Abstract
Premature ovarian failure (POF) is a disease closely related to the apoptosis of granulosa cells (GCs) in the follicle. In this study, exogenous melatonin (Mel) was used to interfere with POF model mice, so as to provide reference for Mel prevention and treatment of POF. Mel could promote estrogen secretion and improve ovarian physiological function in mice. In GCs, mitochondrial membrane potential increases and ATP content increases, LC3/LC3-LL and Beclin1 expression increases, p62 expression decreases, which promoted the occurrence of autophagy. Intersecting target screening, GO and KEGG enrichment analysis of Mel and POF revealed that estrogen receptor 1 (ESR1) was the most compatible target for Mel action; meanwhile, Mel had a high enrichment value in the PI3K-AKT-mTOR pathway. It was detected that Mel could increase the expression of ESR1 and inhibit the phosphorylation levels of PI3K, AKT, and mTOR to promote autophagy and reduce apoptosis of GCs.
Similar content being viewed by others
Introduction
Premature ovarian failure (POF) refers to a heterogeneous disease of the ovaries that occurs in females at a relatively young age and is characterized by disorders of estrogen secretion and a decrease in follicular maturation resulting in a gradual decline or complete loss of ovarian function1. As the partner of the oocyte, granulosa cells (GCs) are the largest group of cells that make up the follicle, and can help the growth and development of the oocyte and promote follicular maturation by secreting nutrients and hormones2. It has been demonstrated that the primary pathological mechanisms of POF are excessive apoptosis and impaired autophagy in GCs, which are accompanied by a reduction in estrogen levels and an increase in estrogen levels3,4. It follows that the protection of GCs’ autophagy, the maintenance of cellular homeostasis, and the alleviation of POF are key objectives.
Autophagy, an intracellular self-degradation process that removes misfolded toxic protein aggregates and damaged organelles through the formation of autophagic lysosomes, prevents cellular damage and maintains cellular homeostasis in the face of energy or nutrient shortages in response to a variety of cytotoxic insults5. Thus, autophagy has a cytoprotective function and needs to be tightly regulated to correctly respond to the different stimuli experienced by the cell in order to adapt to the changing environment6. During autophagy, the marker protein microtubule-associated protein light chain 3 (LC3) marks autophagosomal vacuole production, and the transition from LC3 I type to LC3 type II represents an increase in autophagic flux and an increase in autophagy level7. Beclin1 also represents a change in autophagic activity and is involved in the formation of the Beclin1-Vps34-Vps15 complex, which positively promotes the localization of autophagy-associated proteins to autophagic precursors and autophagosome nucleation, and assists in the regulation of autophagy and membrane transport involved in physiological and pathological processes8. P62 (also known as SQSTM1 protein) is a widely studied autophagy substrate that acts as a bridge linking LC3 and polyubiquitinated proteins during autophagosome formation and is selectively encapsulated into the autophagosome, after which it is degraded by proteolytic hydrolases in autophagic lysosomes, so that the expression of the p62 protein is negatively correlated with autophagic activity9. However, autophagy amplification induced by various stimuli is thought to be closely related to apoptosis, and apoptosis of GCs may contribute to the development of follicular atresia and polycystic ovary syndrome.
Estrogen receptor (ESR), a member of the steroid hormone receptor superfamily, plays a crucial role in organisms as a highly specific transcription factor capable of forming strong bindings to specific ligands such as estrogen, thereby activating relevant signaling pathways10. Phosphatidylinositol 3-kinase (PI3K) can mediate a variety of cellular effects, especially in cells of tumor tissues, whereas Akt is a downstream molecule of PI3K, and PI3K-AKT is an important intracellular signaling pathway for cellular autophagy regulation11. Paromomycin protein (mTOR) is a protein that is highly conserved in living organisms and is chemically characterized as a serine/threonine kinase. This protein is functionally important in that it initiates and regulates a series of complex signaling pathways by binding to specific sites on the cell membrane. Choi et al. found that inhibition of the PI3k-AKT-mTOR pathway resulted in activation of autophagy, leading to apoptosis and degeneration of GCs, which in turn triggered follicular atresia12. Gong et al. demonstrated that oxidative stress induces apoptosis and down-regulates the PI3K-Akt signaling pathway in ovarian GCs in PCOS, but growth hormone antagonizes this effect and stores the activity of the PI3K-Akt pathway13.
Melatonin (Mel), an endogenous hormone secreted mainly by pineal gland synthesis, is a broad-spectrum antioxidant with anti-inflammatory and anti-apoptotic effects14,15,16. Studies have shown that Mel significantly reduces the level of reactive oxygen species in oocytes, decreases early oocyte apoptosis, and repairs mitochondrial integrity17. Mel slows down ovarian aging, regulates ovarian biorhythms, promotes follicle formation and improves oocyte quality18,19. Meanwhile, the autophagy regulation of ovarian GCs by Mel has been mentioned in many studies, which can activate GCs viability in POF patients by activating FOXO3a to promote autophagy20. Mel has a dual role for autophagy regulation, either activating organismal autophagy to protect GCs to reduce apoptosis or inhibiting autophagy to exert cytoprotective effects. The purpose of this study was to use exogenous Mel to intervene in the POF model, to assess whether Mel can reduce apoptosis of follicular GCs and maintain normal physiological function of the ovary by regulating autophagy, and to provide a basis for the prevention and treatment of POF with exogenous Mel.
Results
D-gal successfully constructs a mouse POF model and Mel intervention improves ovarian function
The flowchart of the in vivo test is shown in Fig. 1 A. At the end of the 42 d test, CAT, GSH-Px, MDA, and SOD activities were examined in the ovarian tissues, and compared with the Con group, the D-gal group significantly decreased the activities of SOD, GSH-Px, and CAT (p < 0.001), and markedly elevated the activity of MDA (p < 0.01). While Mel intervention, relative to the D-gal group, in the DML group elevated the activities of SOD, GSH-Px and CAT (p < 0.01) and decreased the activity of MDA (p < 0.01), whereas in the DMH group elevated GSH and CAT (p < 0.05) but did not have a significant effect with SOD and MDA (p > 0.05) (Fig. 1B–E). ATP content was also examined and the results were significantly lower in the D-gal group relative to the Con group (p < 0.001) and elevated in the DML and DMH groups (p < 0.05) (Fig. 1F). The above results suggest that the D-gal group caused mitochondrial damage, redox imbalance in the cells of ovarian tissues, resulting in POF; Mel inhibited the occurrence of oxidative stress in the ovary by increasing the activity of antioxidant enzymes.
