Abstract
Inhaled corticosteroid (ICS) is recommended by Global Initiative for Asthma 2022 Guidelines for patients to attain asthma control. However, inhalational short-acting β2-agonist (SABA) is needed for reliever therapy and emits greenhouse gases (GHG). Despite evidence supporting ICS-formoterol’s superior effectiveness as both maintenance and reliever therapy, and more recent guidelines as the preferred treatment option, some patients continue to be prescribed with ICS. The study aimed to quantify GHG from adults on ICS inhalers treated in primary care in Singapore, including their SABA use, and compare good versus suboptimal asthma control. Data from nine public primary care clinics in eastern Singapore were retrieved from the electronic medical records, comprising asthma-diagnosed patients aged 21 years and older. Records contained their demography, clinical diagnoses, asthma control test (ACT) scores and ICS (beclomethasone pMDI, budesonide DPI and fluticasone pMDI) dispensed from in-house pharmacies. Patients with ACT ≥ 20 were classified as having good asthma control. Total GHG resulting from ICS and SABA were calculated for pMDI or DPI inhalers. Between 2015 and 2023, patients on ICS decreased from 3647–2265, while proportion of well-controlled asthma improved from 53.6–82.5%. Annual GHG emissions showed substantial reductions: ICS-only emissions fell from 101,685–71,899 kgCO2e, and ICS+SABA emissions decreased from 629,989–316,283 kgCO2e. Individual patient emissions dropped from 173 kgCO2e (2015) to 140 kgCO2e (2023). Patients using Fluticasone propionate inhalers had the highest GHG emissions (227 kgCO2e/year). Patients with suboptimal asthma control produced significantly higher GHG emissions, exceeding those with good control by 30 kgCO2e /year (p < 0.001). GHG emissions declined with fewer ICS-treated patients over the years, accompanied by a reduction in per patient GHG emission. Suboptimal asthma control was associated with higher GHG, demonstrating the interdependency between asthma outcomes and environmental sustainability.
Similar content being viewed by others
Introduction
Direct inhalation of medication is the evidence-based effective mode of pharmacotherapy in asthma management1. The medication is delivered to the airway via inhalers, either assisted by propellent in pressurized metered-dose inhalers (pMDI), direct inhalation of powdered medication via dry powder inhalers (DPI) or soft mist inhalers (SMI). Inhaled corticosteroids (ICS) are the primary treatment of asthma to curb the underlying airway inflammation2. Inhaled short-acting β2-agonist (SABA) is needed to dilate the airways in rescue therapy when a person experiences bronchoconstriction during an acute asthma exacerbation.
Currently, ICS delivered in pMDI form is more commonly prescribed. However, usage of pMDI results in elevated carbon footprints due to its hydrofluorocarbons (HFCs) propellants, which are potent greenhouse gases (GHG) that contribute to global warming3. DPIs which are void of propellants, have significantly lower carbon footprint compared to pMDIs. Furthermore, patients with suboptimal asthma control might experience an asthma exacerbation and in turn require rescue therapy using SABA, which are often delivered by pMDI, further aggravating HFC damage to the environment4.
Singapore is an island state in which the lifetime prevalence of asthma among its Asian population is estimated at 10.5%, and current asthma prevalence of 3.9% among adults aged 18–69 years5. These figures are comparable to global patterns, with the United States reporting an asthma prevalence of 8.7% and five pooled European countries reporting a prevalence of 6.7%6,7. However, the burden of acute asthma care in Singapore is particularly high, based on the hospitalization rate of approximately 80 episodes per 100,000 from 2003–2019 – nearly three times higher than the United States’ rate of 29 per 100,0008. Additionally, Emergency Department (ED) visit rate in Singapore reached 390 per 100,000 in 20199. Based on these acute asthma exacerbation rates, the expected GHG emissions from the current asthma control status of patients will be significant.
However, patients with asthma are largely managed in primary care in Singapore. They can access asthma care in the private General Practitioner (GP) clinics or public primary care clinics known as polyclinics, where they can receive rescue therapy during asthma exacerbation10. Alternatively, they can also choose to access ED directly for rescue therapy. In primary care clinics, evacuation to ED for further treatment will occur in the event where rescue therapy fails to relieve the patients from their bronchoconstriction. Nevertheless, the burden of asthma management in primary care and the impact on environment has yet to be elucidated.
