Abstract
Electronic inhalers provide information about patterns of routine inhaler use. During a 12-week study, 360 asthma patients using albuterol Digihaler generated 53,083 inhaler events that were retrospectively analyzed. A total of 41,528 (78%) of the recorded inhalation events were suitable for flow analysis (having a PIF ≥ 18 L/min and <120 L/min). Median PIF, inhalation volume, inhalation duration, and time to PIF for these events steadily decreased between the first and last 10 days of the study, by 5.1%, 12.6%, 15.9%, and 6.4%, respectively. Continuous short-acting beta2-agonist (SABA) overuse, defined as ≥2 SABA inhalations/week throughout the study period, was seen in 29% (n = 104) of patients. Of 260 patients with ≥1 instance of acute short-term SABA overuse, 55 (21%) had a confirmed exacerbation. Electronic recording of real-life inhaler use can capture valuable, objective information that could inform disease management and clinical decision-making.
Similar content being viewed by others
Introduction
Inhalation therapy is the cornerstone of asthma management, delivering medication to the site of action. However, this treatment is reliant upon patients demonstrating correct inhaler technique to allow for the optimal deposition of the medication to achieve a therapeutic effect1,2,3,4,5. Despite acceptable inhaler technique demonstrations in clinical practice or during study visits, patients typically do not maintain correct technique over time6,7,8. Suboptimal inhaler technique can result in impaired drug delivery9,10 and poor clinical outcomes7,11,12,13,14,15. For the past 40 years, the proportion of patients incorrectly using their inhalers has not changed16.
Short-acting beta2-agonists (SABAs) are bronchodilators used for as-needed symptom relief. The extent of SABA use can be reflective of disease status and outcomes. High SABA use is common in uncontrolled asthma17,18,19,20,21,22,23,24 and associated with increased asthma-related unscheduled care, morbidity, and mortality risk20,21,23,25,26,27,28,29. Another challenge in asthma management is poor treatment adherence to controller medications30,31.
Traditional inhalers utilizing dose counters do not typically provide information on whether a dose was inhaled and if the inhaler technique was correct32,33. Digital inhaler systems do provide objective data, including time of use and, in some cases, quality of inhalations34. Such objective data can assist in identifying patients with SABA overuse, suboptimal treatment adherence, and/or poor inhaler technique35,36. Digital inhalers also offer other potential advantages, including monitoring other more functional and clinically meaningful aspects of inhaler use, such as peak inspiratory flow (PIF) (related to using a DPI with a fast inhalation) and inhalation volume (related to exhaling before an inhalation then inhaling for as long as possible)3,5,14,37,38,39. This technology can be utilized to facilitate objective clinical decision making and the delivery of personalized care to patients with respiratory disease.
The ProAir® Digihaler®40 is a Food and Drug Administration (USA)-approved albuterol multidose dry powder inhaler (DPI) with an integrated electronic module (Fig. 1). This Digihaler inhaler objectively measures and records inhalation parameters (PIF, inhalation volume, inhalation duration, and time to PIF), while also providing a time-stamped recording every time the inhaler is used. A recently reported study of asthma patients with a history of exacerbations, using the albuterol Digihaler for 12 weeks, retrospectively analyzed the data downloaded from their inhalers at the end of the study38. A machine-learning model predictive of impending exacerbations was developed with features that included increased SABA use and deteriorating inhalation parameters.
The main aim of the current post-hoc analyses of this above-mentioned study38 was to explore patterns of inhaled reliever medication use (i.e. episodic increases and overall levels of SABA use) as an objective measure of symptom burden, complemented by real-time data on inhalation parameters in patients using the albuterol Digihaler. We also sought to determine whether information provided by the Digihaler inhaler could be used as a tool to assess inhaler technique.
Results
Study population
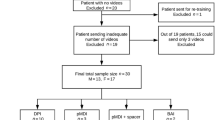
Overall, 397 patients across 45 investigational centers in the US were enrolled to the intention-to-treat (ITT) population. Of these patients, 381 (96%) completed the study and 360 (91%) made at least one PIF-confirmed inhalation using the albuterol Digihaler (Fig. 2). Baseline demographics and maintenance medication use for the 360 patients who completed the study with at least one PIF-confirmed inhalation using the albuterol Digihaler are shown in Table 1; 80.8% of patients were female and the mean age was 49.9 years.
Inhalation events
Across the population of patients who completed the study, 53,083 inhaler events were recorded from a total of 736 inhalers from 360 patients. The distribution of PIF across inhalation events is shown in Table 2. A total of 41,528 (78%) of the recorded inhalation events were suitable for flow analysis (having a PIF ≥ 18 L/min and <120 L/min). Their median (interquartile range [IQR]) PIF and inhalation volume were 70.3 (30.1) L/min and 1.18 (0.87) L, respectively. Median (IQR) inhalation duration and time to PIF were 1.33 (0.9) seconds and 0.4 (0.3) seconds, respectively.