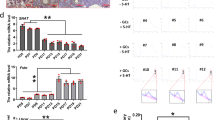
A Schematic diagram of animal model establishment and specimen collection. B Content of SOD in mice ovaries of each group. C Content of GSH-px in mice ovaries of each group. D Content of CAT in mice ovaries of each group. E Content of MDA in mice ovaries of each group. F Content of mitochondrial ATP in mice ovarian tissue. G Contents of FSH,LH,AMH,E2 and P hormones in each group of mice. H Protein bands and quantification results of FSHR in various groups of mice. I Plot of HE results of mice ovaries (400X).
Detection of peripheral blood levels of FSH, AMH, LH, P, and E2 hormones in each group of mice revealed that the D-gal group caused an increase in FSH and LH hormone levels (p < 0.01) and a decrease in AMH, P, and E2 hormone levels (p < 0.01) when compared with the Con group, suggesting that D-gal led to disruption of hormone secretion of the gonadotropic axis of the mice (Fig. 1G). Compared with the D-gal group, the DML and DMH groups could reduce FSH and LH hormone levels and increase AMH, P and E2 hormone levels (p < 0.05), with the DML group having the best effect. Detection of the FSH hormone receptor FSHR revealed reduced expression in the D-gal group compared to the Con group (p < 0.001) and elevated expression in both the DML and DMH groups compared to the D-gal group (p < 0.05) (Fig. 1H). The above results suggest that Mel can alleviate D-gal-induced disruption of gonadal axis hormone secretion. In the ovarian HE staining Fig. 1I we found that follicles and corpus luteum in the Con group were at different stages of maturation, and the D-gal group had a decrease in ovarian volume and a significant increase in the number of atretic follicles, which is an important feature of the effect of POF on follicles. However, in the DML and DMH groups, the number of follicles at different stages of maturation was significantly higher than that in the D-gal group, and atretic follicles were not obvious. It suggests that Mel can promote the development of follicles and reduce the occurrence of atretic follicles.
Mel improves autophagy in mice ovarian GCs
To investigate whether Mel has a role in regulating autophagy in POF, autophagy assay was performed on ovarian tissues in this experiment. In the immunofluorescence assay of LC3 on ovarian tissue sections (Fig. 2A), the fluorescence comparison showed that the brightness of the D-gal group was significantly lower than that of the Con group, whereas administration of the Mel group suppressed this decrease in the amount of fluorescence, and the best effect was observed in the DML group.
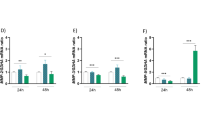
The autophagy markers P62, Beclin1 and LC3II/I protein and mRNA expression were further examined by western blotting and qRT-PCR. The results of protein assay showed that the expression of LC3I/II and Beclin1 was decreased (p < 0.05) and the expression of P62 was significantly elevated (p < 0.001) in the D-gal group relative to the Con group, which was inhibited in the MT group, where the DMH group had a better effect, which significantly decreased the expression of P62 and elevated the expression of Beclin1 and the LC3II/I protein levels (p < 0.05) (Fig. 2B, C). The results of mRNA assay were consistent with the protein results (Fig. 2D), which showed that the expression of LC3 and Beclin1 was decreased and the expression of P62 was significantly increased in the D-gal group relative to the Con group (p < 0.01), and in both the DML and DMH groups, the gene levels of P62 and Beclin1 and LC3 were significantly decreased and increased relative to the D-gal group (p < 0.01). These results suggest that melatonin can promote autophagy in granulosa cells in mice ovarian tissues.
Mel promotes autophagy in KGN cells and inhibits mitochondrial damage
Using Mel to intervene in D-gal-treated KGN cells, the results showed that cell activity decreased sequentially with increasing D-gal concentration, and cell viability was 60% of that of the blank group when the D-gal concentration was 30 mg/ml, whereas Mel had the best cell viability at 1 nm (Fig. 3A–C). The cell test flow chart is illustrated in Fig. 3D. ATP content was assayed, and the results showed that the content of D-gal group was significantly lower, and the content of D-gal+Mel group was significantly higher compared to D-gal group (p < 0.001) (Fig. 3E). The cellular mitochondrial membrane potential was examined by fluorescence assay, which resulted in a significant decrease in red fluorescence and an increase in green fluorescence in the D-gal group compared to the Con group, while one was suppressed in the D-gal+Mel group (p < 0.001) (Fig. 3F). Autophagy proteins were assayed and the results showed that relative to the Con group, the D-gal group resulted in a decrease in LC3II/I, Beclin1, and an increase in P62 (p < 0.001), whereas the D-gal+Mel group showed a decrease in P62 and an increase in LC3II/I, Beclin1 (p < 0.05) (Fig. 3G, H).
A Different concentration of melatonin KGN cell vitality. B Different concentration of D - galactose KGN cell vitality. C D - galactose and different concentrations of melatonin KGN cell vitality test flow chart. D Cell test flow chart. E Mitochondrial ATP content of KGN cells. F KGN cell fluorescence graph. G P62, Beclin1, LC3I protein bands/II representative images. H Proteins of P62, Beclin1 and LC3I/II were quantified in (G).
The autophagy inhibitor Bafilomycin A1 (BafA1) was involved in the trial for validation. Proteins were assayed and their results showed that relative to the D-gal+Mel group, LC3, P62 was elevated and Beclin1 protein was decreased when the inhibitor was added (p < 0.001) (Fig. 4A, B). After performing mRNA assay its result was a decrease in LC3, Beclin1 and an increase in P62 in the D-gal+Mel+Baf A1 group (p < 0.05) (Fig. 4C). Immunofluorescence detection of LC3 revealed decreased fluorescence in the D-gal group relative to the Con group and enhanced fluorescence in the D-gal+Mel group, but the fluorescence brightness of this group was again reduced upon addition of the inhibitor (Fig. 4D). Cells were subjected to MDC autophagy assay, and the results showed that the fluorescence intensity was significantly enhanced in the D-gal+Mel group relative to the fluorescence in the D-gal group, but there was little difference in intensity between the D-gal+Mel+Baf A1 group and the D-gal group (Fig. 4E). The above results indicated that D-gal caused damage to the mitochondria of KGN cells and induced autophagy, and Mel could maintain cellular homeostasis by promoting autophagy.
Visualization of interaction targets in Mel and POF active ingredients
In order to further reveal the potential mechanism by which Mel affects autophagy in GCs, a network pharmacological assay was performed to screen targets for Mel and POF. A total of 222 Mel targets were obtained after screening targets and removing duplicates from Swiss Target Prediction, Pharm Mapper Server, and Drug Bank databases. Cytoscape 3.8.0 was utilized to construct the Mel target network, as shown in Fig. 5A, with 25 known targets, or 11.3% of the total targets, and 197 putative targets, or 88.7% of the total targets, with 10 intersecting targets between the potential and known targets. A total of 2955 POF target genes after de-emphasis were obtained from Drug Bank, Genecards, and OMIM databases. The Veeny tool associated MT targets with POF target genes and obtained 121 crossover genes (Fig. 5B), which were entered into the STRING 12.0 database to obtain PPI network information, with 187 edges, an average node degree of 3.09, and an average local clustering coefficient of 0.42 (Fig. 5C). We used CytoNCA, a plug-in for Cytoscape, to analyze the topology of PPI networks. A total of 22 relevant and significant targets were obtained based on the mean values of degree centrality (DC), betweenness centrality (BC) and proximity centrality (CC) of 3.0, 85.51 and 0.25, respectively (Fig. 5D). The larger the node shape the higher the degree value, the more nodes it interacts with.
