Abstract
Clinical high-risk (CHR) is a prodromal period before psychosis characterized by attenuated, transient, or intermittent psychotic symptoms and declining functioning. They exhibit eye movement abnormalities and brain functional damage compared with schizophrenia, potentially increasing vulnerability to psychosis. This study investigates eye movement dysfunction and brain activity alterations in CHR and first-episode schizophrenia (FSZ) individuals to identify early biomarkers for psychosis progression. Twenty-seven drug-naïve FSZ, 25 CHR, and 28 healthy controls (HCs) were recruited for eye-tracking tasks and resting-state functional magnetic resonance imaging to evaluate eye movement and regional homogeneity (ReHo) differences. Machine-learning algorithms were used to differentiate FSZ from CHR. In combination with the Allen Human Brain Atlas (AHBA), transcriptome-neuroimaging analysis was applied to identify ReHo-related gene expression profiles. FSZ exhibited a wide range of eye movement abnormalities across multiple tasks, while certain abnormalities were already present in CHR. Abnormal ReHo alterations were found in orbitofrontal gyrus, temporal gyrus, and cingulum among three groups, associated with specific eye movement parameters. These differences in eye movement and ReHo allowed for high-accuracy discrimination between them. Genetic analysis identified significant genes in FSZ and CHR, enriched in various biological functions and pathways (all corrected p < 0.05). FSZ and CHR exhibited different eye movement and ReHo patterns, indicating potential as early biomarkers. Our findings reveal correlations between these ReHo patterns and gene expression profiles using AHBA database, shedding light on possible genetic mechanisms underlying brain function in FSZ and CHR.
Similar content being viewed by others
Introduction
Clinical high-risk (CHR) phase refers to the prodromal period before the onset of psychosis, characterized by attenuated, transient, or intermittent psychotic symptoms and declining functioning among non-schizophrenic individuals1. Around 20% of CHR individuals progress to full-blown psychosis within 2 years and 36% at a 3-year follow-up2,3. Identifying reliable biomarkers on CHR could provide a window of opportunity for better understanding the trajectory from elevated risk to well-established illness and decrease the rate of transition to psychosis through early intervention before symptoms worsen4.
Oculomotor dysfunction is a common symptom of schizophrenia, with several studies highlighting eye movement abnormalities in both schizophrenia patients and their relatives5,6. Common findings include reduced gain, greater positional differences, more frequent saccades in smooth pursuit eye movement (SPEM) tasks7, increased saccadic amplitude and scan path in fixation stability tasks8, and reduced accuracy along with prolonged latency in antisaccade tasks9. These characteristics can accurately distinguish patients from healthy controls (HCs), positing their potential as biomarkers for the disorder10. Despite this, there has been limited exploration of eye movement studies in CHR. One study by Obyedkov et al. discovered that error rate in CHR intermediates between HCs and schizophrenia in antisaccade tasks11, suggesting eye movement dysfunction may manifest early in the disease.
Animal studies suggest that cerebral cortex plays a crucial role in controlling SPEM12. Research in rhesus monkeys has shown that SPEM is impaired when the middle temporal area (MT) and medial superior temporal area (MST) are damaged13. Additionally, the frontal eye field (FEF) is essential for generating SPEM, as electrical stimulation in this region induces spontaneous pursuit14. Lesions in the FEF can disrupt smooth tracking, while this region also regulates gain and predicts object motion15. However, the precise neuropathological mechanisms underpinning eye movement abnormalities in schizophrenia remain unclear.