Of the events that were excluded, 6635 (12.5%) had a PIF below the 18 L/min MEMS threshold to record flow or no inhalation, and 4331 (8.2%) had a PIF ≥ 120 L/min. Technical errors, such as unexpected multiple inhalations recorded as a single event, timeout and unexpected exhalations, accounted for 589 events (1.1%) (Table 2).
There was a significant difference between the median of the inhalation parameters over the first 10 days and the median over the last 10 days of the study (Table 3). The change in median PIF, inhalation volume, and inhalation duration across the full study period can be seen in Fig. 3. The median percentage change between the first and last 10 days for PIF, inhalation volume, inhalation duration, and time to PIF was −5.1%, −12.6%, −15.9%, and −6.4%, respectively. A typical patient example from this study highlighting these inhalation parameter trends over time is shown in Supplementary Fig. 1.
SABA use
Of the 53,083 inhaler events from patients who completed the study, 36.2% occurred as episodes of single inhalations; the remaining events (63.8%) occurred as episodes of two (44.5%), three (6.5%), four (2.4%), and more than four consecutive inhalations (10.4%) within 60 seconds. The mean number of inhalations per patient per day was 1.70. Most patient days had zero inhalations (56.2%), while 9.0% of patient days had one inhalation, and 14.2% had two inhalations (Fig. 4).
Of the 360 patients who completed the study with at least one PIF-confirmed inhalation using the albuterol Digihaler, 260 (72.2%) patients had a total of 476 bursts of SABA use (Table 4). The mean number of SABA bursts per patient over the 3-month duration of the study was 1.32; 98 (38%) patients had one SABA burst, 111 (43%) had two SABA bursts, 48 (18%) had three SABA bursts, and three (1%) patients had four SABA bursts. Of the 260 patients with SABA bursts, 55 (21%) patients had confirmed American Thoracic Society/European Respiratory Society-defined exacerbations, compared with nine (9%) of the 100 patients who did not have a SABA burst (Z = 2.702; two-tailed p = 0.007). Of all exacerbations reported during the study (n = 74), 40 (54%) were concomitant with a burst of SABA use.
Continuous SABA overuse was seen in 28.9% of patients and a larger proportion of SABA overusers had SABA bursts (96.2%), compared with patients who were not classified as SABA overusers (62.5%) (Z = 6.462; two-tailed p < 0.001). SABA overusers also had a greater mean number of SABA bursts per patient than SABA non-overusers (2.07 vs. 1.02; Table 4) (T = 9.663; two-tailed p < 0.001). Individual examples of SABA use patterns are shown in Fig. 5. Figure6 shows a patient example from this study of a patient with a period of SABA overuse and a subsequent period with extremely high SABA use ( ≥ 40 puffs/day). This patient displayed a decrease in inhalation volume between the first and second periods of overuse.
a SABA bursts without a confirmed exacerbation. b SABA burst with a confirmed exacerbation. c Continuous SABA overuse without SABA bursts or a confirmed exacerbation. Shaded blue areas represent SABA bursts, the red line represents a confirmed exacerbation, and the green line represents trend in SABA use. SABA bursts were defined as a daily mean of at least three inhalations in the last 2 days and an increase in daily mean inhalations in the last 2 days, compared with the previous 2 weeks. Multiple SABA bursts within a 7-day period were counted as one burst. SABA overuse was defined as at least two inhalations per week, every week, over the study period.
Safety
Of the 397 patients in the ITT population, 127 (32.0%) experienced any adverse event (AE). Two AEs were deemed by the investigators to be possibly related to treatment; both of these were asthma-related events of moderate severity. Six patients had ≥1 serious AE, none of which were deemed possibly related to treatment. Two patients had ≥1 AE leading to discontinuation, none of which were deemed possibly related to treatment. There were no deaths during the study.
Discussion
The findings of this study enhance our understanding of the day-to-day burden of asthma, through accurate and objective recording of patterns of real-life SABA use and inhalation parameters. The results demonstrate a high burden of asthma, with continuous SABA overuse and SABA bursts both being frequently observed. While the vast majority of patients maintained sufficient PIF to generate drug dose emission, PIF and inhalation volume were observed to decline from the first 10 days of the study. Reductions in inhalation flow and volume were also observed a few days before reported asthma exacerbations38. These findings support the hypothesis that the use of a digitally enhanced SABA inhaler, may provide clinically meaningful data about inhaler technique, level of asthma control and physiological changes during worsening episodes, that could be used to enhance the clinician decision-making process.
