thalamic nuclei, other studies have focused on the involvement of higher-order relay thalamic nuclei in cognitive manifestations in PD. In this vein, research has highlighted that the degeneration of the centromedian (CM) and parafascicular (Pf) thalamic nuclei may contribute to certain cognitive deficits, particularly in attentional set-shifting, frequently observed in PD patients12. PD cognitive deterioration may also be linked to anterior thalamic nuclear regions such as the anteroventral (AV) nucleus, which is typically associated with episodic memory processes8, and the anterodorsal nucleus, which has been related to spatial navigation and visual memory13. In contrast, the pulvinar complex and its nuclei do not seem to have a clear role in PD degeneration. However, hypointensity susceptibility mapping in these nuclei has proven to predict cognitive deterioration as well as visual hallucinations after deep brain stimulation in PD patients14,15. Finally, medial nuclei such as the mediodorsal medial magnocellular (MDm) have widespread connections within networks involved in attention, working memory, and emotional control16 via their thalamocortical and corticothalamic projections to PFC17. A longitudinal study associating MDm free water changes with changes in cognition in early PD implicated the MDm in PD cognitive deterioration18.
Considering the specialized functions of thalamic nuclei and the degeneration of specific thalamic nuclei and PD, the present study was aimed at investigating volume changes in thalamic nuclei associated with cognitive and motor manifestations in PD. To this end, we used the segmentation of thalamic nuclei using a probabilistic atlas developed by combining high-resolution ex vivo MRI and histology6, along with between-group analysis of variance (ANOVA) and partial least squares (PLS) correlation analysis19. On the one hand, these thalamic nuclei atlas and segmentation enable the delineation of the thalamus into 26 specific nuclei using high-resolution T1-weighted structural MRI images from individual subjects. Notably, this atlas has demonstrated excellent test-retest reliability, robustness to changes in MRI contrast, and the ability to detect differential thalamic effects in neurological disorders such as Alzheimer’s disease20. On the other hand, PLS correlations will be used here to link changes in thalamic nuclei volume and their hemispheric lateralization asymmetries with individual cognitive and motor variables in PD. PLS allows for a multivariate exploratory analysis as it directly relates image features to behavioral variables19. Identifying specific thalamic nuclei changes in PD is expected to significantly contribute to our understanding of the pathophysiology of both motor and cognitive manifestations in PD. Specifically, based on previous evidence, we make the following four predictions: (a) the CM will be affected due to its importance in attentional set-shifting tasks, (b) the anterior nuclei may also be affected, potentially reflecting memory problems; (c) attention-related thalamic nuclei, such as the MDm in the medial group, may show associations with cognitive symptoms; and (d) motor deterioration will be driven by thalamic nuclei in the ventral group, especially the VL.
Results
Initially, we considered a total of 247 participants (mean age, 59.96 ± 9.96 years, 88 females), having 165 PD patients and 82 HCs from the initial sample after quality control. Table 1 presents the clinical characteristics of the groups. PD patients have significantly lower scores in the MoCA, digit modality, and semantic fluency tests relative to HCs.
First, we conducted group ANOVAs to examine differences between PD patients and HCs in terms of thalamic nuclei volumes and thalamic nuclei volume laterality. No significant group differences were found after Bonferroni correction for multiple comparisons (see Table 2). Therefore, we focus our reporting on the analysis of thalamic volume and lateralization in the PD patients’ group only. We performed a PLS regression analysis between the thalamic nuclei volumes and the clinical and cognitive test scores. Next, we also carried out a PLS regression analysis between the thalamic nuclei volume laterality and the clinical and cognitive variables.