A Melatonin - the putative target network; green nodes represent potential targets; blue nodes represent the known targets; red nodes indicate the intersection of potential and known targets. B Venn diagram of the intersection targets of melatonin and DOR. C PPI network of the intersection targets. D Topological analysis of melatonin and POF core targets. Node size and color according to the degree of value in descending order from big to small, from orange to green said. E GO enrichment analysis and KEGG pathway analysis of top 20 enriched biological processes. F 20 potential therapeutic targets. A, D are from Cytoscape 3.9.1 software, B is from Venny2.1.0 website, C is from STRING 12.0 database, E, F are from bioinformatics database.
The biological functions of Mel associated with POF resistance were determined by graphene oxide enrichment analysis in terms of biological processes (BP), cellular components (CC) and molecular functions (MF) (Fig. 5E). It is suggested that Mel may be related to the response to exogenous stimuli, positive regulation of cell proliferation, signal transduction, and phosphatidylinositol 3-kinase signaling thereby participating in the regulation of the ovarian cell’s response to the protein serine/threonine/tyrosine kinase activity, steroid binding, protein kinase activity, estrogen-responsive element binding, ATP binding, and phosphatidylinositol 3-kinase activity to ameliorate POF. The potential mechanism of Mel to improve POF was explored by KEGG enrichment analysis, which showed that Mel was highly enriched for signaling pathways such as cancer pathway, endocrine resistance, estrogen signaling pathway, and PI3K-Akt signaling pathway. Mel regulates biological processes through these signaling pathways and plays a role in alleviating POF (Fig. 5F). Interestingly, we found that the PI3K/Akt pathway associated with ovarian ovulation was significantly enriched in the top 20 enriched pathways, suggesting that Mel may regulate the role of the PI3K/Akt pathway in ovarian function.
The screening of Mel and POF network pharmacology yielded 12 key hub genes within the topological network, including SRC, ESR1, PIK3CA, PIK3CD, PIK3CB, and EGFR. Furthermore, the molecular docking results demonstrated that Mel exhibited binding affinity to the ESR1 active site, resulting in the lowest binding energy arrangement and the optimal 3D molecular docking structure. Mel can bind to GLU amino acid residue 353 of receptor protein ESR1 through two hydrophobic forces of 3.4 Å, to PRO amino acid residue 324 of receptor protein ESR1 through two hydrophobic forces of 3.5 Å and 3.7 Å, and to PRO amino acid residue 325 of receptor protein ESR1 through hydrogen bonding of 3.2 Å. Furthermore, a two-dimensional force analysis yielded analogous results (Fig. 6A, B). Mel can establish non-bonding interactions with the ESR1 protein within the molecular complex, leading to the formation of forces characterized by electrostatic potential energy, van der Waals forces, and hydrophobic bonds (Fig. 6C, D). These forces facilitate the formation of a stable and compact complex between the two entities. The lowest binding energies observed for the five molecules were all below -6 kcal/mol, with the lowest binding energy recorded at −7.0 kcal/mol (Fig. 6E).
A The small molecule melatonin is designated as a ligand and the target protein ESR1 as a receptor, for small molecules–protein. B Melatonin ligand docking and ESR1 receptor. C The two-dimensional force melatonin ligand and ESR1 electrostatic potential diagram. D Melatonin receptor ligands and ESR1 receptors static 2 D figure, among them, the red dashed lines hydrophobic forces, the green dashed line indicates the lowest binding energy localization of the hydrogen bond. E Melatonin-ESR1 molecular docking. A is from pymol software, B is from Discovery studio software, C is from pymol software, Dis from ligplot software, E is a table made according to the combination of Auto dock docking.
Mel inhibits the PI3K/AKT signaling pathway via ESR1
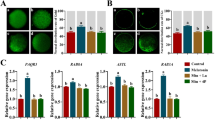
Ovarian tissue sections were subjected to ESR1 immunofluorescence assay, as shown in Fig. 7A, the results of which revealed that the fluorescence brightness of the D-gal group was significantly reduced relative to the Con group. Relative to the D-gal group, the fluorescence intensity groups of the DML and DMH groups significantly increased the fluorescence brightness. The results of ESR1, PI3K, AKT and mTOR protein assays showed that the protein expression of ESR1 was significantly lower in the D-gal group relative to the Con group (p < 0.001), but significantly higher in the Mel group, and the best effect was observed in the L group (p < 0.01). Meanwhile, the protein expression ratios of p-AKT/AKT, p-PI3K/PI3K, and p-mTOR/mTOR were significantly higher in the D-gal group relative to the Con group (p < 0.001), but the expression ratios of these proteins were decreased in the DML and DMH groups relative to the D-gal group (p < 0.05) (Fig. 7B, C).
In the in vitro assay, KGN cells were subjected to ESR1 immunofluorescence assay, the results of which are shown in Fig. 8A. It was found that the fluorescence brightness of the D-gal group was significantly reduced relative to the Con group, and that the fluorescence brightness of the D-gal+Mel group was significantly increased relative to the D-gal group. The protein content of ESR1, PI3K, AKT, and mTOR of KGN cells was examined, and the expression of ESR1 was decreased in the D-gal group (p < 0.001), but significantly increased in the D-gal+Mel group (p < 0.001). Meanwhile, the protein expression ratios of p-AKT/AKT, p-PI3K/PI3K, and p-mTOR/mTOR were significantly higher in the D-gal group relative to the Con group (p < 0.001), but decreased in the D-gal+Mel group relative to the D-gal group (p < 0.05) (Fig. 8B, C).To determine whether Mel mediates autophagy through inhibition of the PI3K-AKT signaling pathway, AKT was activated in cells using SC79, resulting in elevated AKT expression (Supplementary Material 1). Immunofluorescence detection of LC3 on KGN cells showed that the amount of LC3 fluorescence was significantly lower in the D-gal group than in the Con group (p < 0.001), and this decrease in fluorescence was inhibited in the D-gal+Mel group relative to the D-gal group (p < 0.001), but the fluorescence intensity in the SC79 group was significantly reduced when the inhibitor was added (p < 0.001) (Fig. 8D). For the autophagy protein assay, compared with the D-gal+Mel group, the SC79 group showed elevated P62 content, decreased Beclin1 content and LC3II/LC3I ratio (p < 0.01) (Fig. 8E, F), and the above results indicated that the autophagy-promoting effect of Mel was blocked. Meanwhile, immediately after we stained the cells for autophagy MDC, the staining results showed that autophagy was significantly decreased in the SC79 group after adding SC79 inhibitor relative to the D-gal+Mel group (Fig. 8G). These findings indicate that melatonin promotes autophagy through ESR1 pathway, which is mediated by the PI3K/AKT/mTOR pathway.