This research supports established evidence that subjectively observed patient inhaler technique wanes within the days following single episodes of standard-of-care inhaler training8,40,41. However, for the first time, these results show that correct inhaler technique maintenance after training is more complex than just suboptimal PIF. In parallel with the decline in PIF, previously unreported deterioration in both inhalation volume and inhalation duration were also observed. The albuterol Digihaler is a multidose DPI with an integrated electronic module42. When using a DPI, the recommended generic technique for the inhalation maneuver is to slowly exhale as far as comfortable (not in the device), inhale as fast as possible from the start of the inhalation and continue inhaling for as long as possible3. The CritiKal study5 found that errors made during any of these inhalation maneuver steps are clinically important. Inhalation volume ensures that the prepared dose leaves the inhaler and is then delivered to the airway. A reduction in the inhalation volume and the length of the inhalation can be caused by not exhaling before an inhalation or not inhaling for as long as possible. Furthermore, both these are reduced leading up to and during an acute exacerbation38. PIF and time to PIF provide the ‘force’ inside the inhaler to de-aggregate the prepared dose into respirable particles with the greatest likelihood to be deposited into the airways. A reduction in these can be caused by not inhaling as fast as possible. Also leading up to and during exacerbations PIF is reduced38. Thereby, this research provides further insights on the importance of inhaler technique and its potential links to disease outcomes. The results highlight the importance of counselling patients to exhale first and then to inhale as fast and as long as possible when using their DPI, demonstrated by the highest values of the inhalation parameters directly after training during the enrollment visit.
While a majority of patients were able to achieve a minimum PIF, analysis of PIF values indicates that 12.5% of the inhalations were below 18 L/min. As explained in the methodology section, these inhalations were most likely instances where patients prepared a dose by opening the cap but did not inhale. Other errors were technical issues including multiple inhalations recorded as one event, timeout and unexpected exhalations (1%). Hence, of the attempted inhalations, only 2% had a PIF 18– < 30 L/min while the other 98% were 30 L/min or greater. Dose emission studies have shown consistent albuterol dose emission from the Digihaler inhaler when the PIF is greater than 30 L/min43. Despite decline in inhalation parameters throughout the course of the study, the majority of inhalation events were PIF-confirmed inhalations. While the patients in this study did not have access to consistent feedback on inhalation quality via the Digihaler App, it is reasonable to expect that improving clinician and patient access to inhalation parameters data could provide opportunities to address poor inhaler technique.
The Digihaler App, which was not used in this study, enables patients to receive information on the quality of their inhalations. Patients who open the cap but do not inhale would be prompted to make an inhalation, while those patients with a PIF below 30 L/min would be encouraged to try to inhale faster. When the inhalation is 120 L/min or greater, the user could be alerted to be careful about their lips or fingers being too close to the air inlet vent. The Digihaler App defines and presents inhalation data based on PIF, i.e. “good inhalation” (PIF ≥ 45– < 200 L/min), “fair inhalation” (PIF 30– < 45 L/min), “low or no inhalation” (PIF < 30 or no inhalation recorded within 1 minute of the cap being opened), “exhalation” (exhaling/breathing out into the Digihaler inhaler), and “air-vent block” (PIF ≥ 200 L/min). In a study of the Digihaler System (DS), including App and dashboard, inhalation technique based on PIF was maintained throughout the duration of the study, suggesting the effectiveness of technique feedback and monitoring44. Those with a history of a gradual reduction in their inhaled volume could be encouraged to exhale before they inhale and continue inhaling for as long as possible.
The results described here illustrate how future versions of the App could support enhanced patient management. Acute increases in SABA use, reductions in PIF and/or reductions in inhaled volume relative to the patient’s typical values for these parameters could prompt reports or alerts to patients and HCPs. These changes have previously been reported to predict the onset of an acute exacerbation38. Similarly, the information could be used to improve inhaler technique; for example, if there is a reduction in inhaled volume (which will be linked to a decrease in the inhalation length), the patient could be advised to ensure that they exhale before their inhalation and to inhale for as long as they can. In this regard, it bears highlighting that inhalation parameter values recorded by the Digihaler inhaler are not numerically comparable with values for the analagous spirometric parameters. Notably, the median inhaled volume measurements reported here are markedly lower compared with previously reported forced vital capacity measurements obtained via spirometry in patients with asthma. This is consistent with the findings of a previous validation study: among a group of adults with asthma whose mean FVC was 2.82 L, the mean inhaled volumes measured by an Inhalation Profile Recorder and by the Digihaler were 2.10 L and 1.96 L, respectively45. This can be explained in part by the greater internal resistance of the DPI relative to the spirometer—the Digihaler inhaler, having a moderately high internal resistance, does not require a high inspiratory flow for adequate dose delivery; as such, inhaled volumes when using the inhaler tend to be low relative to expiratory parameters measured by spirometry46,47. Moreover, patients typically use maximal effort when performing spirometry or using an inhaler in controlled and supervised conditions that contrast with the unsupervised routine use of the Digihaler inhaler in this study. It is therefore not unexpected that the real-world inhaled volumes reported here are lower than those recorded by Chrystyn and colleagues45. Clinicians should take this into account when interpreting inhalation parameter data from the DS.