Second, the volume PLS analysis yielded one significant latent component (LC), which explained 47.38% of the covariance between thalamic brain volumes and the clinical and cognitive variables (p = 0.002). This LC revealed a strong multivariate association, mainly indicating that variations in the volume of a subset of thalamic nuclei systematically covaried with the MoCA cognitive, digits and symbols, and letters and numbers tests (Fig. 1A). The thalamic nuclei correlating with these cognitive measures were bilaterally the AV, the lateral geniculate nucleus (LGN) and the VPL nuclei, as well as the left hemisphere mediodorsal lateral (MDl), pulvinar inferior (PuI), and ventral anterior (VA) nuclei (Fig. 1B, C). We next examined the correlation between these cognitive measures and nuclei volumes individually, as shown in Fig. 1D. The MoCA test correlated bilaterally with the AV (left: r(162) = 0.280, CI:[0.13, 0.42], p = 0.0003, %LOO = 100%; right r(162) = 0.187, CI:[0.04, 0.33], p = 0.016, %LOO = 100%) and the VPL (left: r(162) = −0.204, CI:[−0.35, −0.05], p = 0.008, %LOO = 100%; right: r(162) = −0.212, CI:[−0.35, −0.06], p = 0.0062, %LOO = 100%) pointing to these nuclei as possible identifiers of early cognitive decline. The digits and symbols test (assessing attention) correlated bilaterally with visual nuclei, LGN (left: r(162) = 0.194, CI:[0.04, 0.34], p = 0.013, %LOO = 100%; right r(162) = 0.263, CI:[0.12,0.40], p = 0.0006, %LOO = 100%) and left PuI (r(162) = 0.172, CI:[0.02, 0.32], p = 0.027, %LOO = 98.18%). Finally, the letters and numbers test (assessing working memory) correlated with the right LGN (r(162) = 0.187, CI:[0.04,0.33], p = 0.016, %LOO = 100%) and right VPL (r(162) = −0.167, CI:[−0.31, −0.03], p = 0.029, %LOO = 98.79%).
Third and last, the laterality-PLS analysis also yielded one significant LC, which explained 38.95% of the covariance between the laterality of thalamic brain volumes and the clinical and cognitive variables (p = 0.000199). This LC was mostly explained by the MDS-UPDRS-III motor test, followed by disease duration and MoCA (Fig. 2A). These clinical variables were linked to the volume lateralization asymmetries of the AV, CeM, CM, LD, LGN, MDm, pulvinar anterior (PuA), and pulvinar medial (PuM) (Fig. 2B, C). In individual regression analyses of volume laterality with the behavioral tests, the MDS-UPDRS-III motor test was positively associated with the volume lateralization asymmetries of CM (r(162) = 0.164, CI:[0.01, 0.31], p = 0.035, %LOO = 96.97%), MDm (r(162) = 0.187, CI:[0.04, 0.33], p = 0.016, %LOO = 100%), PuA (r(162) = 0.204, CI:[0.05, 0.35], p = 0.008, %LOO = 100%) and PuM (r(162) = 0.20, CI:[0.05, 0.34], p = 0.010, %LOO = 100%) (see Fig. 2D). Furthermore, disease duration was significantly associated with the volume laterality of AV (r(162) = -0.178, CI:[−0.32, −0.03], p = 0.021, %LOO = 98.18%), and LD (r(162) = −0.197, CI:[−0.34, −0.05], p = 0.011, %LOO = 100%). However, these correlations should be considered with caution as the variability in disease duration was minimal.
A Laterality-PLS 1st latent component saliences showing the contribution to covariance of cognitive and motor tests. B Saliences for the thalamic nuclei laterality indexes. The saliences shown are the stable ones that contribute significantly to the covariance. C Axial anatomical sections from a representative subject showing the thalamic nuclei with significant contributions to the covariance, using the same color-code used in (B). D Scatterplots showing statistically significant associations, after regressing out age from volume laterality and clinical and cognitive measures. Only statistically significant regressions are shown.
Discussion
In this study, we explored the covariance between thalamic nuclei volumes, laterality, and cognitive and motor assessments in PD. We examined the volume of 26 thalamic nuclei that were segmented using a probabilistic atlas of human thalamic nuclei, together with group comparisons and a multivariate PLS approach. The group analysis did not reveal any significant differences in thalamic volume and thalamic volume laterality between individuals with PD and HCs. However, analyses within the PD patients group showed that changes in cognitive performance (i.e., letters and numbers, digit and symbols and MoCA tests) were associated with volume differences in several thalamic nuclei including bilateral AV, LGN and VPL nuclei, left MDl, PuI and VA. Notably, the MoCA test, a well-established tool for assessing cognitive function and diagnosing MCI in PD, was associated bilaterally with volume changes in AV and VPL. In addition, the volume laterality PLS analysis highlighted that changes in motor function and MoCA tests were associated with volume laterality changes in the AV, CeM, CM, LD, LGN, MDm, PuA and PuM nuclei. Specifically, the CM, MDm, PuA and PuM showed increased volume lateralization asymmetries with increased motor dysfunction. These results will be discussed next.