A ESR1 fluorescence of KGN cells. B ESR1, PI3K, and AKT, mTOR protein bands representative images. C In (B), ESR1, PI3K, AKT and mTOR proteins were quantified. D LC3 fluorescence of KGN cells. E Representative picture of autophagy marker protein bands. F This autophagy marker protein is quantified in (E). G MDC staining of autophagy in KGN cells.
Mel reduced the apoptosis of GCs
The above tests have shown that Mel enhances autophagy and that cellular autophagy reduces apoptosis. TUNEL staining results showed that the apoptosis level was increased in the D-gal group compared to the Con group (p < 0.001), and significantly decreased in the DML and DMH groups compared to the D-gal group (p < 0.01) (Fig. 9A). Protein assay of apoptotic factors in ovarian tissues showed that the protein level of Bax was significantly increased in D-gal tissues compared to Con group (p < 0.001), the expression level of Bax was decreased after administration of Mel (p < 0.001) and decreased significantly in DMH group, the protein expression of Bcl-2 was decreased in the D-gal group (p < 0.001), and compared to the D-gal group expression was significantly higher in the DML and DMH groups (p < 0.01) compared to the D-gal group (Fig. 9B, C).
A TUNEL staining diagram. B Representative images of apoptosis-marker protein bands in ovarian tissue. C Apoptosis marker proteins were quantified in (B). D Flow cytometry results. E Each flow cytometry quantitative marker protein bands. F KGN cell apoptosis representative images. G This apoptosis marker protein is quantified in (F).
In the cell assay, flow cytometry results showed that the apoptosis rate was significantly higher in the D-gal group compared to the Con group (p < 0.001), significantly lower in the D-gal+Mel group compared to the D-gal group (p < 0.001), and higher in the SC79 group (Fig. 9D, E). Cells were subjected to apoptotic protein assay, shown to result in significantly higher Bax expression and significantly lower Bcl-2 expression levels in the D-gal group (p < 0.001). In the D-gal+Mel group Bax expression was decreased and Bcl-2 expression was increased (p < 0.001), and in the SC79 group Bax expression was significantly elevated and Bcl-2 expression was significantly decreased (p < 0.01) (Fig. 9F, G). These findings indicate that Mel exerts an anti-apoptotic effect through the promotion of autophagy.
Correlation analysis of PI3K/AKT/mTOR signaling pathway with autophagy, oxidative stress and apoptosis
Intergene correlation coefficients were calculated and visualized using the R package corrplot (Fig. 10). mRNA expression of PI3K and its downstream target genes was negatively correlated with the levels of Bax, Caspase-3, Caspase-9, and P62 in the ovary, but positively correlated with GSH, SOD, CAT, Bcl-2, LC3, Beclin1, and ATG7 activities. In summary, PI3K/AKT/mTOR and cellular autophagy and oxidative stress were positively correlated, and apoptosis was significantly negatively correlated.
Discussion
The main pathology of POF is hormonal disruption leading to increased activation of primordial follicles and decreased maturation rate resulting in decreased ovarian reserve function1. Hormone replacement therapy is currently the most common form of treatment, but it also has certain treatment pitfalls21,22. D-gal, a molecule widely used in biology and medical research, is noted for its excellent performance in constructing models of aging, a chemical that can mimic a wide range of physiological changes that occur during the aging process in vivo, including alterations in cellular morphology, DNA damage, and disruption of protein homeostasis23,24. Thus, D-gal is a chemical substance that can be used to construct whole-body senescence models with the advantages of a simple modeling process, high reproducibility, and low cost, providing a reliable tool for understanding the mechanisms of aging. Many studies have proved that Mel is essential for the growth and development of the female reproductive system and plays a pivotal role in maintaining the normal mechanism of reproductive organs25,26. In the present study, we used D-gal to model POF, investigate the protective effect of Mel against POF and reveal its mechanism. The assay of serum hormones in mice showed that D-gal increased FSH and LH levels, decreased AMH, P, and E2 levels, and decreased the protein expression of FSHR, however, the reversal occurred after Mel intervention, and the best effect was achieved with the intervention at a dose of 10 mg/kg. As observed by HE sections of ovaries from all groups of mice, D-gal resulted in a significant increase in atretic follicles and a decrease in the number of mature follicular cells, and Mel intervention significantly reduced atretic follicle occurrence and increased follicular maturation rate. Mitochondria are the primary site of reactive oxygen species (ROS) generation in organisms. Under normal conditions, these free radical molecules can act as a key signaling molecule within the cell, regulating a variety of physiological processes including, but not limited to, protein folding, DNA repair, and signaling. However, when the cell is subjected to damage or stress, this equilibrium is disturbed, and the mitochondria not only become the hardest hit by ROS during this period, but also are highly vulnerable to damage themselves. In the present study, we found that in vivo and in vitro, D-gal significantly reduced intracellular ATP content, which was restored after Mel intervention. At the same time, D-gal decreased the activities of antioxidant enzymes SOD2, GSH-px and CAT and increased the level of MDA, indicating that ovarian tissues have more serious lipid peroxidation damage, and the activities of antioxidant enzymes in ovarian tissues were increased and the level of MDA was significantly decreased after Mel intervention, which suggests that Mel administration can significantly reduce lipid peroxidation of tissues.
When intracellular mitochondrial damage occurs, if the level of mitochondrial protective mechanisms is not sufficient to recover from the damage, cellular autophagy will be induced to phagocytose the damaged mitochondria to avoid triggering apoptosis.27. It was shown that mTOR significantly increased the level of autophagy in ovarian tissues after injury, reduced apoptosis of GCs, and maintained the overall function of the ovary28. Wang et al. showed that lycopene alleviated renal fibrosis by promoting cellular macroautophagy to destroy damaged mitochondria29. Qiao et al. showed that Mel had the effect of reducing the degree of mitochondrial oxidative stress and swelling as well as restoring mitochondrial membrane potential30. We showed that D-gal increased P62 levels and decreased Beclin 1 and LC3II/LC3I ratios, but when administered Mel decreased P62 levels and increased Beclin 1 and LC3II/LC3I ratios. The addition of Baf-Al further increased the LC3II/LC3I ratio and the expression of P62 and decreased the content of Beclin1 in the cells, indicating that Mel could promote the first half of autophagy. Detection of overall intracellular autophagy by MDC fluorescence resulted in a decrease in autophagy fluorescence intensity by D-gal, and Mel intervention could further enhance the autophagy fluorescence intensity. Based on the above results we hypothesized that Mel intervention may reduce apoptosis by promoting the formation of autophagosomes and converting autophagosomes to autophagolysosomes, which in turn phagocytose a large number of damaged organelles and restore mitochondrial function.