This research has also identified potentially new indicators of poorly controlled asthma and/or disease ‘flare-ups’ through digital capture in real time of acute increases in SABA use, or ‘SABA bursts’. It is established that SABA overuse is associated with increased risks of exacerbation and mortality20,21,25,26,28,29, and the increasing trend in SABA overuse48 is worrisome as this medication does not target the underlying inflammatory pathology that leads to the asthma symptoms and exacerbations49,50. In addition to inhaler technique, this study demonstrated a new perspective on the prevalence of SABA overuse and overreliance. Over the course of this study, those patients who continuously overused SABA were more likely to have exacerbations as well as SABA bursts than patients with lower overall SABA use. The Digihaler System allows for a more reliable way to assess control, based on the objective measure of real-life SABA use instead of a patient’s recollection. There were some patterns of SABA bursts seen in this study that appeared to be similar to patterns associated with exacerbations but did not coincide with a reported exacerbation. These episodes are probably undiagnosed exacerbations as, in the real world, patients forget or do not report them, and HCPs miss the opportunity to diagnose such exacerbations. In this respect, it is interesting to note that all of the patients who had exacerbations and overused SABA during the study recorded at least one SABA burst. These exacerbation-like events are indicative of clinical deterioration or undiagnosed exacerbations, and are probably clinically significant. Further studies are needed to fully understand the implications of such SABA use patterns and to evaluate behavioral systems designed to reduce SABA overreliance and increase use of preventer medication.
These analyses have several limitations. Firstly, because patients in this study did not have access to the Digihaler App, the inhalation event timestamps were not collected in real time but downloaded from the inhaler at the end of the study. In some instances, this data collection occurred several weeks after study completion, which could have resulted in minor inaccuracies in the time of inhalation recorded by the inhaler. Additionally, exacerbations were identified in retrospect via monthly telephone follow-ups by the clinical sites, and not captured in real time. Therefore, some of the exacerbation events may have been subject to recall bias. Furthermore, the definition of continuous SABA overuse excludes patients who used their inhaler continuously for periods of a week or more at a time but did not meet the criteria for an overuser (which stipulated use during every week of the study). In addition, opportunities to provide timely feedback to patients may have been missed owing to the App not being used in this study. This may have contributed to the technique waning effect described above, as patients with suboptimal technique were not alerted to seek medical assistance which might have prevented exacerbations or flare ups.
Although nebulizers were prohibited outside of an exacerbation, use was not recorded and there was no way to prevent patients from using nebulizers during periods of worsening disease. This methodology possibly resulted in periods where albuterol Digihaler use was underestimated around the time of an exacerbation for some patients. Nine (9%) patients in this study had exacerbations without evidence of SABA bursts. This finding illustrates that additional recording and assessment, such as lung function, symptom questionnaires or acoustic monitoring, may be helpful in identifying patients at risk of an exacerbation51.
In conclusion, the albuterol Digihaler captures objective information about inhalation events, inhaler parameters, and patterns of SABA use and could be used as a tool to assess inhaler technique. This technology has the potential to ‘profile’ different phenotypes of asthma patients using a combination of inhaler use patterns and inhalation parameters. If these data were routinely available, they could support earlier detection of inhaler technique errors and decline, as well as early warning signs to predict exacerbations. These data could facilitate informed self-management and disease management by clinicians through improved coaching of inhalation technique and clinical decision-making based on objective data. Analysis of these patterns may provide additional information and learnings about long-term asthma outcomes, especially in patients who have fewer documented exacerbations. Understanding this behavior may also help identify markers of uncontrolled disease, such as frequent reliever medication use, highlighting patients who are at risk for poor outcomes.
Methods
Study design and participants
This open-label study consisted of a 2-week screening period followed by a 12-week data capture period across 45 study centers in the United States (US) between February 2017 and February 2018 (NCT02969408). Patients included in the study were adults with a physician diagnosis of asthma, which was poorly controlled as defined by an Asthma Control Questionnaire-5 score of ≥1.552, and at least one severe asthma exacerbation during the 12 months prior to screening. Eligible patients were required to be using moderate or high doses of inhaled corticosteroids, equivalent to at least 440 µg daily of fluticasone propionate, with or without other asthma controller medications (long-acting beta2-agonist, leukotriene antagonist, long-acting antimuscarinic agent, biologic, or maintenance oral corticosteroids). Patients with any confounding underlying lung disorders other than asthma or who had used any investigational drugs within five half-lives of discontinuation were excluded.