Associations between AV volume bilaterally and the MoCA test are relevant given that the AV nucleus is densely interconnected with medial temporal lobe structures, an area typically affected in MCI in PD21,22 which is crucial for memory processing. Moreover, noradrenaline loss has been reported in the AV nucleus in PD, leading to alterations in neuronal activity within this region8. In addition, in the atlas used to segment thalamic nuclei, the anterior nuclei, including the anterodorsal nucleus, were all subsumed under the AV nucleus as a result of difficulties assigning boundaries between the different anterior nuclei due to the number of fibers observed in these anterior areas in the histological study used to develop this atlas (see6. Previous research indicates that the degeneration of the anterodorsal nucleus, along with cognitive decline, is observed in Lewy body diseases, such as PD, characterized by the accumulation of α-synuclein protein23. Neuropathological studies also suggest that the anterodorsal nucleus is one of the primary sites of degeneration in PD, showing severe neuronal loss and neurofibrillary Tau protein tangle formation24.
The VPL nucleus volume showed a bilateral negative association with cognitive decline measured with the MoCA test, and the right VPL volume was also negatively associated with the letters and numbers test, which mainly assesses working memory. The VPL receives sensory information from the spinal cord and innervates the sensorimotor cortex, which is known to be affected in PD8,25,26. Moreover, regions within the sensorimotor cortex are crucial for higher-order processes such as verbal creativity or cognitive control of language27,28. Our data revealed that only the VPL shows negative correlations with performance measurements. Since the developmental trajectories of thalamic nuclei volume are still unknown in both health and disease, it might be possible that a reduced VPL volume is associated with better performance on general cognitive assessments in typically developing individuals. Future studies should further investigate the thalamic nuclei increases and reductions in volume and their relationship with cognitive performance in PD.
Interestingly, the LGN showed a positive bilateral association with the digits and symbols test, and the same was true for left PuI. This cognitive assessment requires the engagement of the visuospatial system29,30, where the LGN and PuI have a main role due to their connections with the visual cortex. Furthermore, the integration of visual sensory input and executive processes, which is crucial for such tasks, is compromised in cases of blast-related brain injury when the visual pathway network is impaired31. Additionally, the volume of the right LGN also showed a positive association with the letters and numbers task that evaluates working memory, in line with evidence indicating that the LGN coordinates neuronal activity in V1 as a function of modulations induced by attention-demanding and working memory tasks32,33.
Laterality analysis highlighted the importance of the thalamus in motor functions, with several nuclei showing statistically significant positive associations between volume lateralization asymmetries and the number of motor symptoms. In this regard, the association between the MDm nucleus and the MDS-UPDRS-III motor test highlights its relevance in motor function in PD. This observation may appear contrary to the classification of MDm as part of the high-order nuclei. However, MDm neurons process information related to motor responses and participate in working memory processes, which makes this nucleus a pivotal one for both motor and cognitive function34. Furthermore, longitudinal research examining free water imaging in MDm showed early cognitive deterioration in PD patients and, subsequently, highlighted the relevant role of this nucleus in signaling PD progression18. The lateralization asymmetries in the volumes of the PuM and PuA also showed associations with the MDS-UPDRS-III motor test, potentially indicating motor deficits that could be linked to the pulvinar role in visual motor planning, particularly concerning spatial choices35. This function is typically attributed to the Pulvinar projections to the frontal and parietal eye fields35,36. Finally, the lateralization asymmetries in the volume of the CM showed a positive correlation with the MDS-UPDRS-III that could be linked to the influence of the CM through its participation in the direct basal ganglia pathway with the motor cortex potentially affecting motor and nonmotor manifestations37,38,39. Regarding motor manifestations, although further research is needed, deep brain stimulation (DBS) of the CM nucleus has been shown to alleviate levodopa-induced dyskinesias, freezing episodes, and tremors in patients with advanced Parkinson’s disease40,41. Meanwhile, CM/PF neurons play a key role in attentional shifts and, through interactions with the dopaminergic system, which may influence action bias, goal-directed learning, and reward responses to motivational stimuli42. Also, the CM/PF complex has been found to be degenerated in idiopathic PD, with a 30–40% loss in patients5.