Granulosa cells are the primary components of follicle development and maturation. Their apoptosis is the fundamental cause of follicular atresia. A complex link has been revealed between granulosa cell autophagy and follicular atresia by scientists. Autophagy represents a form of self-repair mechanism whereby cells may absorb and decompose a portion of their material in response to environmental stress or damage, thereby enabling their survival. While autophagy itself does not directly cause follicular atresia, it may indirectly affect granulosa cell function, thereby altering the entire reproductive cycle. Jinshan Xing et al. demonstrated that ferredoxin 1 is a pivotal regulator of follicular development, influencing apoptosis, autophagy, and proliferation31. Min Gao et al. showed that PFOS induced apoptosis and autophagy by inhibiting the PI3K/AKT/mTOR pathway in KGN cells32. In the present study, TUNEL assay of ovarian tissues showed that D-gal increased the apoptosis of granulosa cells around the oocytes, while Mel reduced the apoptosis of granulosa cells. Flow cytometry and in vitro, D-gal caused KGN apoptosis rate increased, after dosing Mel can reduce the apoptosis rate of cells, but, after joining SC79 Mel KGN apoptosis inhibition effect disappeared, further illustrate Mel through PI3K-AKT-mTOR pathways play a role.The results of apoptosis marker protein analysis in vitro and in vivo demonstrated that D-gal elevated Bax expression and diminished Bcl-2 expression, whereas Mel administration markedly enhanced the expression of apoptotic proteins. These findings suggest that Mel can exert an anti-apoptotic effect on granulosa cells.
In order to explore the potential targets of action of Mel in regulating granulocyte autophagy, the intersecting targets of Mel and POF diseases were screened by network pharmacology, combined with network topology analysis, PPI network analysis indicated, GO enrichment analysis and KEGG pathway enrichment analysis. It was hypothesized that the core target of the drug acting on POF is ESR1 and the pathway of action is PI3K/AKT/mTOR. In addition, molecular docking of Mel and ESR1 was performed, and it was found that Mel could bind to ESR1 protein to form a stable structure, and the coupling was stable, and the five molecules with the lowest binding energies were all below −6 kcal/mol, with the lowest binding energy of −7.0 kcal/mol, which suggests that Mel can directly act on ESR1 targets. There are two subtypes of estrogen receptor (ER), encoded by the estrogen receptor 1 (ESR1) and 2 (ESR2) genes, estrogen receptor α and β, respectively. These are designated estrogen receptors α and β33. The autophagy-inducing function of ERβ has been demonstrated in a variety of cell types, such as breast cancer cells, human spermatogonial tumor cells, and osteosarcoma cells34,35. Wentao Fan et al. demonstrated that estrogen receptor α activation inhibited colitis by promoting NLRP6-mediated autophagy, reduced inflammatory factors, maintained intestinal epithelial cell homeostasis and promoted colitis tissue repair36. We examined ESR1 content ex vivo and in vivo by immunofluorescence and protein blotting, and the results were that D-gal decreased ESR1 content and Mel intervention increased ESR1 expression, suggesting that Mel promotes ESR1 expression within GCs. PI3K, an intracellular phosphatidylinositol kinase, is regulated by multiple growth factors and can mediate autophagy and apoptosis, cell proliferation37. AKT is a major regulator of cell survival under stress conditions, which regulates not only nutrient intake but also the expression of growth factors38. The PI3K/AKT/mTOR signaling pathway has emerged as an important regulatory pathway upstream of autophagy. Studies have shown that estrogen receptor α mediates the PI3K/AKT/mTOR pathway to regulate oocyte growth, inhibit ovarian granulosa cell apoptosis, and prevent follicular pool hyperactivation39,40. We validated PI3K/AKT/mTOR signaling in vitro and in vivo, and our results showed that the phosphorylation levels of PI3K, AKT, and mTOR were elevated in the D-gal group and decreased in the Mel group, suggesting that Mel intervention inhibited the phosphorylation of PI3K, AKT, and mTOR, and further suggesting that Mel is the most effective tool to regulate autophagy through the PI3K/AKT/mTOR signaling pathway to regulate autophagy. When the AKT activator SC79 was added in vitro, the activation of mitochondrial autophagy by Mel was found to be weakened by the detection of autophagy proteins, and the ratios of Beclin1 and LC3II/I were reduced, and the content of P62 was elevated, and the role of Mel in regulating autophagy through the PI3K/AKT/mTOR pathway was suppressed, which verified our hypothesis and demonstrated that Mel promotes autophagy in ovarian GCs by inhibiting the PI3K/AKT/mTOR pathway to promote autophagy in ovine GCs.
In summary, Mel participates in autophagy and apoptosis of ovarian GCs by acting on ESR1 targets and regulating the PI3K/AKT/mTOR signaling pathway to reduce atretic follicles and promote the restoration of ovarian function (Fig. 11).
Methods
Reagents and antibodies
Melatonin (S20287) was purchased from Shanghai Yuanye Biological Co., LTD. (Shanghai, China). D-galactose (D6310) was purchased from Biotoppet (Beijing, China). SC79 (M5153), Bafilomycin A1 (M4953) purchased from AbMole (Shanghai, China). LC3 α/β (1:500, WL01506), P62 (1:500, WL02385), Beclin1 (1:500, WL02508), FSHR (1:500, WL04496), PI3K (1:500, WL03380), p-PI3K (1:500, WL02240), AKT (1:500, WL0003b), p-AKT (1:500, WLP001a), mTOR (1:500, WL02477), p-mTOR (1:500, WL03694), ESR1 (1:500, WL00940), Bax (1:500, WL01637), Bcl-2 (1:500, WL01556) purchased from Wanleibio (Shen Yang, China). β-actin (1:3500, bs-12581R), HRP-labeled goat anti-rabbit (1:5000, bs-0295G-HRP) were purchased from Bioss (Beijing, China).
Network pharmacological analysis
Use the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), “melatonin” as keywords, obtain the Mel Canonical SMILES information and 3d structure; Upload the Mel SMILES to Swiss Target Prediction database (http://www.swisstargetprediction.ch/), 3D structure of Mel was uploaded to the Pharm Mapper website (http://www.lilab-ecust.cn/pharmmapper/) to predict potential Mel targets. Using DrugBank (https://www.drugbank.com/) and TCMSP database (https://tcmspw.com/tcmsp.php/) to determine the Mel known targets. All the retrieved target name use UniProt database (https://www.uniprot.org/) correction is the official symbol. After correction, Excel spreadsheet was used to remove the weight to obtain the target of Mel. “Premature ovarian failure” was the key word, In GeneCards (https://www.genecards.org/), OMIM database (http://www.omim.org/), Drug Bank database (https://go.drugbank.com/) to obtain disease-related targets after screening and integration.