During the baseline visit on study Day 1, patients were trained on correct inhaler technique and, following demonstration of competency, received seven albuterol Digihalers (considered sufficient for the study duration; albuterol 90 μg/dose; up to 1–2 inhalations every 4 hours) for use, as needed, alongside their usual maintenance therapy. For this study, exacerbations were defined based on the 2009 American Thoracic Society/European Respiratory Society recommendations53. Moderate exacerbations were defined as worsening asthma that required administration of systemic corticosteroids (SCS) above baseline for at least 3 days, or an unscheduled healthcare provider visit (e.g. doctor’s office or emergency care) associated with an increase in asthma therapy. Severe exacerbations needed both an unscheduled healthcare provider visit and administration of SCS as above.
Patients were required to discontinue all other reliever therapy containing SABA or short-acting antimuscarinic agents and replace them with the albuterol Digihaler as their only reliever inhaler for the duration of the study. Use of nebulized albuterol for treatment of acute exacerbations at home or at the hospital was permitted if deemed necessary by the patient or their physician; this was not recorded. Patients could also continue use of other medications, with any modifications at the discretion of their treating physician. Patients did not have use of the mobile phone software application (App) connected to the Digihaler inhaler, as this was not approved for patient use at the time the study was conducted. The data were downloaded from the Digihaler inhalers at the end of the study; therefore, any patient treatment modifications during the study were based on reports from the patients and not on the data from the albuterol Digihaler. Patients were contacted monthly by phone for collection of information regarding exacerbations (including date of occurrence), maintenance medication, and adverse events.
Analysis of inhalation parameters
A microelectromechanical system (MEMS) sensor in an integrated electronic module located within the top of the Digihaler inhaler detects changes in pressure generated by inhalation airflow at the mouthpiece. These changes were recorded by the Digihaler inhaler and inhalation parameters (PIF, inhalation volume, inhalation duration, and time to PIF) were calculated45. The electronic module also recorded a timestamp upon each use (date and time of inhalation). Each recorded timestamp represented one inhaler use event, i.e. an inhalation.
The MEMS sensor within the Digihaler inhaler has a lower threshold of 18 L/min, below which it cannot discriminate changes in pressure from ambient pressure fluctuations. When this occurs, the Digihaler records a PIF of zero. Previous studies using an Inhalation Profile Recorder to measure PIF (accurate below 5 L/min) involving a total of 300 patients45,54, each making three inhalations using either the Digihaler inhaler or the equivalent non-electronic version of this inhaler, have reported that only 2 inhalations (made by the same 7 year old child) had a PIF value that was below 18 L/min. Therefore, recorded PIF values below 18 L/min would almost entirely be due to the patient opening the cap (to activate the electronic module) and making no attempt to inhale. Furthermore, in the aforementioned studies, no patient inhaled with a PIF greater than 110 L/min. A PIF of 120 L/min or greater when using the Digihaler inhaler was, therefore, due to the patient’s lips partially impeding the airflow through the inlet of the device’s air vent. This action would cause a change in the overall resistance of the inhaler, leading to higher internal pressure changes and unrealistically faster PIF and larger inhaled volume values. Inhalations with a PIF ≥ 120 L/min were, therefore, not included in the analysis of the inhalation parameters.
Continuous SABA overuse was defined as use of at least two SABA inhalations per week every week throughout the study period. For the purposes of this analysis, we differentiated continuous SABA overuse from acute short-term high use, and referred to the latter patterns of use as “SABA bursts”. “SABA bursts” were defined as a daily mean of at least three inhalations in the last 2 days and an increase in daily mean inhalations in the last 2 days compared with the previous 2 weeks. Multiple SABA bursts within a 7-day period were counted as one burst.
Statistical analysis
For the analysis of the inhalation parameters over the 12-week period, those with PIF not detected (less than 18 L/min) or with PIF 120 L/min or greater were excluded, as these were considered to represent patients opening and closing the cap, and possible air vent impediment due to accidental covering of the air vent by the patient, respectively (as previously described above in the methods). Analysis of the PIF measurements revealed a non-normal distribution (Kolmogorov-Smirnov test with p < 0.0001). Inhalation parameters for each patient were aggregated into 10-day periods to account for natural intra-individual fluctuations between the inhalations. Descriptive statistics were used to report demographics and SABA use, but no statistical comparisons were made. Statistical analyses were performed using R Statistical Software (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
Safety
Adverse events were recorded during the study in line with the study protocol, from baseline up to Week 12.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data sets used and/or analyzed for the study described in this manuscript are available upon reasonable request. Qualified researchers may request access to patient level data and related study documents including the study protocol and the statistical analysis plan. Patient level data will be de-identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please visit www.clinicalstudydatarequest.com to make your request.