This study represents a first attempt to specifically link thalamic nuclei volume with cognitive and motor symptoms of PD. However, several limitations of the present study and potential directions for future studies must be considered. First, using baseline visit data from the PPMI dataset provided us with a reasonable sample size. This allowed us to limit to some extent undesired variability, such as data collected in MRI devices of different types or with different field strengths. However, in applying these selection criteria, the majority of PD subjects in this study had relatively normal cognitive function, which may explain the lack of group differences in the ANOVAs between PD patients and HCs, and, within the PD patients’ group, may have limited the sensitivity to predict more severe motor and cognitive manifestations. Future studies using longitudinal and/or follow-up data could provide valuable insights in this regard. Second, a more comprehensive cognitive assessment, including multiple cognitive tests for each domain, would be advantageous to more precisely evaluate MCI status and progression. Finally, the study only assessed volumetric measurements. Although examining volumetric differences and laterality changes in volume can provide us with relevant information about the involvement of thalamic nuclei and thalamic circuitries in PD and MCI manifestations, future studies should combine volumetric and diffusion measures to further characterize the role of grey and white matter, as well as potential interactions between them in predicting motor and cognitive deterioration in PD.
In sum, our findings point to two relevant and differentiated phenomena. Cognitive changes are related to a set of thalamic nuclei volume changes (AV, LGN and VPL, PuI and VA) rather than to specific nuclei differences, with a noteworthy positive association between the AV and VPL nuclei volume with cognitive decline. Motor deterioration, on the other hand, is reflected in specific volume lateralization asymmetries profiles of the CM, MDm, PuI, and PuM nuclei. An unexpected finding was the negative correlations observed between VPL volume and clinical as well as cognitive measures, an observation that warrants further investigation in the context of thalamic nuclei volume development trajectories across typical development and disease. Overall, these findings align well with previous neuroanatomical and neuropathological studies and provide valuable insights in regard to the links between specific thalamic nuclei and initial critical cognitive and motor manifestations in PD.
Methods
Participants
In this work, we used the Parkinson’s Progression Markers Initiative (PPMI) database43. PD patients and healthy controls (HCs) from this dataset were included based on the availability of a T1-weighted MPRAGE image obtained in a Siemens Trio 3 T MRI scanner, to avoid inter-scanner variability, and the Montreal cognitive assessment (MoCA) scores at baseline. In total, 184 patients with PD (age 61 [37–77], gender male 105 [63.6%]) and 82 HCs (age 58 [30–80], gender male 54 [65.8%]) were selected from this database (https://www.ppmi-info.org/). After data quality control for motion or artifacts in the structural images, a total of 19 PD patients were excluded, leaving 165 PD patients in the final study sample. Most of the 165 PD patients were medication naïve, with only 18 being on medication at baseline. The remaining 147 PD patients started medication after baseline: either 3 months later (48 PD patients), 6 months later (42 PD patients), or beyond 6 months (57 PD patients). Thus, PD medication should have minimal impact on the effects reported in the present work. Table 1 presents the clinical characteristics of the groups. PD patients have significantly lower scores in the MoCA, digit modality, and semantic fluency tests relative to HCs. The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Neuropsychological and clinical examination
Clinical and neuropsychological examinations of PD participants were performed while they were under the effects of their habitual antiparkinsonian medication. Parts I, II, III, and IV of the Movement Disorders Society Unified Parkinson Disease Rating Scale (MDS-UPDRS) were used to evaluate non-motor issues, daily living activities, motor features, and motor complications, respectively44. The MoCA test was used as a general cognitive screening test45. Exclusion criteria included a history of head trauma, psychiatric or neurological disorders other than PD, other major medical comorbidities, alcohol or drug dependence or abuse. PD patients with dementia, as diagnosed according to the International Parkinson and Movement Disorder Society (MDS) Task Force criteria, were also excluded46. In addition to the previous tests, each subject performed three cognitive assessments: Attention, comprising alertness, was measured by the inverse digit span memory and symbol digit modality tests30; Working memory maintenance and manipulation was evaluated by the letters-numbers test47; and Language was evaluated by the semantic fluency test48.
MRI acquisition
PPMI MRI scans were obtained from 11 sites mainly from the United States of America and Europe (https://www.ppmi-info.org/about-ppmi/ppmi-clinical-sites) on 3 Tesla SIEMENS MAGNETOM TRIO using a standardized acquisition protocol for the T1-weighted image acquired with a 3D magnetization-prepared rapid gradient echo (MPRAGE) sequence with the following parameters: 176 sagittal slices with thickness = 1.0 mm and no gap; repetition time = 2300 ms; echo time = 2.98 ms; flip angle = 9°; field of view = 240 × 256 mm; matrix size= 240 × 256; inversion time = 900 ms; voxel size = 1 × 1 × 1 mm3.
