Abstract
There is growing interest in combining chemical complexity with external stimuli like pressure, field, and light for property control in van der Waals solids. This is because extreme conditions trigger the development of new states of matter and functionality. In this work, we bring together synchrotron-based infrared absorption, Raman scattering, and diamond anvil cell techniques with first-principles calculations of the lattice dynamics and energy landscape to reveal the series of structural phase transitions in CrSBr. By tracking how the phonons change under pressure, we uncover a remarkable chain of complex symmetry modifications, interlayer interactions, and chemical reactions. A group-subgroup analysis suggests that CrSBr undergoes an orthorhombic Pmmn → monoclinic P2/m transition at 7.6 GPa, and based upon a comparison with model oxychlorides like FeOCl and CrOCl, we propose that changes in the pendant halide groups drive the system to a P21/m-like space group above 15.3 GPa. Compression above 20.2 GPa is irreversible, resulting in the formation of an entirely new compound that is metastable for months. This work opens the door to the use of pressure and possibly strain to control the properties of CrSBr.
Similar content being viewed by others
Introduction
van der Waals solids are legendary for their complex energy landscapes, sensitivity to external stimuli, and property control through chemical substitution. Amongst the various external stimuli, such as magnetic field, strain, and light, pressure is a potent tool for tuning properties because it acts directly on bond lengths and angles, controls the c/a ratio, and modifies the van der Waals gap1,2,3,4,5. In transition metal dichalcogenides like MoS2 and WS2, work has focused on pressure-driven insulator-to-metal transitions, interactions between superconductivity and competing states, and the mechanisms of solid-state lubrication6,7,8,9. There are many exciting high-pressure properties and states of matter in the MPX3 family of materials (M = Fe, Cr, Mn, Co, Ni, and X = S, Se) as well10. Significantly less is known about other van der Waals solids under compression. While efforts have been made to reveal the phase diagrams of magnetic insulators like CrSiTe3, CrI3, and Mn3Si2Te611,12,13, van der Waals magnets such as CrSBr14,15,16 have received markedly less attention, which is surprising since CrSBr is reported to be air stable. This system is a metamagnet, formed by anisotropic ferromagnetic layers coupled antiferromagnetically to one another, exhibiting antiferromagnetic ordering below 133 K, a direct bandgap of 1.5 eV, and spin-mediated exciton-phonon coupling17,18,19,20,21,22,23,24. CrSBr exfoliates easily, and van der Waals heterostructures of this material have been used in magnetic tunnel junctions and spin valves, offering a route toward atomically thin memory devices23,24. Despite many promising discoveries in the few- and single-layer limit25,26,27,28,29,30,31,32,33,34,35, understanding how external stimuli like pressure impacts the properties of this class of materials is highly under-explored. To our knowledge, there has been only a single study on the impact of chemical and physical pressure on the magnetic properties of CrSBr36. While chemical pressure in the form of Cl substitution for Br drives a crossover in antiferromagnetic to ferromagnetic coupling, compression systematically reduces the Néel temperature, smoothly evolves the lattice parameters, and modifies the exchange interactions due to local structure changes in the 0–1.9 GPa range36. These early results suggest that still greater pressures could trigger even more interesting magnetic and excitonic behavior36 as well as other highly advantageous processes.
In this work, we combined diamond anvil cell techniques and synchrotron-based infrared absorption and Raman scattering with a group-subgroup symmetry analysis, lattice dynamics calculations, and an analysis of the energy landscape to reveal the properties of CrSBr under pressure. From an examination of the phonon behavior under compression, we identify a series of structural phase transitions at 7.6, 15.3, and 20.2 GPa associated with a remarkable chain of symmetry modifications, interlayer interactions, and chemical reactions. For instance, at PC,1 = 7.6 GPa, we find a continuous volume change accompanied by a significant modification of the infrared pattern which, when combined with a group-subgroup analysis, is consistent with an orthorhombic Pmmn to monoclinic P2/m transition. PC,2 is the pendant halide transition, akin to what is observed in FeOCl37,38,39. By contrast, PC,3 functions as an irreversibility limit which, when surpassed, triggers a chemical reaction in this system upon pressure release. The resulting state is metastable for months, offering an interesting platform for additional investigation. These results highlight the rich behavior of CrSBr under pressure and the potential for compression (and presumably strain) to control properties for future applications40,41.