References
Chrystyn, H. et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim. Care Respir. Med 27, 22 (2017).
Labiris, N. R. & Dolovich, M. B. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharm. 56, 588–599 (2003).
Laube, B. L. et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 37, 1308–1331 (2011).
Levy, M. L. et al. Inhaler technique: facts and fantasies. A view from the Aerosol Drug Management Improvement Team (ADMIT). NPJ Prim. Care Respir. Med 26, 16017 (2016).
Price, D. B. et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J. Allergy Clin. Immunol. Pr. 5, 1071–1081.e1079 (2017).
Dhadge, N. et al. Monitoring of inhaler use at home with a smartphone video application in a pilot study. NPJ Prim. Care Respir. Med 30, 46 (2020).
Melani, A. S. et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir. Med 105, 930–938 (2011).
Ovchinikova, L., Smith, L. & Bosnic-Anticevich, S. Inhaler technique maintenance: gaining an understanding from the patient’s perspective. J. Asthma 48, 616–624 (2011).
Sulaiman, I. et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 195, 1333–1343 (2017).
Sulaiman, I. et al. The impact of common inhaler errors on drug delivery: Investigating critical errors with a dry powder inhaler. J. Aerosol Med Pulm. Drug Deliv. 30, 247–255 (2017).
Basheti, I. A., Reddel, H. K., Armour, C. L. & Bosnic-Anticevich, S. Z. Improved asthma outcomes with a simple inhaler technique intervention by community pharmacists. J. Allergy Clin. Immunol. 119, 1537–1538 (2007).
Harnett, C. M. et al. A study to assess inhaler technique and its potential impact on asthma control in patients attending an asthma clinic. J. Asthma 51, 440–445 (2014).
Kocks, J. W. H. et al. Systematic review of association between critical errors in inhalation and health outcomes in asthma and COPD. NPJ Prim. Care Respir. Med 28, 43 (2018).
Usmani, O. S. et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir. Res 19, 10 (2018).
Vestbo, J. et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax 64, 939–943 (2009).
Sanchis, J., Gich, I. & Pedersen, S. Systematic review of errors in inhaler use: Has patient technique improved over time? Chest 150, 394–406 (2016).
Amin, S., Soliman, M., McIvor, A., Cave, A. & Cabrera, C. Usage patterns of short-acting β(2)-agonists and inhaled corticosteroids in asthma: A targeted literature review. J. Allergy Clin. Immunol. Pr. 8, 2556–2564.e2558 (2020).
Azzi, E. A. et al. Understanding reliever overuse in patients purchasing over-the-counter short-acting beta(2) agonists: an Australian community pharmacy-based survey. BMJ Open 9, e028995 (2019).
Bloom, C. I. et al. Asthma-related health outcomes associated with short-acting β(2)-agonist inhaler use: An observational UK study as Part of the SABINA Global Program. Adv. Ther. 37, 4190–4208 (2020).
Lugogo, N. et al. Real-world patterns and implications of short-acting β(2)-agonist use in patients with asthma in the United States. Ann. Allergy Asthma Immunol. 126, 681–689.e681 (2021).
Nwaru, B. I. et al. Overuse of short-acting β(2)-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur. Respir. J. 55, 1901872 (2020).
O’Byrne, P. M. et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N. Engl. J. Med 378, 1865–1876 (2018).
Quint, J. K. et al. Short-acting beta-2-agonist exposure and severe asthma exacerbations: SABINA findings from Europe and North America. J. Allergy Clin. Immunol. Pr. 10, 2297–2309.e2210 (2022).
Slejko, J. F. et al. Asthma control in the United States, 2008-2010: indicators of poor asthma control. J. Allergy Clin. Immunol. 133, 1579–1587 (2014).
Global Initiative for Asthma. Global strategy for asthma management and prevention, https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf (2022).
Bateman, E. D. et al. Short-acting β(2)-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study. Eur. Respir. J. 59, 2101402 (2022).
Levy, M. L. The national review of asthma deaths: what did we learn and what needs to change? Breathe (Sheff.) 11, 14–24 (2015).
Spitzer, W. O. et al. The use of beta-agonists and the risk of death and near death from asthma. N. Engl. J. Med 326, 501–506 (1992).
To, T. et al. Is overreliance on short-acting β(2)-agonists associated with health risks in the older asthma population? ERJ Open Res 8, 00032–02022 (2022).
Averell, C. M. et al. Impact of adherence to treatment with inhaled corticosteroids/long-acting β-agonists on asthma outcomes in the United States. Ther. Adv. Respir. Dis. 16, 17534666221116997 (2022).