MRI anatomical segmentation
Automated voxel-based subcortical segmentation and cortical parcellation were extracted from the T1-weighted MPRAGE images using FreeSurfer (v7.2, https://surfer.nmr.mgh.harvard.edu)49. Two independent raters graded each subject’s recon-all outcome based on three features: the presence of motion artifacts in the images, skull stripping, and white matter (WM) and grey matter (GM) segmentation accuracy. Each parameter was assessed on a scale of one to three. The inter-judge agreement was 76.44% in motion artifacts, 56.5% in skull stripping and 71.10% in GM/WM matter segmentation accuracy. Disagreement on ratings was resolved by discussing each individual case. As noted above, 19 PD subjects who presented extreme motion, bad segmentation, poor skull striping, abnormal intracranial structures, or a strong signal dropout were excluded. If necessary, images were manually corrected by modifying the skull stripping, the WM watershed or adding control points until segmentation imperfections were minimized.
Subsequently, the thalamic nuclei volumes were obtained based on a segmentation tool included in FreeSurfer (v7.2) that derives from a probabilistic atlas of the human thalamus which was built combining high-resolution ex vivo MRI of six autopsy samples with manual delineation of 26 thalamic nuclei in each hemisphere on the serial histology of twelve whole thalami from the same samples (see Fig. 3)6. Out of the 26 nuclei, six (i.e., limitans-suprageniculate, parafascicular, paratenial, paracentral, ventromedial, and reticular) were excluded due to their small size and having large coefficients of variation in terms of mean volume at a standard resolution of 1 mm³. The remaining 20 thalamic nuclei volume measurements were normalized by regressing out the age and the whole thalamus volume of each subject’s hemisphere obtained with this same thalamic atlas, to account for differences in brain size.
AV anteroventral, CeM central medial, CL central lateral, CM centromedian, LD laterodorsal, LGN lateral geniculate, LP lateral posterior, MDl Mediodorsal lateral parvocellular, MDm Mediodorsal medial magnocellular, MGN Medial geniculate, MV (re) reuniens (medial ventral), PuA pulvinar anterior, PuI pulvinar inferior, PuL pulvinar lateral, PuM pulvinar medial, VA ventral anterior, VAmc ventral anterior magnocellular, VLa ventral lateral anterior, VLp ventral lateral posterior, VPL ventral posterolateral.
In addition, we sought to examine thalamic nuclei volume lateralization asymmetries, as this is one of the potential hallmarks of PD progression and could be associated with its motor and/or cognitive manifestations50. We computed the laterality index [LI; see Eq. (1)] of each thalamic nucleus with the following formula, with positive values defining leftward asymmetries and negative values defining rightward asymmetries:
Statistical analysis
First, group ANOVAs were conducted for each thalamic nuclei volume and each thalamic nuclei volume laterality to examine potential differences between PD patients and HCs. No covariates were added since age and the whole thalamus volume of each subject’s hemisphere were regressed out from the nuclei variance for normalization. Second, to investigate the relationship between thalamic nuclei features and PD motor and cognitive manifestations, we focused our analyses on the PD group and carried out a PLS correlational analysis19 between the GM volume of thalamic nuclei and the scores of the following clinical and cognitive measurements: disease duration, MoCA, MDS-UPDRS-III motor test, semantic fluency test, letters-numbers test and digit and symbols test. Third, an equivalent PLS correlation analysis was performed with the LI measures of the thalamic nuclei and the same clinical and cognitive scores. Before conducting the PLS analyses, we regressed out subjects’ age from clinical and cognitive measurements. It is worth noting that we also run both the volume PLS and the volume laterality PLS analyses with the HCs group only, but these analyses did not yield any statistically significant results after correcting for multiple comparisons.
In brief, PLS involves the singular value decomposition of the covariance matrix between the behavioral variables and the thalamic nuclei, resulting in latent components (LC) corresponding to behavioral and imaging scores, respectively. Each LC has a set of behavior weights and brain weights, which indicate how strongly each behavioral and brain variable contributes to the brain-behavior covariance. The significance of each LC-explained correlation was determined by permutation testing (50,000 iterations)19. The LC was considered significant if the corresponding p-value was less than 0.05, applying Bonferroni’s correction for multiple comparisons. The stability of brain and behavior weights was obtained using bootstrapping (50,000 iterations), selecting as stable those weights whose 95% confidence interval did not include 0.