SingHealth Polyclinics currently comprises ten polyclinics located in eastern Singapore which provide primary care services to the residents in the neighborhood, including continuing care for patients with stable asthma control and rescue therapy for those who walk in for acute exacerbations. The polyclinic clinicians, including physicians, trained advanced practice nurses and pharmacists prescribe inhaled asthma medications, which are dispensed from the in-house pharmacy. The electronic medical record (EMR) and e-prescription systems provide invaluable data to estimate the GHG produced resulting from the longitudinal asthma care and related prescriptions of the patients served by these polyclinics. The data will provide insight into the asthma care burden in the community and lays the foundation for a comprehensive action plan to address both the gaps and environmental concerns.
The retrospective longitudinal study aimed to report the asthma control status of patients who were treated in a primary care institution from 2015–2023, quantify the inhaled asthma medications dispensed from the in-house pharmacies and to estimate the GHG resulting from the asthma treatment.
Methods
Study design
This retrospective longitudinal study leverages on the data from the electronic medical record (EMR) of routine clinical asthma management in SingHealth Polyclinics. The study period covers from 2015–2023 and includes 8 public primary clinics located in eastern Singapore.
Study sites and population
All patients aged 21 years old and above with clinical diagnosis of asthma within the study period were included. The extracted clinical and administrative data includes their demographics, Asthma Control Test (ACT) scores, dispensed asthma-related medication, Rescue Therapy (RT) records. RT refers to the institution protocol-based administration of inhalational salbutamol and ipratropium via space chamber to patients at the polyclinic who presented with wheezing and/or breathlessness and had rhonchi detected on auscultation of the lungs by healthcare professionals such as doctors and trained nurses. The polyclinics allow walk-in access to patients presenting with acute medical conditions, including RT for asthma exacerbations to increase healthcare access to any residents. Empanelment of patients with chronic diseases such as asthma to specific polyclinic in Singapore is strongly encouraged but is not mandatory by regulations.
Patients were included if they had at least one ACT score available in the year, reflecting an asthma consultation at the clinic. Patients were excluded if they had incomplete or missing ACT records as they can visit a clinic for other purposes. Those who had a switch in inhaler, with either a switch in type (DPI or pMDI) or brand, or switch to ICS-LABA inhalers were also excluded. The asthma medications were dispensed directly from the in-house pharmacy in each polyclinic in accordance with clinic’s formulary. The prescription and dispensing data were captured in the EMR.
Data management
To comply to national bioethics regulations, the data extracted from the Electronic Health intelligence System (eHINTS), a single enterprise data repository for clinical data, was de-identified by an independent approved trusted third-party in SingHealth Medical Information Office prior to analysis by the study team members. A detailed list of variables extracted are as follows: demographic (age, gender, and ethnicity), ACT records, RT records with treatment intensity, dispensed inhaled ICS medication (Beclomethasone Dipropionate, Budesonide and Fluticasone propionate) and dispensed inhaled beta-agonist (Salbutamol). The RT utilization records were only accessible from 2016 onwards, following the integration of these records into standardized clinical documentation. Only the de-identified dataset availed to the study team members for analysis.
Outcome measures
The primary outcome was inhaler usage, assessed based on the number of canisters dispensed, inhaler type and brand. Each inhaler included in the analysis was assigned a carbon footprint value, determined by the propellant used, with values sourced from literature11,12. More details on carbon footprint values are provided in Supplementary Table 1. The carbon footprint value for each canister represents the total greenhouse gas emissions associated with its usage, measured in kilograms of carbon dioxide equivalent. The carbon footprint calculation for RT was based on the standard dosage of 10 puffs of Ventolin per RT cycle, in accordance with the SHP’s clinical guideline. The total carbon footprint calculation is as follow:
where, Ni = Number of canisters dispensed for inhaler type iCFi = Carbon footprint per canister of inhaler type i (kgCO2e)
ACT scores were used to categorize patients into two groups: suboptimal controlled (ACT < 20) and well-controlled (ACT ≥ 20). Patients with at least 1 recorded ACT of less than 20 within a calendar year were classified as suboptimal. A comparative analysis of the GHG emission was done between patients who had suboptimal asthma control versus those with well-controlled asthma status.
Data cleaning
Data wrangling was performed to ensure the accuracy, reliability, and consistency in the desired data format. Inhaler usage data was reviewed and filtered to ensure that patients remained on the same inhaler type and form throughout the year. Duplicated ACT records from the same visit were removed. The final dataset was structured in a longitudinal format for analysis, where a single patient could have records in multiple years within the study period. Patients with missing data on demographics and ACT score were excluded to ensure that patients who were regularly reviewed and managed by the respective polyclinics were included in the study population. Only complete case records were analyzed.