Jansen, E. M. et al. Global burden of medication non-adherence in chronic obstructive pulmonary disease (COPD) and asthma: a narrative review of the clinical and economic case for smart inhalers. J. Thorac. Dis. 13, 3846–3864 (2021).
Cushen, B. et al. The clinical impact of different adherence behaviors in patients with severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 197, 1630–1633 (2018).
Hesso, I., Nabhani Gebara, S., Greene, G., Co Stello, R. W. & Kayyali, R. A quantitative evaluation of adherence and inhalation technique among respiratory patients: An observational study using an electronic inhaler assessment device. Int J. Clin. Pr. 74, e13437 (2020).
Bosnic-Anticevich, S., Bakerly, N. D., Chrystyn, H., Hew, M. & van der Palen, J. Advancing digital solutions to overcome longstanding barriers in asthma and COPD management. Patient Prefer Adherence 17, 259–272 (2023).
Chan, A. H. Y. et al. Digital inhalers for asthma or chronic obstructive pulmonary disease: A scientific perspective. Pulm. Ther. 7, 345–376 (2021).
Mosnaim, G. S., Greiwe, J., Jariwala, S. P., Pleasants, R. & Merchant, R. Digital inhalers and remote patient monitoring for asthma. J. Allergy Clin. Immunol. Pr. 10, 2525–2533 (2022).
Mahler, D. A. Peak inspiratory flow rate: An emerging biomarker in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 199, 1577–1579 (2019).
Lugogo, N. L. et al. A predictive machine learning tool for asthma exacerbations: Results from a 12-week, open-label study using an electronic multi-dose dry powder inhaler with integrated sensors. J. Asthma Allergy 15, 1623–1637 (2022).
Kocks, J. et al. Identifying critical inhalation technique errors in Dry Powder Inhaler use in patients with COPD based on the association with health status and exacerbations: findings from the multi-country cross-sectional observational PIFotal study. BMC Pulm. Med 23, 302 (2023).
Takemura, M. et al. Repeated instruction on inhalation technique improves adherence to the therapeutic regimen in asthma. J. Asthma 47, 202–208 (2010).
Takemura, M. et al. Relationships between repeated instruction on inhalation therapy, medication adherence, and health status in chronic obstructive pulmonary disease. Int J. Chron. Obstruct Pulmon Dis. 6, 97–104 (2011).
FDA. ProAir® Digihaler® Prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/205636Orig1s012lbl.pdf (2018).
Chrystyn, H., Safioti, G., Buck, D., Ruiter, F. & Blair, J. Justification of the inhalation parameters to provide patients with dose confirmation feedback when they use a digitally enabled albuterol multidose dry powder inhaler (eMDPI). Am. J. Resp. Crit. Care Med 197, A3941 (2018).
Hoyte, F. C. L. et al. Effectiveness of a digital inhaler system for patients with asthma: A 12-week, open-label, randomized study (CONNECT1). J. Allergy Clin. Immunol. Pr. 10, 2579–2587 (2022).
Chrystyn, H. et al. Investigating the accuracy of the Digihaler, a new electronic multidose dry-powder inhaler, in measuring inhalation parameters. J. Aerosol Med Pulm. Drug Deliv. 35, 166–177 (2022).
Azouz, W. et al. The inhalation characteristics of patients when they use different dry powder inhalers. J. Aerosol Med Pulm. Drug Deliv. 28, 35–42 (2015).
Chrystyn, H., Azouz, W. & Tarsin, W. Dry Powder Inhalers: From Bench to Bedside. J. Aerosol Med Pulm. Drug Deliv. 36, 324–335 (2023).
Sadatsafavi, M., Tavakoli, H., Lynd, L. & FitzGerald, J. M. Has asthma medication use caught up with the evidence?: A 12-year population-based study of trends. Chest 151, 612–618 (2017).
O’Byrne, P. M., Jenkins, C. & Bateman, E. D. The paradoxes of asthma management: time for a new approach? Eur. Respir. J. 50, 1701103 (2017).
Stanford, R. H., Shah, M. B., D’Souza, A. O., Dhamane, A. D. & Schatz, M. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann. Allergy Asthma Immunol. 109, 403–407 (2012).
Levy, M. L. et al. Wheeze detection: recordings vs. assessment of physician and parent. J. Asthma 41, 845–853 (2004).
Juniper, E. F., Svensson, K., Mörk, A. C. & Ståhl, E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir. Med 99, 553–558 (2005).
Reddel, H. K. et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med 180, 59–99 (2009).
Azouz, W., Chetcuti, P., Hosker, H., Saralaya, D. & Chrystyn, H. Inhalation characteristics of asthma patients, COPD patients and healthy volunteers with the Spiromax® and Turbuhaler® devices: a randomised, cross-over study. BMC Pulm. Med 15, 47 (2015).