To interpret the relationships revealed in a latent component, we also examined associations between clinical and cognitive test scores and the volume and LI of the nuclei that contributed to a significant LC, calculating the partial correlation considering age as a nuisance covariate. To assess the reliability of the correlational effects, we conducted a leave-one-out (LOO) analysis for all correlations with clinical and cognitive covariates that were statistically significant. This analysis was performed iteratively, excluding one individual at a time for each significant correlation. We then calculated the percentage of iterations in which the correlation remained statistically significant. A value of 95% or higher was considered indicative of a reliable correlational effect. This percentage (i.e., %LOO) is reported in the Results section for all correlations that met the statistical significance threshold and demonstrated a %LOO of 95 or above.
Data availability
We utilized the Parkinson’s Progression Markers Initiative (PPMI) database43. Parkinson’s disease patients and healthy controls from the Baseline phase of this dataset were included (https://www.ppmi-info.org/access-data-specimens/download-data), RRID:SCR_006431. Data used in the preparation of this article were initially obtained on [2019-04-29]. For up-to-date information on the study, visit http://www.ppmi-info.org.
Code availability
The data and code related to this work can be found at https://osf.io/8b4wp/.
References
Damier, P., Hirsch, E. C., Agid, Y. & Graybiel, A. M. The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122, 1437–1448 (1999).
Cools, R., Barker, R. A., Sahakian, B. J. & Robbins, T. W. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb. Cortex 11, 1136–1143 (2001).
Fang, C., Lv, L., Mao, S., Dong, H. & Liu, B. Cognition deficits in Parkinson’s disease: mechanisms and treatment. Parkinson’s. Dis. 2020, 2076942–11 (2020).
Alexander, G. E., DeLong, M. R. & Strick, P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381 (1986).
Halliday, G. M. Thalamic changes in Parkinson’s disease. Parkinsonism Relat. Disord. 15, S152–S155 (2009).
Iglesias, J. E. et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. NeuroImage 183, 314–326 (2018).
Park, K. Y. et al. Mapping language function with task-based vs. resting-state functional MRI. Plos One 15, e0236423 (2020).
Pifl, C., Kish, S. J. & Hornykiewicz, O. Thalamic noradrenaline in Parkinson’s disease: Deficits suggest role in motor and non-motor symptoms. Mov. Disord. 27, 1618–1624 (2012).
Gross, R. E. et al. Histological analysis of the location of effective thalamic stimulation for tremor: Case report. J. Neurosurg. 100, 547–552 (2004).
Charyasz, E. et al. Functional mapping of sensorimotor activation in the human thalamus at 9.4 Tesla. Front. Neurosci. 17, 1116002 (2023).
Stepniewska, I., Sakai, S. T., Qi, H.-X. & Kaas, J. H. Somatosensory input to the ventrolateral thalamic region in the macaque monkey: a potential substrate for parkinsonian tremor. J. Comp. Neurol. 455, 378–395 (2003).
Villalba, R. M., Wichmann, T. & Smith, Y. Neuronal loss in the caudal intralaminar thalamic nuclei in a primate model of Parkinson’s disease. Brain Struct. Funct. 219, 381–394 (2014).
Boelens Keun, J. T. et al. Structural assessment of thalamus morphology in brain disorders: a review and recommendation of thalamic nucleus segmentation and shape analysis. Neurosci. Biobehav. Rev. 131, 466–478 (2021).
Matsuura, K. et al. Low pulvinar intensity in susceptibility-weighted imaging may suggest cognitive worsening after deep brain stimulation therapy in patients with Parkinson’s disease. Front. Neurol. 10, 1158 (2019).
Matsuura, K. et al. Pulvinar quantitative susceptibility mapping predicts visual hallucinations post-deep brain stimulation in Parkinson’s disease. Brain Behav. 13, e3263 (2023).
Golden, E. C., Graff-Radford, J., Jones, D. T. & Benarroch, E. E. Mediodorsal nucleus and its multiple cognitive functions. Neurology 87, 2161–2168 (2016).
Mengxing, L., Lerma-Usabiaga, G., Clascá, F. & Paz-Alonso, P. M. High-resolution tractography protocol to investigate the pathways between human mediodorsal thalamic nucleus and prefrontal cortex. J. Neurosci. 43, 7780–7798 (2023).
Guttuso, T. et al. Thalamic dorsomedial nucleus free water correlates with cognitive decline in Parkinson’s disease. Mov. Disord. 37, 490 (2021).