Statistical analyses
Descriptive statistics were used to evaluate the annual impact of GHG based on dispensed medication from the study population. The mean values were calculated to summarize the aggregated GHG emission with respect to the different types of medication. Generalized Estimating Equations (GEE) were used to analyze trends in GHG emissions over a 9-year study period, comparing between patients with well-controlled and suboptimal asthma control. The GEE model was chosen to account for repeated measured within patients over the study period and handle within-subject correlation due to non-consecutive records of patients across different years. An exchangeable correlation structure was employed to handle within-subject correlation. The model accounted for fixed effects in asthma control and year, with patient ID as the clustering variable. The GHG emission associated with each medication type were further analyzed and compared. A p value < 0.05 was deemed statistically significant. All statistical analysis were carried out using Python 3.11.2 and Tableau 2023.3.
Results
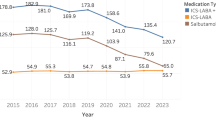
A total of 10,042 unique patients across the 9-year period was analyzed. Despite a 38% reduction in patients on ICS inhalers (3647–2265) between 2015 and 2023, the proportion of well-controlled patients improved from 54–82%. In 2023, 83.5% of the patients were treated with Beclomethasone Dipropionate, followed by Budesonide (10.6%) and Fluticasone propionate MDI (5.9%). Between 2015–2023, total annual GHG emission reduced by approximately half, from 629,988–317,571 kgCO2e (Table 1). Salbutamol usage contributed substantially to the annual GHG emission, with approximately 5.2 and 3.4 times that of dispensed ICS inhalers in 2015 and 2023 respectively. The annual GHG emissions attributed to Rescue Therapy declined from 0.7% (2016) to 0.4% (2023) of total emissions.
On average, each patient emits 139.6 kgCO2e yearly, a decline from 172.7 kgCO2e in 2015. Dispensed salbutamol was the main contributor to GHG emission, ranging from 108–145 kgCO2e per patient, an equivalent of approximately 5 canisters yearly. In contrast, ICS inhalers contributed to 32 kgCO2e per patient and increased slightly over the years, mainly driven by increased dispensing of Fluticasone propionate canisters (4.6–4.8). Contribution from RT to GHG emissions reduced over the years, declining from 1.2–0.6 kgCO2e per patient. Annual GHG emission per patient are summarized and reflected in Fig. 1.
Figure 2 shows that GHG emission was higher in patients with suboptimal asthma control, contributed vastly by inhaled Salbutamol. In 2023, they emitted 40 kgCO2e more as compared to those well-controlled. The GEE model revealed that patients with suboptimal asthma control had a significantly higher GHG emission, averaging 29.5 kgCO2e more per year (p < 0.001). Additionally, the annual GHG emission per patient showed a declining trend, decreasing by 2.3 kgCO2e per year (p < 0.001) (Table 2). However, comparison on ICS-only inhalers revealed that patients with suboptimal control had consistently lower GHG emission by approximately 3 kgCO2e compared to those with good control due to fewer canisters of dispensed ICS inhalers.
In comparison between the three brands of ICS among the suboptimal control group, those treated with Fluticasone propionate contributed more GHG (Fig. 3). Between types of ICS inhalers, the annual GHG emission per patient were substantially greater for ICS-pMDI by 14–45 times compared to the approximately 2 kgCO2e emitted via ICS-DPI. The addition of Salbutamol into the computation led to a reduction in the difference in annual GHG emitted by 35–43 kgCO2e for ICS-DPI in both suboptimal and well-controlled groups of patients. Dispensed Salbutamol was highest in Beclomethasone Dipropionate, averaging 3.9 canisters per patient, and was similar for patients on Budesonide and Fluticasone propionate (3.6 canisters) (Table 3).
From Fig. 4, usage of Ventolin for RT was highest in suboptimal patients using Budesonide and Fluticasone propionate inhalers (1.9 kgCO2e). A substantial dip in RT consumption pattern was observed in 2022. Utilization of RT services remain similar in patients with good asthma control across all three ICS inhalers (0.4 kgCO2e).