Recent resting-state functional magnetic resonance imaging (rs-fMRI) has increasingly served as a non-invasive tool to reveal regional activity and functional alterations in schizophrenia and CHR16,17,18,19. Findings in CHR have frequently reported intermediate changes between schizophrenia and HCs20. One promising neuroimaging metric is regional homogeneity (ReHo), which assesses local synchronization of neuronal activity in specific brain areas by calculating similarity of time series between adjacent voxels21. ReHo has high test-retest reliability by minimizing disruptions of tempo-spatial noise and outliers compared to conventional fMRI metrics22. Previous studies have shown increased ReHo in the superior and middle frontal cortex, and inferior parietal lobule in schizophrenia patients, while decreased ReHo has been observed in the cingulate, superior temporal gyrus (STG), fusiform gyrus, and precuneus23,24,25,26. However, these findings are inconsistent due to confounding factors such as antipsychotic medication and disease duration. To date, few studies have utilized ReHo to compare spontaneous brain activity and establish an association with eye movements between first-episode schizophrenia (FSZ) and CHR27.
Moreover, schizophrenia is a highly heritable disorder, with a recent genome-wide association study (GWAS) reporting 287 distinct genomic loci associated with the disease28. However, the biological consequences and causal associations between these genes and disease phenotypes are not yet fully understood. Transcriptome-neuroimaging spatial association analyses, which integrate genomic data with neuroimaging findings, offer a promising approach for linking genetic variations to specific brain abnormalities. Utilizing whole-brain gene expression databases such as the Allen Human Brain Atlas (AHBA)29, accumulating studies have identified genes linked to abnormal brain structural and functional properties across several psychiatric illnesses30,31,32,33. Previous studies have revealed genetic mechanisms underlying cortical anatomical variations and cerebellar functional connectivity in schizophrenia33,34,35,36.
In this study, we combine multiple eye-tracking tasks and ReHo analysis to assess relationship between oculomotor dysfunction and brain activity alterations in FSZ and CHR. We will use machine learning algorithm to identify key differences between these two groups. Furthermore, transcriptome-neuroimaging analyses were applied to identify genes linked to abnormal ReHo patterns and to explore their functional signatures using the AHBA database. We hypothesized that (1) Schizophrenia patients have a wide range of eye movement abnormalities across multiple tasks, while certain eye movement abnormalities are already apparent in CHR individuals; (2) These eye movement abnormalities are associated with abnormal brain function activity in the frontal and temporal regions; (3) These functional activities would be associated with specific gene expression profiles.
Materials and methods
Participants and clinical measures
Twenty-seven outpatients with drug-naive FSZ were enrolled at the Second Xiangya Hospital of Central South University. Patients were all independently diagnosed by two senior psychiatrists according to the Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria and were diagnosed with the first episode of the disease and onset less than three years ago. Positive and Negative Syndrome Scale (PANSS) was used to assess different symptoms in schizophrenia patients, including positive symptoms, negative symptoms, and general mental symptoms. Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB) was utilized to evaluate cognitive function, including multiple cognitive dimensions such as processing speed, working memory, attention, executive functioning, and problem-solving ability. Higher scores on each dimension represent better functional performance. Twenty-five CHR individuals were recruited from outpatient department of the hospital. Structured Interview for Prodromal Syndromes (SIPS) was administered to define the CHR criteria, including brief intermittent psychotic syndrome (BIPS), attenuated positive syndrome (APS), and genetic risk and deterioration syndrome (GRD). SIPS (19 items) comprises four symptom clusters: positive symptoms, negative symptoms, disorganization, and general symptoms. Twenty-eight HCs were enrolled from broader community. They were excluded if their first-degree relatives had a history of psychosis. All participants in this survey were all Han Chinese, right-handed, aged between 16 and 45 years.
Exclusion criteria for all participants were: (1) any other psychosis that meets DSM-5, prior history of psychiatric illness, current medication use; (2) prior neurological condition, used substances in the past six months, and history of head trauma or head injury; (3) strabismus, color blindness, visual field loss, naked/corrected visual acuity < 1.0 or other severe visual impairment; (4) pregnancy or lactation; (5) abnormal brain structural alteration after MRI scan; (6) MRI contraindications.