McIntosh, A. R. & Lobaugh, N. J. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage 23, S250–S263 (2004).
Wu, Y. et al. Abnormal functional connectivity of thalamic subdivisions in Alzheimer’s disease: a functional magnetic resonance imaging study. Neuroscience 496, 73–82 (2022).
Brandão, P. R. et al. Mapping brain morphology to cognitive deficits: a study on PD-CRS scores in Parkinson’s disease with mild cognitive impairment. Front. Neuroanat. 18 (2024).
Groen, T. van., Kadish, I. & Wyss, J. M. Role of the anterodorsal and anteroventral nuclei of the thalamus in spatial memory in the rat. Behav. Brain Res. 132, 19–28 (2002).
Brooks, D. & Halliday, G. M. Intralaminar nuclei of the thalamus in Lewy body diseases. Brain Res. Bull. 78, 97–104 (2008).
Xuereb, J. H. et al. Nerve cell loss in the thalamus in Alzeheimer’s disease and Parkinson’. S Dis. Brain 114, 1363–1379 (1991).
Remy, P., Doder, M., Lees, A., Turjanski, N. & Brooks, D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128, 1314–1322 (2005).
Westlund, K. N., Zhang, D., Carlton, S. M., Sorkin, L. S. & Willis, W. D. Chapter 5 - Noradrenergic innervation of somatosensory thalamus and spinal cord. in Progress in Brain Research (eds Barnes, C. D. & Pompeiano, O.) vol. 88 77–88 (Elsevier, 1991).
Feng, Q. et al. Verbal creativity is correlated with the dynamic reconfiguration of brain networks in the resting state. Front. Psychol. 10 (2019).
Tabassi Mofrad, F., Jahn, A. & Schiller, N. O. Dual function of primary somatosensory cortex in cognitive control of language: evidence from resting state fMRI. Neuroscience 446, 59–68 (2020).
Jaeger, J. Digit symbol substitution test. J. Clin. Psychopharmacol. 38, 513–519 (2018).
Smith, A. The Symbol-Digit Modalities Test: A Neuropsychologic Test of Learning and Other Cerebral Disorders Learning Disorders. (Special Child Publications, In, Helmuth J., ed., Seattle, 1973).
Lee, E.-Y. et al. Visual working memory deficits in patients with Parkinson’s disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain 133, 2677–2689 (2010).
Matar, E., Brooks, D., Lewis, S. J. G. & Halliday, G. M. Limbic thalamus atrophy is associated with visual hallucinations in Lewy body disorders. Neurobiol. Aging 112, 122–128 (2022).
van Kerkoerle, T., Self, M. W. & Roelfsema, P. R. Layer-specificity in the effects of attention and working memory on activity in primary visual cortex. Nat. Commun. 8, 13804 (2017).
Watanabe, Y. & Funahashi, S. Thalamic mediodorsal nucleus and working memory. Neurosci. Biobehav. Rev. 36, 134–142 (2012).
Wilke, M., Turchi, J., Smith, K., Mishkin, M. & Leopold, D. A. Pulvinar inactivation disrupts selection of movement plans. J. Neurosci. 30, 8650–8659 (2010).
Dominguez-Vargas, A.-U., Schneider, L., Wilke, M. & Kagan, I. Electrical microstimulation of the pulvinar biases saccade choices and reaction times in a time-dependent manner. J. Neurosci. 37, 2234 (2017).
Benarroch, E. What is the role of the intralaminar thalamic input to the striatum and its potential implications in Parkinson disease?. Neurology 101, 118–123 (2023).
Galvan, A. & Smith, Y. The primate thalamostriatal systems: Anatomical organization, functional roles and possible involvement in Parkinson’s disease. Basal Ganglia 1, 179–189 (2011).
Mandelbaum, G. et al. Distinct cortical-thalamic-striatal circuits through the parafascicular nucleus. Neuron 102, 636–652.e7 (2019).
Peppe, A. et al. Deep brain stimulation of CM/PF of thalamus could be the new elective target for tremor in advanced Parkinson’s Disease?. Parkinsonism Relat. Disord. 14, 501–504 (2008).
Testini, P., Min, H.-K., Bashir, A. & Lee, K. H. Deep brain stimulation for tourette’s syndrome: the case for targeting the thalamic centromedian–parafascicular complex. Front. Neurol. 7 (2016).