Results and discussion
Ambient pressure vibrational properties of CrSBr
Figure 1 displays the infrared spectrum of CrSBr at ambient conditions. Group theory predicts a total of six infrared-active vibrational modes for an orthorhombic material with the Pmmn space group: 2B1u+ 2B2u+ 2B3u20. This is precisely what we find, although the broad band between 300 and 350 cm−1 must be fit with model oscillators to resolve the individual peaks. An example of this deconvolution process is shown in Supplementary Note 4. Vibrational mode assignments were made using our complementary lattice dynamics calculations. The details are summarized in Table 1. For instance, we describe the motion of the B2u mode at 92 cm−1 as shearing of the outer Br atoms against the inner CrS bilayer. The high frequency modes are various types of Cr...S stretches.
Synchrotron infrared spectrum of CrSBr at ambient conditions and the theoretically calculated mode displacement patterns. Blue, yellow, and red represent chromium, sulfur, and bromine atoms, respectively. The inset shows a close-up view of the weak 1B2u phonon with its displacement pattern. The positions of the 2B3u, 2B2u, and 2B1u modes were determined using traditional oscillator fitting techniques as discussed in the text.
Figure 2 displays the Raman scattering response of CrSBr at ambient conditions. There are three strong peaks at 114, 245, and 343 cm−1. These features are assigned as 1Ag, 2Ag, and 3Ag phonons, in excellent agreement with our lattice dynamics calculations [Table 1]. Each manifests varying degrees of interlayer character, exhibiting motion along the stacking direction. The lowest frequency 1Ag mode consists of bromine atoms vibrating toward adjacent layers, whereas the high frequency 3Ag mode shows intralayer character along the c-axis primarily involving the inner CrS bilayer with suppressed Br motion42. Bg symmetry features are also allowed in the Raman scattering response42,43, although they are extremely weak. Similar behavior is seen in other layered materials37,39,44.
Raman scattering spectrum of CrSBr taken near ambient conditions inside the diamond anvil cell along with our theoretically calculated mode displacement patterns. The three Raman-active Ag modes are easily identified. The Bg symmetry modes are not clearly observed here43.
Structural phase transitions in CrSBr under pressure
Figure 3 summarizes the infrared properties of CrSBr under pressure. All six phonons harden under compression, a normal trend for a material in a diamond anvil cell45. The 1B2u mode is weak and disappears near 7 GPa. Figure 3b focuses on the behavior of the 1B3u and 1B1u modes at 179 and 225 cm−1. While the 1B1u peak diminishes and eventually disappears, the 1B3u peak appears to split into a doublet near 15 GPa, although this “splitting” is actually new peak activation. The third panel summarizes the response of the broad band, which, as discussed previously, can be deconvoluted into three separate features using standard peak-fitting techniques. These phonon modes become more distinct under compression because their frequencies harden at different rates. The 2B3u mode moves systematically under compression, indicating that the symmetry element it represents is unperturbed. The 2B2u mode also hardens with only modest changes in its overall trend. The behavior near the high-frequency 2B1u mode is the most interesting. A new peak appears above 7.5 GPa and again at approximately 20 GPa. These changes are quite dramatic and signal strong symmetry breaking with the activation of new modes due to the development of lower symmetry phases under pressure.
We combine these trends to generate the frequency versus pressure plot shown in Fig. 3d. The mode trends define three critical pressures, separating four structural phases, including the Pmmn phase at ambient conditions. We find PC,1 = 7.6 GPa. It is defined by the disappearance of the 1B2u mode and the development of a new peak near the high-frequency 2B1u phonon. PC,2, the second critical pressure, presents itself at approximately 15.3 GPa and is evidenced by the disappearance of the 1B1u phonon mode and the appearance of the peak near 175 cm−1. The additional peak that develops near the high-frequency 2B1u mode defines PC,3 at approximately 20 GPa. Since various peaks appear and disappear, we know that CrSBr has both symmetry restorations and reductions under pressure. We can use these spectral changes to reveal the symmetry behavior of the high-pressure phases. Even though the phonon modes differ, six infrared modes are active in the ambient pressure phase and across PC,1 and PC,2. The highest pressure phase above PC,3 has a total of seven infrared-active modes, indicating an overall decrease in the symmetry. No evidence of metallicity is observed even up to 50 GPa. This is different than many other complex chalcogenides that host insulator-to-metal transitions under pressure10.