This study was reviewed and approved by the Second Xiangya Hospital of Central South University Medical Research Ethics Committee. All participants provided informed consent, and principles of ethical research were thoroughly adhered to in the study.
Eye movement tasks
Eye movements were recorded using an EyeLink 1000 eye tracker (SR Research, Ontario, Canada). Hole-in-the-card test was used to determine the dominant eye. Tracking of the dominant eye was achieved through the use of a 25 mm camera lens. The stimuli were presented on a 24-inch screen with a resolution of 1920 × 1080 pixels and a refresh rate of 144 Hz. Participants were 70 cm away from the screen and positioned their heads on a chin/forehead rest to minimize head movements. A 9-point calibration mode was used to begin each task, and drift correction was automatically applied throughout each task. Before each task, a uniform instruction was read to the subjects. If the subjects showed large head movements during the task, the task had to be re-collected. We used multiple eye movement tasks, including picture free-viewing, SPEM, and fixation stability tasks. Details on these tasks can be found in Supplementary Materials 1.
Image acquisition
Resting-state functional imaging data were acquired using an SIEMENS 3.0 T MRI machine with an echo planar imaging (EPI) sequence. Participants were instructed simply to lie supine, keep their eyes closed, and remain awake. Foam padding and soft earplugs were used to minimize scanner noise and head motion. Imaging parameters were as follows: TR/TE = 2000ms/30 ms, flip angle = 90°, field of view = 220 mm × 220 mm, 64 × 64 matrix, 4 mm slice thickness, 39 slices, and 200 volumes.
Data processing and ReHo analysis
A toolbox for Data Processing & Analysis for Brain Imaging (DPABI) software (http://rfmri.org/DPABI) was used to preprocess neuroimaging data37. The first 10 time points were discarded to obtain more stable signals. Subsequently, slice timing and head motion correction were conducted. Participants who had over 2 mm maximal translation and/or 2° maximal rotation were discarded. Then, corrected data were spatially normalized to Montreal Neurological Institute (MNI) space and resampled to 3 × 3 × 3 mm³ voxel size to improve signal-to-noise ratio. Friston-24 head motion parameters, signals from white matter centered region and cerebrospinal fluid were removed as covariates. Finally, the data were bandpass filtered (0.01 to 0.08 Hz) and linearly detrended to reduce high-frequency physiological noise interference.
ReHo analysis was conducted on a voxel-wise basis by calculating Kendall’s Coefficient of Concordance (KCC) for each voxel and its surrounding 26 neighboring voxels to measure the similarity of the time series. The ReHo value of each voxel was then subtracted from the whole-brain average ReHo value, divided by the standard deviation. The final ReHo images were smoothed using a 4 mm full-width at half-maximum (FWHM) isotropic Gaussian kernel and Fisher Z-transformed to enhance normality22.
Statistical analysis
An analysis of variance (ANOVA) analysis was conducted to examine group differences in age, education years, and scale scores across three groups using SPSS 26 (https://www.ibm.com/spss). A chi-square test was used to compare sex distributions, with the significance level set at p < 0.05. Kruskal-Wallis tests were employed for eye movement data that did not follow a normal distribution, and Bonferroni correction was used for the post-hoc t-tests of variables with the significance level set at q < 0.05. ReHo values of the three groups were subjected to ANOVA analysis with Bonferroni correction at q < 0.05 and were corrected by Gaussian random field theory (GRF) (voxel p < 0.001, cluster p < 0.01). Sex, age, and head movement parameters were taken as covariates.
Spearman’s correlation analysis was applied to assess the relationship between abnormal eye movement or ReHo values and clinical symptoms and cognitive function scores in three groups with Bonferroni correction at q < 0.05.
Classification analysis
In this study, we selected eye movement parameters that are significantly different between the groups for classification analysis. To reduce computational complexity and minimize the risk of overfitting, we applied several widely used feature selection techniques, including logistic regression (LR), least absolute shrinkage and selection operator (LASSO), random forest (RF), and support vector machine-recursive feature elimination (SVM-RFE). Feature selection was performed using R (version 4.3.2, https://www.r-project.org).