Discussion
This retrospective longitudinal study aimed to report the asthma control status, quantify the inhaled asthma medications dispensed from the in-house pharmacies and estimate the GHG resulting from the asthma treatments. From 2015–2023, the number of patients using ICS-only inhaler declined by 38%. Consequently, the GHG emitted from ICS inhalers declined from 101,684–71,899 kgCO2e. A larger reduction in ICS-only users was observed from 2020, which could be attributed to patients switching to ICS-formoterol. This was in-line with growing evidence showing ICS-LABA being more effective in reducing asthma exacerbation13,14, as well as the Global Initiative for Asthma (GINA) recommendation of as-needed ICS-formoterol inhalers in place of daily ICS-only inhalers for patients classified as Step 215.
In recent years, the environmental impacts of medical treatment have become an essential consideration in patient care16. Patients with suboptimal asthma control emitted 20–40 kgCO2e more GHG compared with those with well-controlled asthma yearly, primarily driven by more salbutamol inhalers used. The environmental impact becomes particularly pertinent when considering the cumulative effect across the entire asthma patient population. With an asthma prevalence of 3.9%5, about 163,000 adult residents in Singapore will have asthma, potentially amounting up to 6.5 million kgCO2e more GHG emissions for suboptimal asthma control. Besides good asthma control, adherence to ICS inhaler can result in reduced use of salbutamol and its resultant GHG17. Hence, educating patients on the importance of adherence to ICS inhaler needs to be a key priority.
Though use of salbutamol showed a decreasing trend, it plays an essential role as a reliever for some patients with asthma. Even within the well-controlled asthma patients, salbutamol still contributed a substantial 100 kgCO2e of GHG per patient per annum. Salbutamol in traditional pMDI preparation is the biggest contributor to GHG. An alternative would be to use powdered-form salbutamol which was developed after pMDI, but is less frequently used and currently not available in our setting. The literature has proven equal effectiveness between salbutamol in pMDI and DPI preparations18. Thus, the provision of salbutamol in DPI could help in reducing GHG.
This study found an improvement in asthma control despite reduced ICS usage. While mandatory mask-wearing regulations during the COVID-19 pandemic likely contributed to improved asthma control by reducing exposure to airborne irritants, established clinical processes appear to be equally important. These processes include structured asthma counselling and regular inhaler technique assessments conducted by trained nurses, which help ensure optimal medication adherence and proper inhaler usage. These support initiatives likely improve patients’ self-management of asthma. The combination of robust clinical support may have contributed to better asthma outcomes despite lower ICS utilization. However, more efforts are required to manage the subset of the patients with suboptimal asthma control.
Patients with suboptimal asthma control demonstrated increased RT utilization across all three ICS inhaler brand, resulting in higher GHG emissions. The relationship between suboptimal asthma control and elevated environmental impact underscores the benefit of attaining optimal asthma management. This aligns with the CARBON study in UK which reported 8.1 times higher GHG emission in patients with poorly controlled asthma19. Notably, a decline in GHG emissions observed from 2019–2022 might be attributed to the COVID-19 pandemic, with reduced acute respiratory infections (ARI) attendance observed19. During the outbreak with restricted movement in the community, quarantine of infected patients and mandatory wearing of mask, the incidence of ARI, a common asthma trigger, was reduced by approximately 50%19. It could be associated with reduced access to healthcare facility for mild asthma exacerbation due to fear of cross infection with COVID. Besides direct GHG contribution from RT, the utilization of RT necessitates travel to the polyclinics. These journey, whether by public or private transport, contribute additional GHG emissions to overall carbon footprint of asthma care. The cascading environmental impact further emphasize the importance of achieving good asthma control. Thus, understanding both the environmental impact of RT and frequent clinic visits could lead to increased awareness and act as motivation for better asthma self-management.
Study strengths and limitations
This study reports real-world data based on the institution EMR. SHP manage over 2500 patients with asthma on ICS annually and longitudinally. Collected as routine clinical records, the data is less subjected to patients’ recall bias, or errors from manual data entry from data collection forms.
However, the study is limited by the absence of data from patients who bought their inhalers from pulmonologist, private general practitioner (GP) clinics or community pharmacies. The number is likely to be small due to higher expenses from these sources of purchase. The inhaler dispensing records may not reflect actual utilization by patients. This potentially overestimates greenhouse gas emissions, which only occur when inhalers are actuated.
Conclusion
Raising awareness on the environmental impact of suboptimal asthma could nudge patients in adhering to the medication regime and improve self-management ability. The dual benefit of improved asthma outcomes and reduced environmental impact presents a compelling case for optimizing asthma management. To ensure a more sustainable asthma care, healthcare providers should consider incorporating environmental impact data when making treatment decisions, without compromising on health outcomes and patient suitability.