Figure 4 summarizes the Raman scattering response of CrSBr under compression. The most dramatic effect is that while the 2Ag and 3Ag modes harden under pressure, the 1Ag phonon softens overall. Typical mechanisms for mode softening include phase transitions, electron-phonon coupling, temperature changes, doping, and hydrogen bonding, among others46,47,48, although the situation is different here. As discussed below, we attribute the 1Ag mode softening to buckling of the pendant halide groups. Figure 4a highlights these trends. There is a slight hardening of the 1Ag mode up to approximately 5 GPa, above which the peak softens considerably with each consecutive pressure step until about 15 GPa, after which it hardens again. As a reminder, this mode is extremely sensitive to out-of-plane Br motion. The phonon softening and peak activation in the Raman scattering response is consistent with the series of structural distortions and three critical pressures established above. Figure 4d summarizes the frequency versus pressure trends for the Raman-active modes. Again, we observe the same critical pressures of 7.6, 15.3, and 20.2 GPa. Notice that PC,1 coincides with the pressure at which the 1Ag mode begins to soften.
Symmetry analysis across the structural phase transition at 7.6 GPa
We employed the Bilbao Crystallographic Server as a tool to unravel the symmetry progression across PC,1 from the orthorhombic Pmmn phase of CrSBr at ambient conditions to a new space group above PC,149. We used group-subgroup relations (based on Landau’s theory of phase transitions) to determine potential space groups associated with the symmetry changes. This approach is successful because the irreducible representations of distinct crystal phases exhibit the full crystal symmetry50,51. Many candidate space groups and pathways were evaluated based on the following caveats. Due to the inherent nature of the space groups containing translational symmetry elements, the order in which these symmetry changes may very well result in different sets of space groups, so we evaluated all reasonable possibilities. The mode progression across PC,1 contains symmetry restoration and symmetry breaking [Figs. 3 and 4]. Therefore, the first step is to realize that all subgroups and supergroups related to the ambient pressure phase must be evaluated to provide a thorough understanding of which structure ultimately results. Table 2 shows several simplified candidate pathways for PC,1. Focusing first on the infrared-active modes, we recall that the 1B2u mode disappears near 7.6 GPa, whereas a new feature appears at 420 cm−1 alongside the 2B1u mode. All Raman-active modes harden on approach to PC,1. The 1Ag mode softens dramatically after the transition is complete, although there is no change in the number of features. The other key finding is that six infrared-active modes are present in the ambient pressure phase and six in the high-pressure phase. There is no change in the number of Ag modes within our sensitivity, so the symmetry operations that correspond to these modes are unchanged. Furthermore, none of the spectral features overlap [Fig. S5, Supplementary Note 6], meaning that the rule of mutual exclusion is active. This implies that inversion symmetry is not broken, so we can eliminate polar space groups from consideration. Additional detail is available in Supplementary Note 6. The space groups assumed in each step of the symmetry analysis were further refined such that the symmetry elements of each subgroup must also be present in the parent group to have a viable pathway between one another. These vibrational mode patterns are distinct for each phase of CrSBr.
Table 2 summarizes the results of our analysis. We show several different subgroup and supergroup pathways, the subsequent space group, and the predicted number of vibrational modes for that space group. Using the Bilbao Crystallographic Server, we also calculated the different infrared- and Raman-active modes for the resulting space group(s) of each pathway. Several stringent criteria help us refine the results in Table 2. Most importantly, we require six infrared-active phonons and at least three Raman-active phonons in the high-pressure phase. We also require an inversion center (due to the rule of mutual exclusion). With these candidate pathways, it is easy to realize that the only viable path is orthorhombic Pmmn to monoclinic P2/m across PC,1. The other pathways do not agree with the experimental spectra because their phonon modes would require the same activity in both infrared absorbance and Raman scattering, which is not the case given our observations.