Support Vector Machine (SVM) is a powerful classification algorithm that excels in high-dimensional data and is effective at handling complex datasets. It works by finding the optimal hyperplane that best separates data points into distinct classes. We then applied SVM to construct classifiers based on the eye movement features selected through the aforementioned feature selection methods and differential ReHo values separately, aiming to identify the most relevant features for differentiating between groups. The SVM was implemented using the LIBSVM package (http://www.csie.ntu.edu.tw/~cjlin/libsvm/) in MATLAB. More details can be found in Supplementary Materials 1.
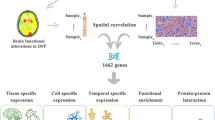
Brain gene expression data processing and spatial correlation analysis
Brain gene expression data were obtained from AHBA dataset (http://www.brain-map.org)29, including more than 20,000 genes detected by 58,692 probes at 3702 distinctive brain tissue samples from six healthy adult donors38. All data were processed with “Abagen” toolbox (version 0.1.4, https://github.com/rmarkello/abagen)39. After discarding 2 regions that do not contain genes, we finally obtained a gene expression matrix of 121 × 6864 (region × genes).
A 3 mm radius sphere was then defined, centered on the MNI coordinate of the Brainnetome 246 Atlas (http://atlas.brainnetome.org/). The average ReHo value of the voxels within this sphere in the ReHo difference maps was calculated to explore spatial associations with gene expression profiles. Partial least squares (PLS) regression is a statistical method used to model the relationship between two sets of variables by extracting latent components that maximize the covariance between them. In our study, average ReHo value was response variables and gene expression as predictors. PLS1 identified gene combinations explaining the most variance in the ReHo map. Bootstrapping (5000 iterations) assessed each gene’s contribution, and significant genes were selected after Benjamini-Hochberg correction (FDR-BH, q < 0.05)34. Details on the pipeline can be found in Supplementary Materials 1.
Gene enrichment analysis
We performed gene enrichment analyses on the significant PLS1 genes to explore their relationship with abnormal function changes in the FSZ and CHR. Gene-related biological functions and interactions were determined by the Gene Ontology (GO) enrichment analysis, including three ontologies: molecular functions (MF), cellular components (CC), and biological processes (BP). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and Disease Ontology (DO) enrichment were used to identify gene-related signal pathways and categories of diseases separately. All above enrichment analyses were conducted using “ClusterProfiler” package in R software. Furthermore, to identify candidate cell populations, tissue distribution, and time-specific expressions to be disrupted in FSZ and CHR, we utilized tissue-specific expression analysis (TSEA) via the “TissueEnrich” R package (https://github.com/Tuteja-Lab/TissueEnrich/)40, and time-specific and cell type-specific expression analysis (CSEA)41,42 accessible online at https://sites.wustl.edu/doughertylab/. Significant multiple comparison results were set with FDR-BH q value < 0.05.
Protein-protein interaction analysis
Protein-protein interaction (PPI) analysis was used to understand how ReHo-related genes interact with each other and form biological networks by STRING (vision 12.0, https://string-db.org/). The minimum required interaction score was set with the highest confidence of 0.9. Betweenness centrality method was used to identify the most important hub genes by calculating the number of shortest paths for a nude to all other nudes.