Active ingredient for clear Mel targets with POF disease targets, the relationship between the two targets of import Venny2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/index.html) draw the Venn diagram, determining the target intersection of genes. Will cross genes into STRING 12.0 database (https://stringdb.org/), and use the confidence score >0.9, hidden in the network link node, the other parameters remain the same. The results were imported into Cytoscape3.9.1 software in text format to generate protein interaction network for visualization.
GO enrichment and enrichment of KEGG analysis using microscopic letter database (https://www.bioinformatics.com.Cn/). Enrichment analysis analyzed the enrichment levels of proteins and genes from three aspects: biological process (BP), cellular component (CC), and molecular function (MF).
Molecular docking
The binding ability of Mel to the key targets of POF was verified by molecular docking. The crystal structure of ESR1 of the core target was obtained from the Protein Data Bank (PDB format). Protein and ligand data were saved in PDBQT format using AutoDockTools-1.5.6. Finally, using AutoDockVina.exe for molecular docking and the results are calculated. The docking results of key target proteins with the strongest binding ability were displayed using PyMOL software.
Grouping and handling of experimental animals
Female C57BL/6 mice aged 6–8 weeks were purchased from Liaoning Changsheng Biotechnology Co., LTD. (Shenyang, China). Mice were randomly divided into the following 5 groups: control group (Con), melatonin group (Mel), model group (D-gal), D-gal+ melatonin low-dose group (DML), D-gal+ melatonin high-dose group (DMH). Each treatment group was repeated 3 times, 5 mice each time, and the injection method was intraperitoneal injection. The treatments for each group were as follows, Con group: normal saline 0.2 mL/mice/day. Mel group: normal saline was injected, on day 13, 10 mg/kg Mel (dissolved in normal saline) was injected. D-gal group: 200 mg/kg D-gal (dissolved in normal saline) was injected. DML and DMH groups: D-gal was injected and on day 13, 10 mg/kg and 20 mg/kg Mel were injected, respectively. After 42 days, all animals were euthanized by injection of barbiturates. Blood samples and isolated ovarian tissues were collected and then stored in a −80 °C freezer. The two reagent reference doses for D-gal and Mel in this study were determined based on published papers and preliminary experiments41,42.
Northeast Agricultural University Institutional Animal Protection and Use Committee (NEAUEC20240391) approved the study. Experiments on animals, abide by the National Institutes of Health on experimental animals live, care and use of the standard. All trials were designed and reported in accordance with the standard for animal studies: Report of in Vivo Studies (ARRIVE).
Determination of peripheral blood hormones in mice
Frozen serum samples were allowed to melt at room temperature, and ELISA kits were allowed to equilibrate at room temperature for 15 min. Carried out in accordance with the first kit biological specification standard sample dilution and point, then the serum samples of sample points, incubate, liquor, washing and enzyme and color. Readings were taken using a fully functional microplate reader, the resulting OD values were analyzed, a standard curve was drawn, and the final results were calculated according to the manufacturer’s instructions. Kits were used to detect anti-Mullerian hormone (AMH), gonadotropin (FSH), luteinizing hormone (LH), estrogen (E2) and progesterone (P) in serum. ELISA kits were purchased from Beijing Chenglin Biotechnology Co., LTD. (Beijing).
Ovarian tissue morphology
Ovaries were fixed in 4% formaldehyde for 24 h, dehydrated in increasing concentrations of ethanol solution and treated with xylene, paraffin embedded and cut into 5 µm sections. Sections were stained with hematoxylin and eosin (H&E) reagent, and follicles were observed morphologically and categorized by microscopy.
Immunofluorescence
Ovarian tissue slices according to normal procedure dewaxing and hydration, antigen epitope repair and closed after the nonspecific binding sites, with a resistance of LC3, ESR1 incubation under 4 °C for the night. After washing with PBS, the sections were incubated with fluorescent secondary antibody at a dilution ratio of 1:300 for 50 min at room temperature in the dark. The nuclei were then stained with DAPI. With resistance to finally fluorescence quenching agent (PVB) sealing piece, fluorescence microscope autophagy marker LC3 and ESSR1 express, and analysis of fluorescence intensity.
TUNEL staining of ovarian tissue
Paraffin sections prepared from ovarian tissue were deparaffinized twice in xylene for 10 min. Finally, in turn, in anhydrous ethanol, 90% ethanol and 70% ethanol hydration in 2 min. In detail according to the TUNEL kit instruction of paraffin section. Add 20 mu g/mL of protease (excluding DNase), 37 °C incubation for 20 min, PBS washing three times. The TUNEL assay solution was prepared with TdT enzyme and fluorescent labeling solution at a ratio of 1:9, mixed thoroughly, added to the sections, incubated at 37 °C in the dark for 1 h, and washed clean with PBS. Using fluorescence quenching of sealing seal tablets in fluorescence microscope and photo collection of images, Image J 6.0 software was used to Image density of fluorescent quantitative analysis.
TUNEL kit (GDP1044) was purchased from Wuhan Sevier Biotechnology Co., LTD. (Wuhan, China).
Cell culture and viability assay
KGN cells, a human ovarian granular tumor cell line, were obtained from EK Bioscience, Shanghai, China. KGN cell culture in adding 10% fetal bovine serum (FBS) and 100 mu, 100 u/ml penicillin g/ml chain mildew DMEM-F12 medium completely, at 37 °C and 5% CO2 under the environment of cultivating, represented a week 2 to 3 times.
The cell count kit-8 (CCK 8) method is used to determine the cell vitality. 0.1 mL of KGN cells (1 × 105 cells /mL) were plated in 96-well plates and different concentrations of D-gal and Mel were added. At the end of the experiment, 10 μL CCK-8 was added and incubated in the incubator for 1.5 h. Finally, a spectrophotometer at 450 nm per hole is used to measure the optical density. D - gal concentration of 0, 10 mg/ml, 20 mg/ml, 30 mg/ml, 40 mg/ml, 60 mg/ml. Mel concentrations were 0, 100 PM, 500 pm, 10 nm, 15 nm, and 30 nm.
Mitochondrial membrane potential (MMP) was measured
Mitochondria are considered to be the bioenergy centers of cells and also play a crucial role in the apoptotic pathway. Reduction of MMP is considered to be a marker of early apoptosis. Membrane potential sensitive probe JC-1 was used to detect the changes in MMP. When MMP is high, the cationic JC-1 dye aggregates in the mitochondrial matrix to form aggregates that emit red fluorescence, whereas when MMP is low, the monomers emit green fluorescence. JC-1 from red fluorescence to green fluorescent show that MMP, less can be used as the early detection of cell apoptosis index. KGN cells were seeded in 6-well plates at a density of 5 × 105 cells/well, cultured overnight, and treated with D-gal and Mel. 0.5 ml of JC-l dye was added to 6-well plates and stained for 15 min according to the instructions. Using fluorescent microscope cell fluorescence.