Structure-property relations in chalco-halides
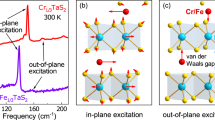
Materials structurally and chemically similar to CrSBr, such as FeOCl and CrOCl, underscore common mechanisms of pressure-induced structural phase transitions. Like CrSBr, FeOCl, and CrOCl host ambient pressure orthorhombic Pmmn space groups with transition metal chalcogen bilayers sandwiched between layers of dangling halides that act to create the van der Waals gap52. Single-crystal X-ray diffraction within a diamond anvil cell reveals that FeOCl displays a Pmmn to monoclinic B21/m phase transition near 15 GPa37. The latter is the same as P21/m but with a different primary axis. CrOCl also displays a Pmmn to incommensurate phase transition near 16 GPa38. Transitions in these materials are attributed to the buckling of the halide centers as they rearrange to accommodate denser packing upon compression37,38. After the buckling occurs, the monoclinic angle for the oxy-halides37,38 is on the order of 90.015∘. Raman scattering corroborates these findings in FeOCl, highlighting significant spectral changes across the 15 GPa critical pressure37. Two new modes emerge as the van der Waals gap narrows and the lattice distorts, leading to a buckling of the halide layers37.
Figure 5 displays the Raman scattering response of CrSBr and FeOCl. Some of the spectral features in FeOCl were not present in ref. 37. Even so, a direct comparison is striking. Both materials display pressure-driven structural phase transitions near 15 GPa, and based upon the prior mechanistic work by Bykov et al. on FeOCl37, the transition in CrSBr is likely due to halide buckling driven by the need to maintain favorable halide...halide distances. That said, the vibrational pattern of the oxy-halide is not a perfect match with that of CrSBr, suggesting that the space group may only be P21/m-like due to the large number of possible buckling configurations. Considering the similarity between these two materials, it may be worthwhile to use infrared techniques to search for a lower-pressure transition in FeOCl. Other oxy-halides like TiOBr and TiOCl host P21/m phases as well39. Even so, we emphasize that CrSBr hosts three critical pressures. Only PC,2 where the halide buckling occurs, is mechanistically similar. The other steps in the sequence are unique.
Frequency versus pressure plots of the Raman-active modes of CrSBr and FeOCl at room temperature. All Raman modes of FeOCl were digitized directly from Bykov et al37. The vertical dashed lines denote the critical pressures for each material. Space groups are indicated. The 15 GPa buckling transition is seen in both materials, and it appears to lead to a P21/m-like space group in CrSBr.
Pressure cycling drives an irreversible structural phase transition in CrSBr
It is well known that pressure cycling can be used to develop new materials51,53,54,55. In the case of CrSBr, vibrational spectroscopy reveals an additional structural phase transition upon decompression. Figure 6 summarizes the spectroscopic response of CrSBr upon compression to 22 GPa and subsequent release. The released form of the material differs from that of the starting compound. New phonon modes appear in the infrared absorption and Raman scattering spectra at approximately 12 GPa when the sample is decompressed from pressures above PC,3. These features are maintained when the sample is fully released. At this time, we do not know much about this new phase except that it has lower symmetry than Pmmn (with both in- and out-of-plane symmetry breaking) and is stable, lasting for at least ten months at ambient pressure outside of the diamond anvil cell. It is therefore amenable to additional investigation. As noted by Pawbake et al., minor changes in the light incidence angle or slight sample tilting at extreme pressures can give rise to angular-dependent phonon modes, such as the Bg modes observed after compressing past PC,343. However, even when accounting for these features, additional vibrational modes emerge in samples compressed beyond the third critical pressure [Fig. S6, Supplementary Note 7]. Interestingly, we can only access this pathway from pressures higher than PC,3 [Fig. S6, Supplementary Note 7]. Cycling to PC,2 or PC,1 does not drive this structural phase transition. This reinforces the existence of PC,3 and provides a route to interesting new functionality that is very much path dependent.