Data availability
The datasets used in the course of the current study are not publicly available as they contain information that is sensitive to the study institution. They may be made available by the corresponding author on reasonable request.
References
Scichilone, N. Asthma control: the right inhaler for the right patient. Adv. Ther. 32, 285–292 (2015).
Global Initiative for Asthma (GINA). 2022 GINA Report, Global Strategy for Asthma Management and Prevention https://ginasthma.org/gina-reports/ (2022).
Rigby, D. Inhaler device selection for people with asthma or chronic obstructive pulmonary disease. Aust. Prescr. 47, 140–147 (2024).
Marques, L. & Vale, N. Salbutamol in the management of asthma: a review. Int. J. Mol. Sci. 23, 14207 (2022).
Jeyagurunathan, A. et al. Asthma prevalence and its risk factors among a multi-ethnic adult population. Yale J. Biol. Med. 94, 417–427 (2021).
Swed, S. et al. Asthma prevalence among United States population insights from NHANES data analysis. Sci. Rep. 14, 8059 (2024).
Khan, A. H. et al. Prevalence and burden of asthma in five European countries: a retrospective cross-sectional study. BMJ Open. 15, e085175 (2025).
Pate, C. A. & Zahran, H. S. The status of asthma in the United States. Prev. Chronic Dis. 21, E53 (2024).
Lim, L. H. M., Chen, W., Amegadzie, J. E. & Lim, H. F. The increasing burden of asthma acute care in Singapore: an update on 15-year population-level evidence. BMC Pulm. Med. 23, 502 (2023).
Koh, M. S. et al. Patient characteristics, management, and outcomes of adult asthma in a Singapore population: data from the SDG-CARE asthma registry. Pragmat. Obs. Res. 15, 209–220 (2024).
Wilkinson, A. & Woodcock, A. The environmental impact of inhalers for asthma: A green challenge and a golden opportunity. Br. J. Clin. Pharmacol. 88, 3016–3022 (2022).
PrescQIPP. Bulletin 295: Inhaler Carbon Footprint https://www.prescqipp.info/our-resources/bulletins/bulletin-295-inhaler-carbon-footprint/ (2021).
Wells, K. E. et al. The relationship between combination inhaled corticosteroid and long-acting β-agonist use and severe asthma exacerbations in a diverse population. J. Allergy Clin. Immunol. 129, 1274–1279.e2 (2012).
Park, H. J. et al. Comparative efficacy of inhalers in mild-to-moderate asthma: systematic review and network meta-analysis. Sci. Rep. 12, 5949 (2022).
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention (2021 Update) https://ginasthma.org/wp-content/uploads/2021/05/Whats-new-in-GINA-2021_final_V2.pdf (2021).
Woodcock, A. et al. The environmental impact of inhaled therapy: making informed treatment choices. Eur. Respir. J. 60, 2102106 (2022).
Vervloet, M. et al. Understanding relationships between asthma medication use and outcomes in a SABINA primary care database study. NPJ Prim. Care Respir. Med. 32, 43 (2022).
Kuek, S. L. et al. Dry-powder inhaler use in primary school-aged children with asthma: a systematic review. ERJ Open. Res. 10, 00455–2024 (2024).
Wilkinson, A. J. K. et al. Greenhouse gas emissions associated with suboptimal asthma care in the UK: the SABINA healthCARe-based envirONmental cost of treatment (CARBON) study. Thorax 79, 412–421 (2024).
Acknowledgements
The authors wish to acknowledge Office of Deputy Group Chief Medical Informatics Officer (Research), SingHealth for de-identification of the EMR dataset, and National Medical Research Council RIE 2025 Centre Grant for SingHealth Polyclinics for supporting the study implementation.
Author information
Authors and Affiliations
Contributions
T.N.C. conceived and designed the study. A.W.K. extracted the data from the Electronic Medical Records. N.D.X. conducted data cleaning, analysis and data visualization. N.D.X. and L.Q.H. drafted the original manuscript. L.Q.H., K.Y.L. and T.N.C. edited and reviewed the manuscript. All authors critically revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Ethics approval was obtained prior to the commencement of the study (SingHealth Centralized Institutional Review Board Ref No. 2023/2362).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ng, D.X., Leow, M.Q.H., Koh, Y.L.E. et al. Resultant greenhouse gases from the use of inhaled corticosteroid based on Global Initiative for Asthma (GINA) guidelines: a primary care used case from Singapore. npj Prim. Care Respir. Med. 35, 35 (2025). https://doi.org/10.1038/s41533-025-00441-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-025-00441-x