Mitochondrial membrane potential detection kit (M8650) was purchased from Solebo Technology Co., LTD. (Beijing, China).
The determination of ATP
The ovarian tissue of each group was weighed accurately, and the ratio of weight (g) : volume (ml) = 1:9 was added with cold double steamed water to make 10% homogenized slurry, which was boiled in a boiling water bath for 10 min, removed the mixture, extracted for 1 min, centrifuged at 3500 r/min for 10 min, and the supernatant was taken for measurement. After the culture supernatant was removed, the lower layer of precipitated cells was obtained. The collected cells were added with 300–500 μL cold double-steamed water, placed in an ice water bath, homogenized and broken, and the cell suspension was heated in a boiling water bath for 10 min. The cell suspension was taken out and mixed and extracted for 1 min, which could be used for determination. According to the kit instruction for ovarian tissue and cell sample for 96 orifice. Use fully functional enzyme standard meter reading is determined, the results were analyzed, and the OD value standard curve drawing, the final result calculated on the basis of the specification.
Adenosine triphosphate content detection kit (A095-1-1) was purchased from Nanjing Jiancheng BioEngineering Institute (Nanjing, China).
Flow cytometry to detect cell apoptosis
The cells cultured in 12-well plates were treated with D-gal and Mel, washed with PBS, digested with trypsin without EDTA, transferred to a centrifuge tube, and centrifuged at 300 r/min for 5 min at 4 °C. They were washed twice with precooled PBS and centrifuged again for 5 min at 4 °C and 300 r/min. Gettering PBS, add 100 mu L 1 × Binding Buffer heavy suspension cells. 5 µL of Annexin V-FITC and 5 µL of PI staining solution were added and mixed gently. After 10–15 min at room temperature away from light incubation, to join 400 (including L 1 × Binding Buffer, using flow cytometry to detect each cell apoptosis.
Annexin V-FITC apoptosis detection kit (C10625) was purchased from Biyuntian Biotechnology Co., LTD. (Wuhan, China).
The MDC staining
MDC is an autofluorescent compound used to label autophagic vacuoles. KGN cells were seeded in 6-well plates at a density of 5 × 105 cells/well, cultured overnight, and treated with D-gal and Mel. According to the manufacturer’s instructions, after processing of cells with a 50 mM MDC in 37 °C for 15 min, incubation with PBS washing three times, 5 min. The fluorescence of the cells was observed using a fluorescence microscope.
Autophagy staining detection kit (G0170) was purchased from Solebo Technology Co., LTD. (Beijing, China).
Western blot analysis
Samples of the ovarian tissue and cells, into the prepared hybrid RIPA cracking liquid. Total protein concentration was determined using the BCA protein assay and quantified to the same concentration. Protein samples were added to each lane of 10 or 15% SDS-PAGE gels and transferred to a PVDF membrane (0.22 μm, 0.45 μm). With TBST (TBS + 0.1% Tween-20) preparation of 5% skim milk 2 h closed PVDF membrane, and then clean with TBST lotion 3 times, each time 10 min. Subsequently, the film and a resistance under the 4 °C incubation for the night. The next day, the cells were washed with TBST and incubated with secondary antibody (1:2000) for two hours. The PVDF membrane was illuminated by a Jan-emitter-coupled logic (ECL) substrate for image capture. Image using Image. J 6.0 software for quantitative analysis of the strength of each band.
Real-time fluorescent quantitative PCR detection
Using TRIzol total RNA was isolated from cultured cells, using Nano Drop 2000 to detect the concentration of the extraction of RNA and purity. The Prime Script RT Kit (Thermo Fisher Scientific) was reverse transcribed to cDNA. The RT-qPCR reagent was 2X SYBR Premix Plus(Tiangen Biotechnology Co., LTD., Beijing, China). The determination was based on the total amount of 10ul in the reaction system. In Mx3000p real-time fluorescent quantitative PCR amplification on the thermal cycler. Finally, melting curve analysis was performed, and gene expression was calculated using the 2−δδCT formula with β-actin as the reference gene. Gene primer sequences are shown in Table 1.
Statistical analysis
The results of this study are representative of at least three independent tests and are presented as mean ± standard deviation. The t-test was used for continuous variables and one-way ANOVA was used for multiple group comparisons; all tests were two-tailed and the statistical significance level was set at 0.05. Graph Pad Prism 8.0.2 was used for statistics and images were derived. *p < 0.05, **p < 0.01, ***p < 0.001 (compared with Con); #p < 0.05, ##p < 0.01, ###p < 0.001 (compared with D-gal) (N = 3, mean ± SD).
Data availability
All data generated or analyzed during this study are included in this article and its supplementary information files. All other data collected and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Bai, X. & Wang, S. Signaling pathway intervention in premature ovarian failure. Front. Med. 9, 999440 (2022).
Zhou, R. & Liu, D. The function of exosomes in ovarian granulosa cells. Cell tissue Res. 394, 257–267 (2023).
Zhou, W. et al. LIPUS combined with TFSC alleviates premature ovarian failure by promoting autophagy and inhibiting apoptosis. Gynecol. Endocrinol. 39, 2258422 (2023).
Hu, B., Zheng, X. & Zhang, W. Resveratrol-βcd inhibited premature ovarian insufficiency progression by regulating granulosa cell autophagy. J. Ovarian Res. 17, 18 (2024).
Kaushik, S. & Cuervo, A. M. Chaperones in autophagy. Pharmacol. Res. 66, 484–493 (2012).
Vargas, J. N. S., Hamasaki, M., Kawabata, T., Youle, R. J. & Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 24, 167–185 (2023).
Ballesteros, U., Alonso, A., Montes, L. R. & Etxaniz A. Vesicle-vesicle fusion promoted by the Atg8-family autophagy protein LC3C: relevance of the N-terminal region. Autophagy 2024;20:2349–2351.
Fujiwara, N., Shibutani, S., Ohama, T. & Sato, K. Protein phosphatase 6 dissociates the Beclin 1/Vps34 complex and inhibits autophagy. Biochem. Biophys. Res. Commun. 552, 191–195 (2021).
Alcober-Boquet, L. et al. The PB1 and the ZZ domain of the autophagy receptor p62/SQSTM1 regulate the interaction of p62/SQSTM1 with the autophagosome protein LC3B. Protein Sci. 33, e4840 (2024).
Burstein, H. J. Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer. N. Engl. J. Med. 383, 2557–2570 (2020).
Peng, J. et al. Aspirin alleviates pulmonary fibrosis through PI3K/AKT/mTOR-mediated autophagy pathway. Exp. Gerontol. 172, 112085 (2023).