Methods
Crystal growth and loading of the diamond anvil cell
Single crystals of CrSBr were synthesized using a modified chemical vapor transport method discussed previously14,17,22,56,57. A small piece was placed in a symmetric diamond anvil cell. The diamond anvil cell was equipped with synthetic type II-as diamonds with 300 μm culets58. A stainless steel gasket was pre-indented to a thickness of 48 μm; the hole size was 150 μm. The pressure-transmitting medium was petroleum jelly and KBr for infrared and Raman scattering measurements, respectively, to achieve a quasi-hydrostatic environment. An annealed ruby ball was employed to determine pressure59. As shown in Fig. S1 of Supplementary Note 1, the ruby fluorescence is symmetric with well-separated R1 and R2 lines over the full pressure range of our work.
Spectroscopic measurements
The vibrational properties of CrSBr were investigated using synchrotron infrared and Raman scattering spectroscopies. High-pressure synchrotron infrared measurements were performed using the 22-IR-2 beamline at the National Synchrotron Light Source II (NSLS-II) at Brookhaven National Laboratory. The infrared spectra were collected in the far-infrared range (50–700 cm−1; 4 cm−1 resolution; transmission geometry) using a Bruker 80v equipped with a helium-cooled bolometer. The sample thickness must be carefully chosen to control optical density. Raman scattering spectra were measured with a Horiba Lab RAM spectrometer, a 532 nm excitation laser, a 100× working distance objective, 1800 line/mm gratings, and a cold CCD detector. Each scan was 60 s and averaged appropriately. Measurements focused on the 0–25 GPa pressure range at room temperature. Pressure was determined by ruby fluorescence [Fig. S1, Supplementary Note 1].
Symmetry analysis
The structural phase transition of CrSBr across the first critical pressure was analyzed using group-subgroup relations. The Bilbao Crystallographic Server was employed to identify the possible space group symmetries based on the number of changes in phonon modes60,61,62,63. The analysis involved plotting the phonon mode frequencies as a function of pressure and identifying the symmetry elements associated with each phase transition via the appearance and disappearances of phonon modes at specific pressures.
First-principles calculations
Frequencies, symmetries, and mode displacement patterns were obtained using first-principles spin-polarized density functional theory (DFT) calculations in the plane wave formalism as implemented in the Quantum ESPRESSO package64. The exchange-correlation energy is calculated using the generalized gradient approximation (GGA) using the Perdew–Burke–Ernzerhof (PBE)65 functional and standard Ultra-soft (USPP) solid-state pseudopotentials extracted from the Materials Cloud Database. The electronic wave functions were expanded with well-converged kinetic energy cut-offs for the wave functions (charge density) of 50 (400). The crystal structures were fully optimized using the Broyden–Fletcher–Goldfarb–Shanno (BFGS) algorithm until the forces on each atom were smaller than 1 × 10−4 Ry/au and the energy difference between two consecutive relaxation steps was less than 1 × 10−5 Ry. Grimme-D2 dispersion corrections were added to account for van der Waals interactions between adjacent layers. The Brillouin zone was sampled by a fine Γ-centered 8 × 8 × 6 k-point Monkhorst-Pack66. The phonon spectrum was calculated using a 3 × 3 × 2 supercell employing the Phonopy code67.
Data availability
Data are available from the corresponding authors upon reasonable request.
References
Grochala, W., Hoffmann, R., Feng, J. & Ashcroft, N. W. The chemical imagination at work in very tight places. Angew. Chem. Int. Ed. 46, 3620–3642 (2007).
Xiao, J.-R., Yang, S.-H., Feng, F., Xue, H.-G. & Guo, S.-P. A review of the structural chemistry and physical properties of metal chalcogenide halides. Coord. Chem. Rev. 347, 23–47 (2017).
Xu, K., Miao, X. & Xu, M. The structure of phase-change chalcogenides and their high-pressure behavior. Phys. Status Solidi RRL 13, 1800506 (2019).
Zhang, L., Wang, Y., Lv, J. & Ma, Y. Materials discovery at high pressures. Nat. Rev. Mater. 2, 1–16 (2017).
Pei, S., Wang, Z. & Xia, J. High pressure studies of 2d materials and heterostructures: a review. Mater. Des. 213, 110363 (2022).
Chi, Z.-H. et al. Pressure-induced metallization of molybdenum disulfide. Phys. Rev. Lett. 113, 036802 (2014).
Nayak, A. P. et al. Pressure-induced semiconducting to metallic transition in multilayered molybdenum disulphide. Nat. Commun. 5, 1–9 (2014).