Matsumoto, N., Minamimoto, T., Graybiel, A. M. & Kimura, M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J. Neurophysiol. 85, 960–976 (2001).
Marek, K. et al. The Parkinson progression marker initiative (PPMI). Prog. Neurobiol. 95, 629–635 (2011).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. J. Mov. Disord. Soc. 23, 2129–2170 (2008).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatrics Soc. 53, 695–699 (2005).
Emre, M. et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 22, 1689–1707 (2007).
Mielicki, M. K., Koppel, R. H., Valencia, G. & Wiley, J. Measuring working memory capacity with the letter–number sequencing task: Advantages of visual administration. Appl. Cogn. Psychol. 32, 805–814 (2018).
Kaplan, E., Goodglass, H. & Weintraub, S. Boston Naming Test. (Lea & Febiger, 1983).
Destrieux, C., Fischl, B., Dale, A. & Halgren, E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 53, 1–15 (2010).
Tomer, R., Levin, B. E. & Weiner, W. J. Side of onset of motor symptoms influences cognition in Parkinson’s disease. Ann. Neurol. 34, 579–584 (1993).
Acknowledgements
The authors would like to thank Jake Vinnacombe and Caroline Handley for proofreading and helpful comments on the manuscript. V.F-G. was supported by a Basque Government predoctoral fellowship (PRE_2019_1_0085); T.E-P. was supported by a predoctoral grant from the Spanish Ministry of Economy and Competitiveness (BES-2016-079489); M.C.R-O. was supported by grants from the Government of Navarra, Instituto de Salud Carlos III, and Centro para el Desarrollo Tecnológico y la Innovación from the Spanish Government; C.C-G. was supported by a grant from the Spanish Ministry of Science and Innovation (PID2023-149410b-100), and from the Programa Investigo of the Basque Government; P.M.P-A. was supported by grants from the Spanish Ministry of Science and Innovation (PID2021-123574NB-I00), from the Basque Government (PIBA-2021-1-0003), and from “la Caixa” Foundation (ID 100010434) under the agreement HR18-00178-DYSTHAL. BCBL acknowledges funding from the Basque Government through the BERC 2022–2025 program and by the Spanish State Research Agency through BCBL Severo Ochoa excellence accreditation CEX2020-001010-S. PPMI—a public-private partnership—is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including 4D Pharma, Abbvie, AcureX, Allergan, Amathus Therapeutics, Aligning Science Across Parkinson's, AskBio, Avid Radiopharmaceuticals, BIAL, BioArctic, Biogen, Biohaven, BioLegend, BlueRock Therapeutics, Bristol-Myers Squibb, Calico Labs, Capsida Biotherapeutics, Celgene, Cerevel Therapeutics, Coave Therapeutics, DaCapo Brainscience, Denali, Edmond J. Safra Foundation, Eli Lilly, Gain Therapeutics, GE HealthCare, Genentech, GSK, Golub Capital, Handl Therapeutics, Insitro, Jazz Pharmaceuticals, Johnson & Johnson Innovative Medicine, Lundbeck, Merck, Meso Scale Discovery, Mission Therapeutics, Neurocrine Biosciences, Neuron23, Neuropore, Pfizer, Piramal, Prevail Therapeutics, Roche, Sanofi, Servier, Sun Pharma Advanced Research Company, Takeda, Teva, UCB, Vanqua Bio, Verily, Voyager Therapeutics, the Weston Family Foundation and Yumanity Therapeutics.
Author information
Authors and Affiliations
Contributions
V.J.F-G.: Methodology, Software, Formal Analysis, Writing (OD), Visualization, Project administration; T.E-P.: Methodology, Formal Analysis; M.C.R-O.: Conceptualization, Writing (RE); C.C-G.: Methodology, Writing (RE), Supervision, Funding acquisition; P.M.P-A.: Conceptualization, Methodology, Project administration, Writing (OD), Supervision, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
M.C.R-O. received financial support for attending scientific meetings and lectures from Boston Scientific, Insightec, Palex, and Medtronic, from advisory boards from Boston Scientific, Medtronic, and Insightec, and from Siemens for research projects outside the submitted work. The authors declare no other conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ferrer-Gallardo, V.J., Esteban-Peñalba, T., Rodriguez-Oroz, M.C. et al. Thalamic nuclei volume changes associated with cognitive and motor manifestations of Parkinson’s disease. npj Parkinsons Dis. 11, 279 (2025). https://doi.org/10.1038/s41531-025-01129-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-01129-2