Acknowledgements
Medical writing support for the development of this article was provided by Ian Grieve, PhD, and Jane Blackburn, PhD, of Ashfield MedComms, an Inizio company, and funded by Teva Branded Pharmaceutical Products R&D, Inc. The study was funded by Teva Branded Pharmaceutical Products R&D, Inc.
Author information
Authors and Affiliations
Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article. M.L.L., G.S., M.R., M.D., N.L.L., T.H. and H.C. contributed to the study conception and design. Data collection was performed by G.S., M.R. and M.D. All authors participated in data analysis and interpretation, participated in the development of the manuscript, provided final approval of the manuscript for submission, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
M.L.L. has received payments from publishers Taylor Francis and from Class Publishing; consulting fees from Smart Respiratory; Respiri; Imperial College, AstraZeneca, Novartis, and TEVA; speaker/writing fees from Chiesi, AstraZeneca, TEVA, and honoraria for manuscript writing and educational events from Consorzio Futuro in Ricerca; fees for expert testimony from HM Coroner, Waltham Forrest, London; support to attend meetings from TEVA; and has held leadership roles (unpaid) in Global Initiative on Asthma (GINA), NHS England, and UK All Party Parliamentary Advisory Group (Asthma). J.W.H.K. reports grants, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from Boehringer Ingelheim, grants and personal fees from Chiesi, grants, personal fees and non-financial support from GSK, non-financial support from Mundi Pharma, grants and personal fees from Teva, personal fees from MSD, personal fees from COVIS Pharma, grants from Valneva outside the submitted work, and holds <5% shares of Lothar Medtec GmbH and 72.5% of shares in the General Practitioners Research Institute. S.B.-A. received support to provide expert opinion, contribute to advisory boards, deliver expert lectures/education and/or undertake independent investigator developed and led research from Teva, AstraZeneca, GSK, Viatris, Boehringer Ingelheim, Ibbvie, Chiesi, Menarini. SB-A is currently employed as Real World Evidence Lead, AstraZeneca, Australia and New Zealand. G.S., M.R., R.B., T.H., and T.L. are employees of Teva Pharmaceuticals. M.D. was an employee of Teva Pharmaceuticals when research was performed, and is currently an employee of Incyte Corporation, Newark, DE, USA. M.C. reports institutional grant funding from NIH, ALA, PCORI, AstraZeneca, GSK, Novartis, Pulmatrix, Sanofi-Aventis, and Shionogi. He receives consulting fees from Allakos, Arrowhead, Genentech, GSK, Merck, Novartis, Sanofi-Aventis, Teva and OM Pharma. He receives payment for speaker’s bureau activities for Amgen, AstraZeneca, Genentech, Regeneron, Sanofi-Aventis, and Teva. He receives royalties from Aer Therapeutics and Elsevier. N.F. receives speaker honoraria from GSK, AstraZeneca, Teva Pharmaceuticals, Regeneron, Genentech, ALK-Abelló and Optinose. N.L.L. received consulting fees from Amgen, AstraZeneca, Avillion, Genentech, GSK, Novartis, Regeneron, Sanofi, and Teva; honoraria for non-speakers bureau presentations from GSK, Niox and AstraZeneca; and travel support from AstraZeneca; her institution received research support from Amgen, AstraZeneca, Avillion, Evidera, Gossamer Bio, Genentech, GSK, Regeneron, Sanofi, Novartis and Teva. She is an honorary faculty member of Observational and Pragmatic Research Institute but does not receive compensation for this role. H.C. has no shares in any pharmaceutical companies. He has received sponsorship to carry out studies, together with Board Membership, consultant agreements and honoraria for presentation, from several pharmaceutical companies that market inhaled products. These include Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Innovata Biomed, Meda, MediSage, Menarini, Mundipharma, Napp Pharmaceuticals, Nemera, NorPharma, Norvartis, Orion, Sanofi, Teva, Truddell Medical International, UCB and Zentiva. Research sponsorship has also been received from grant awarding bodies (EPSRC and MRC).
Ethics approval
This study was conducted in full accordance with the Declaration of Helsinki and International Conference on Harmonisation guidelines for Good Clinical Practice. All study documents and procedures were approved by the appropriate Institutional Review Board or Ethics Committee (IRB #201606556). Written informed consent was obtained from each patient before study participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Levy, M.L., Kocks, J.W.H., Bosnic-Anticevich, S. et al. Uncovering patterns of inhaler technique and reliever use: the value of objective, personalized data from a digital inhaler. npj Prim. Care Respir. Med. 34, 23 (2024). https://doi.org/10.1038/s41533-024-00382-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-024-00382-x