Park, S. et al. Superconductivity emerging from a stripe charge order in IrTe2 nanoflakes. Nat. Commun. 12, 3157 (2021).
O’Neal, K. R. et al. High pressure vibrational properties of WS2 nanotubes. Nano Lett. 16, 993–999 (2016).
Matsuoka, T., Kim, H.-S., Samanta, S., Musfeldt, J. L. & Mandrus, D. G. MPX3 van der Waals magnets under pressure (M = Mn, Ni, V, Fe, Co, Cd; X = S, Se). Front. Mater. 11, 1362744 (2024).
Ghosh, A. et al. Exotic magnetic and electronic properties of layered CrI3 single crystals under high pressure. Phys. Rev. B 105, L081104 (2022).
Olmos, R. et al. Pressure-dependent magnetic properties of quasi-2d Cr2Si2Te6 and Mn3Si2Te6. J. Phys. Chem. C 127, 10324–10331 (2023).
Musfeldt, J. L., Mandrus, D. G. & Liu, Z. Insulator–metal transition in CrSiTe3 triggered by structural distortion under pressure. npj 2D Mater. Appl. 7, 28 (2023).
Beck, J. Über chalkogenidhalogenide des chroms synthese, kristallstruktur und magnetismus von chromsulfidbromid, CrSBr. Z. Anorg. Allg. Chem. 585, 157–167 (1990).
Göser, O., Paul, W. & Kahle, H. Magnetic properties of CrSBr. J. Magn. Magn. Mater. 92, 129–136 (1990).
Tabataba-Vakili, F. et al. Doping-control of excitons and magnetism in few-layer CrSBr. Nat. Commun. 15, 4735 (2024).
Telford, E. J. et al. Layered antiferromagnetism induces large negative magnetoresistance in the van der Waals semiconductor CrSBr. Adv. Mater. 32, 2003240 (2020).
Klein, J. et al. The bulk van der Waals layered magnet CrSBr is a quasi-1d material. ACS Nano 17, 5316–5328 (2023).
Lin, K. et al. Strong exciton–phonon coupling as a fingerprint of magnetic ordering in van der Waals layered CrSBr. ACS Nano 18, 2898–2905 (2024).
Uykur, E. et al. Phonon and magnon dynamics across antiferromagnetic transition in 2d layered van der Waals material CrSBr. Preprint at https://arxiv.org/abs/2405.07853v1 (2024).
Watson, M. D. et al. Giant exchange splitting in the electronic structure of a-type 2d antiferromagnet CrSBr. npj 2D Mater. Appl. 8, 54 (2024).
Boix-Constant, C. et al. Probing the spin dimensionality in single-layer CrSBr van der Waals heterostructures by magneto-transport measurements. Adv. Mater. 34, 2204940 (2022).
Boix-Constant, C. et al. Multistep magnetization switching in orthogonally twisted ferromagnetic monolayers. Nat. Mater. 23, 212–218 (2024).
Chen, Y. et al. Twist-assisted all-antiferromagnetic tunnel junction in the atomic limit. Nature 632, 1–7 (2024).
Esteras, D. L., Rybakov, A., Ruiz, A. M. & Baldoví, J. J. Magnon straintronics in the 2D van der Waals ferromagnet CrSBr from first-principles. Nano Lett. 22, 8771–8778 (2022).
Wilson, N. P., Yao, W., Shan, J. & Xu, X. Excitons and emergent quantum phenomena in stacked 2d semiconductors. Nature 599, 383–392 (2021).
Bae, Y. J. et al. Exciton-coupled coherent magnons in a 2d semiconductor. Nature 609, 282–286 (2022).
Hou, J. et al. Unveiling the localized exciton-based photoluminescence of manganese doped cesium zinc halide nanocrystals. Nano Lett. 23, 3762–3768 (2023).
Diederich, G. M. et al. Tunable interaction between excitons and hybridized magnons in a layered semiconductor. Nat. Nanotechnol. 18, 23–28 (2023).
Zur, Y. et al. Magnetic imaging and domain nucleation in CrSBr down to the 2d limit. Adv. Mater. 35, 2307195 (2023).