Choi, J., Jo, M., Lee, E. & Choi, D. AKT is involved in granulosa cell autophagy regulation via mTOR signaling during rat follicular development and atresia. Reproduction 147, 73–80 (2014).
Gong, Y. et al. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 18, 121 (2020).
Vasey, C., McBride, J. & Penta, K. Circadian rhythm dysregulation and restoration: the role of melatonin. Nutrients 13, 3480 (2021).
Ma, W. Y. et al. Melatonin promotes cardiomyocyte proliferation and heart repair in mice with myocardial infarction via miR-143-3p/Yap/Ctnnd1 signaling pathway. Acta Pharm. Sin. 42, 921–931 (2021).
Wu, X. L. et al. Melatonin receptor agonist ramelteon attenuates mouse acute and chronic ischemic brain injury. Acta Pharm. Sin. 41, 1016–1024 (2020).
Guo, Y. M., Sun, T. C., Wang, H. P. & Chen, X. Research progress of melatonin (MT) in improving ovarian function: a review of the current status. Aging 13, 17930–17947 (2021).
Qi, M. K. et al. Therapeutic effect of melatonin in premature ovarian insufficiency: Hippo pathway is involved. Oxid. Med. Cell. Longev. 2022, 3425877 (2022).
Zhu, R. et al. Melatonin antagonizes ovarian aging via YTHDF2-MAPK-NF-κB pathway. Genes Dis. 9, 494–509 (2022).
Li, P., Dou, Q., Zhang, D., Xiang, Y. & Tan, L. Melatonin regulates autophagy in granulosa cells from patients with premature ovarian insufficiency via activating Foxo3a. Aging 16, 844–856 (2024).
Arnao, M. B. & Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 121, 195–207 (2018).
Olcese, J. M. Melatonin and female reproduction: an expanding universe. Front. Endocrinol. 11, 85 (2020).
Zhao, F. F., Zhou, Y. Z., Gao, L., Qin, X. M. & Du, G. H. Advances in the study of the rat model of aging induced by D-galactose. Acta Pharmaceutica Sin. 52, 347–354 (2017).
Shang, Z. et al. Red ginseng improves D-galactose-induced premature ovarian failure in mice based on network pharmacology. Int. J. Mol. Sci. 24, 8210 (2023).
Reiter, R. J. et al. Aging-related ovarian failure and infertility: melatonin to the rescue. Antioxidants 12, 695 (2023).
Tao, J. et al. Effect of exogenous melatonin on the development of mice ovarian follicles and follicular angiogenesis. Int. J. Mol. Sci. 22, 11262 (2021).
Suman, I. et al. The interplay of mitophagy, autophagy, and apoptosis in cisplatin-induced kidney injury: involvement of ERK signaling pathway. Cell Death Discov. 10, 98 (2024).
Chen, X. et al. Acupuncture regulates the autophagy of ovarian granulosa cells in polycystic ovarian syndrome ovulation disorder by inhibiting the PI3K/AKT/mTOR pathway through LncMEG3. Biomed. Pharmacother. 144, 112288 (2021).
Wang, Y. et al. LYC inhibits the AKT signaling pathway to activate autophagy and ameliorate TGFB-induced renal fibrosis. Autophagy 20, 1114–1133 (2024).
Mei, Q., Diao, L., Xu, J. M., Liu, X. C. & Jin, J. A protective effect of melatonin on intestinal permeability is induced by diclofenac via regulation of mitochondrial function in mice. Acta Pharmacol. Sin. 32, 495–502 (2011).
Xing, J. et al. Ferredoxin 1 regulates granulosa cell apoptosis and autophagy in polycystic ovary syndrome. Clin. Sci. 137, 453–468 (2023).
Gao, M. et al. Perfluorooctane sulfonate (PFOS) induces apoptosis and autophagy by inhibition of PI3K/AKT/mTOR pathway in human granulosa cell line KGN. Environ. Pollut. 344, 123333 (2024).
Richards, J. S. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiological Rev. 60, 51–89 (1980).
Guido, C. et al. Estrogen receptor beta (ERβ) produces autophagy and necroptosis in human seminoma cell line through the binding of the Sp1 on the phosphatase and tensin homolog deleted from chromosome 10 (PTEN) promoter gene. Cell Cycle 11, 2911–2921 (2012).
Su, Q. et al. Induction of estrogen receptor β-mediated autophagy sensitizes breast cancer cells to TAD1822-7, a novel biphenyl urea taspine derivative. Mol. Biol. Rep. 49, 1223–1232 (2022).
Fan, W. et al. Estrogen receptor β activation inhibits colitis by promoting NLRP6-mediated autophagy. Cell Rep. 41, 111454 (2022).
Dong, Z., Zhang, L., Wang, W., Jiang, F. & Ai, H. ZnSO 4 Protects against premature ovarian failure through PI3K/AKT/GSK3β signaling pathway. Theriogenology 207, 61–71 (2023).
Jiang, C. et al. Novel specific pyruvate kinase M2 inhibitor, compound 3h, induces apoptosis and autophagy through suppressing Akt/mTOR signaling pathway in LNCaP cells. Cancers 15, 265 (2022).
Zhou, L. et al. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J. Ovarian Res. 10, 56 (2017).
Jiang, X. et al. Bu-shen-zhu-yun decoction inhibits granulosa cell apoptosis in rat polycystic ovary syndrome through estrogen receptor α-mediated PI3K/AKT/mTOR pathway. J. Ethnopharmacol. 288, 114862 (2021).
Li, N., Wang, J., Wang, X., Sun, J. & Li, Z. Icariin exerts a protective effect against d-galactose induced premature ovarian failure via promoting DNA damage repair. Biomed. Pharmacother. 118, 109218 (2019).
Li, X., Li, X. & Deng, L. Chrysin reduces inflammation and oxidative stress and improves ovarian function in D-gal-induced premature ovarian failure. Bioengineered 13, 8291–8301 (2022).
Acknowledgements
The authors thank the Key Laboratory of the Provincial Education Department of Heilongjiang for Common Animal Disease Prevention and Treatment, College of Veterinary Medicine, Northeast Agricultural University, for providing conditions.
Author information
Authors and Affiliations
Contributions
G.H.Z.: Methodology, Data curation, and Writing-original draft. Y.F.H.: Methodology and Validation. H.L.W.: Methodology. Y.B.L.: Conceptualization. Y.M.C.: Software. Z.L.S.: software, investigation. W.H.Y.: Writing-review & editing. All authors have read the manuscript and have agreed to submit the manuscript in its current form for consideration for publication in this journal.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, Gh., He, Yf., Wang, Hl. et al. Exogenous melatonin alleviates premature ovarian failure by regulating granulosa cell autophagy. npj Regen Med 10, 35 (2025). https://doi.org/10.1038/s41536-025-00422-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41536-025-00422-1