Ziebel, M. E. et al. CrSBr: an air-stable, two-dimensional magnetic semiconductor. Nano Lett. 24, 4319–4329 (2024).
Alapatt, V. et al. Highly polarized single photon emitter from intrinsic localized excitons in a WSe2/CrSBr heterostructure. Preprint at https://arxiv.org/abs/2409.04749v1 (2024).
Rivero-Carracedo, G., Rybakov, A. & Baldoví, J. J. Magnon sensing of NO, NO2 and NH3 gas capture on CrSBr monolayer. Chem. Eur. J. e202401092 (2024).
Komar, R. et al. Colossal magneto-excitonic effects in 2d van der Waals magnetic semiconductor CrSBr. Preprint at https://arxiv.org/abs/2409.00187v2 (2024).
Marques-Moros, F., Boix-Constant, C., Mañas-Valero, S., Canet-Ferrer, J. & Coronado, E. Interplay between optical emission and magnetism in the van der Waals magnetic semiconductor CrSBr in the two-dimensional limit. ACS Nano 17, 13224–13231 (2023).
Telford, E. J. et al. Designing magnetic properties in CrSBr through hydrostatic pressure and ligand substitution. Adv. Phys. Res. 2, 2300036 (2023).
Bykov, M. et al. High-pressure behavior of FeOCl. Phys. Rev. B 88, 014110 (2013).
Bykov, M. et al. Pressure-induced normal-incommensurate and incommensurate-commensurate phase transitions in CrOCl. Sci. Rep. 5, 9647 (2015).
Fausti, D. et al. Symmetry disquisition on the TiOX phase diagram (X = Br, Cl). Phys. Rev. B 75, 245114 (2007).
Burch, K. S., Mandrus, D. & Park, J.-G. Magnetism in two-dimensional van der Waals materials. Nature 563, 47–52 (2018).
Henríquez-Guerra, E. et al. Strain Engineering of Magnetoresistance and Magnetic Anisotropy in CrSBr. Preprint at https://arxiv.org/abs/2504.09920 (2025).
Torres, K. et al. Probing defects and spin-phonon coupling in CrSBr via resonant Raman scattering. Adv. Funct. Mater. 33, 2211366 (2023).
Pawbake, A. et al. Raman scattering signatures of strong spin-phonon coupling in the bulk magnetic van der Waals material CrSBr. Phys. Rev. B 107, 075421 (2023).
Zhang, T. et al. Magnetism and optical anisotropy in van der Waals antiferromagnetic insulator CrOCl. ACS Nano 13, 11353–11362 (2019).
Gu, Y. et al. Phonon mixing in the charge density wave state of ScV6Sn6. npj Quantum Mater. 8, 58 (2023).
Krantz, M., Thomsen, C. & Cardona, M. et al. Raman-active phonons and mode softening in superconducting HgBa2CuO4+δ. Phys. Rev. B 50, 1165 (1994).
Lukačević, I., Gupta, S. K., Jha, P. K. & Kirin, D. Lattice dynamics and Raman spectrum of rutile TiO2: The role of soft phonon modes in pressure induced phase transition. Mater. Chem. Phys. 137, 282–289 (2012).
Susmitha, B., Arjun, K. & Karthikeyan, B. Raman spectral studies on phonon softening, surface temperature, fano resonance, and phase change in MoS2 nanoflakes. Appl. Phys. A 129, 309 (2023).
Kroumova, E. et al. Bilbao crystallographic server: useful databases and tools for phase-transition studies. Phase Transit. 76, 155–170 (2003).
Fan, S. et al. Vibrational fingerprints of ferroelectric HfO2. npj Quantum Mater. 7, 32 (2022).
Musfeldt, J. et al. Structural phase purification of bulk HfO2:Y through pressure cycling. Proc. Natl. Acad. Sci. USA 121, e2312571121 (2024).
Zhang, J. et al. Magnetoelastic coupling in the incommensurate antiferromagnetic phase of FeOCl. Phys. Rev. B Condens. Matter Mater. Phys. 86, 134428 (2012).
Walsh, J. P. & Freedman, D. E. High-pressure synthesis: a new frontier in the search for next-generation intermetallic compounds. Acc. Chem. Res. 51, 1315–1323 (2018).
Klein, R. et al. High-pressure synthesis of the BiVO3 perovskite. Phys. Rev. Mater. 3, 064411 (2019).
Müller, C. J., Schwarz, U., Schmidt, P., Schnelle, W. & Doert, T. High-pressure synthesis, crystal structure, and properties of GdS2 with thermodynamic investigations in the phase diagram Gd-S. Z. Anorg. Allg. Chem. 636, 947–953 (2010).
Rybakov, A. et al. Probing short-range correlations in the van der Waals Magnet CrSBr by small-angle neutron scattering. Small Sci. 4, 2400244 (2024).
Boix-Constant, C. et al. Programmable magnetic hysteresis in orthogonally-twisted 2D CrSBr magnets via stacking engineering. Adv. Mater. 37, 2415774 (2025).
Jayaraman, A. Diamond anvil cell and high-pressure physical investigations. Rev. Mod. Phys. 55, 65 (1983).
Mao, H. K., Xu, J. & Bell, P. M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. Solid Earth. 91, 4673–4676 (1986).
Aroyo, M. I. et al. Crystallography online: Bilbao crystallographic server. Bulg. Chem. Commun. 43, 183–197 (2011).
Aroyo, M. I. et al. Bilbao crystallographic server: I. Databases and crystallographic computing programs. Z. Kristallogr. Cryst. Mater. 221, 15–27 (2006).
Aroyo, M. I., Kirov, A., Capillas, C., Perez-Mato, J. & Wondratschek, H. Bilbao crystallographic server. ii. representations of crystallographic point groups and space groups. Acta Crystallogr. Sect. A 62, 115–128 (2006).
Harms, N. C. et al. Symmetry progression and possible polar metallicity in NiPS3 under pressure. npj 2D Mater. Appl. 6, 40 (2022).
Giannozzi, P. et al. Quantum espresso: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 21, 395502 (2009).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Togo, A. & Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 108, 1–5 (2015).
Acknowledgements
J.L.M. thanks Physical Behavior of Materials, Basic Energy Sciences, U.S. Department of Energy (Contract number DE-SC0023144) for support of this research. Work at the National Synchrotron Light Source II at Brookhaven National Laboratory is funded by the Department of Energy (DE-AC98-06CH10886). Use of the 22-IR-1 beamline is supported by the National Science Foundation Division of Earth Sciences (EAR) SEES: Synchrotron Earth and Environmental Science (EAR-2223273) and Chicago/DOE Alliance Center (CDAC) DENA-0004153. We thank Y. Gu and K. A. Smith for valuable conversations. J.J.B. acknowledges the European Union (ERC2021-StG-101042680 2D-SMARTiES) and the Generalitat Valenciana (grant CIDEXG/2023/1). A.M.R. thanks the Spanish MIU (Grant No FPU21/04195). The authors in Valencia acknowledge the financial support from the Spanish MICIU (Excellence Unit “María de Maeztu” CEX2024-001467-M/AEI/10.13039/501100011033) and the Generalitat Valenciana (PROMETEO Program and a Ph.D fellowship to C.B.-C.). This study forms part of the Advanced Materials program and was supported by MCIN with funding from European Union NextGenerationEU (PRTR-C17.I1) and by the Generalitat Valencia.
Author information
Authors and Affiliations
Contributions
L.J.L. and J.L.M. designed the study. C.B.C. and S.M.V. grew the crystals with guidance from E.C. L.J.L., J.L.M., and Z.L. performed the high-pressure synchrotron infrared and Raman scattering spectroscopies. A.M.R. carried out the theoretical calculations supervised by J.J.B. L.J.L. analyzed the spectral data with guidance from J.L.M. C.B., S.M., and E.C. grew the crystals. L.J.L. and J.L.M. wrote the manuscript. All authors commented on the text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Langston, L.J., Ruiz, A.M., Boix-Constant, C. et al. Pressure-induced structural phase transitions in CrSBr. npj Quantum Mater. 10, 57 (2025). https://doi.org/10.1038/s41535-025-00767-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41535-025-00767-2
This article is cited by
-
Pressure-tuned plethora of ferroelectric phases in CuInP2S6
npj 2D Materials and Applications (2026)