Results
Demographic and clinical characteristics
Table 1 presents demographic and clinical characteristics of three groups. CHR exhibited a lower age than both FSZ and HCs (p < 0.05). A significant difference in education years was observed between CHR and other groups (p < 0.001). As expected, PANSS and SIPS scores were consistent with the disease characteristics of FSZ and CHR. Table 2 exhibits significant inter-group disparities in MCCB tests. FSZ exhibited significant impairments in processing speed, verbal learning, and visual learning compared to HCs (p < 0.05), with CHR showing intermediate impairments. Attention/vigilance was significantly higher in CHR compared to FSZ and HCs (all p < 0.01). Reasoning and problem-solving showed a significant difference between FSZ and HCs (p = 0.033) while working memory did not differ across groups (p = 0.922). These findings suggest that cognitive deficits are most pronounced in FSZ, with CHR individuals showing intermediate impairments in specific domains.
Group differences in eye movement
Table S1-S4 illustrates significant differences in eye movements across three groups in multiple tasks. During free-viewing task, FSZ displayed significant reductions in saccadic duration, amplitude, scan path, and average and peak velocity compared with HCs. CHR demonstrated significant decreases in saccadic duration and scan path compared with HCs. During SPEM task, FSZ showed significantly longer saccadic duration and higher peak velocity compared to CHR, with FSZ also showing increased saccadic duration compared to HCs in the HS4X task. In Lissajous paradigm, FSZ exhibited increased saccadic duration and peak velocity in LS2, LS4, and LS2B tasks, and increased saccadic amplitude in LS4 and LS2B tasks compared to CHR. FSZ also showed increased saccadic duration and amplitude in LS2, LS4, and LS2B tasks, and increased peak velocity in LS2 and LS2B tasks compared to HCs. In fixation stability task, FSZ exhibited increased saccadic number and scan path in simple mode, and increased fixated duration and number, saccadic duration, number, amplitude, peak velocity, and scan path in distracter mode compared to HCs. CHR exhibited increased scan path in simple mode, increased saccadic duration, and peak velocity in distracter mode compared to HCs (all q < 0.05). Overall, FSZ differed significantly from HCs in all tasks, whereas CHR differed mainly in free-viewing and fixation stability tasks, with moderate differences observed between FSZ and HCs.
Group differences in ReHo analysis
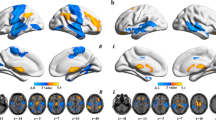
Compared with CHR, FSZ showed decreased ReHo in the right superior orbitofrontal gyrus (OFG) and left inferior temporal gyrus (ITG) and increased ReHo in the right midcingulate cortex (MCC). Relative to HCs, FSZ displayed increased ReHo in the right superior temporal pole (STP) and MCC; and CHR had increased ReHo in the left middle temporal gyrus (MTG) and STG. These findings suggest substantial differences in neuronal activity patterns among three groups in these specific brain regions. All the differences were surveyed after GRF correction (voxel p < 0.001, cluster p < 0.01) (Fig. 1, Table 3).
The FSZ group showed decreased ReHo in the right superior OFG and left ITG and increased ReHo in the right MCC compared with the CHR group and increased ReHo in the right STP and MCC compared with the HC group. The CHR group showed increased ReHo in the left MTG and STG compared with the HC group. The significance level of the p-value was corrected by Gaussian random field theory (GRF) (voxel p < 0.001, cluster p < 0.01). Blue denotes lower ReHo and red denotes higher ReHo. Color bar indicates T values from post-hoc t-tests. FSZ first-episode schizophrenia, CHR clinical high-risk, HC healthy control, ReHo regional homogeneity, OFG orbitofrontal gyrus, ITG inferior temporal gyrus, MCC midcingulate cortex, STP superior temporal pole, MTG middle temporal gyrus, STG superior temporal gyrus.
Correlations results
Figure S1 illustrates the correlation between eye movements, clinical symptoms, and cognitive function scores. Negative correlations were found between SIPS positive scores and LS4 task, especially saccadic amplitude (r = −0.57), average velocity (r = −0.60), and peak velocity (r = −0.61), and between SIPS general scores and peak velocity (r = −0.57) in CHR (all q < 0.05). Moreover, in FSZ, PNASS general scores had positive correlations with average velocity in free-viewing task (r = 0.64) and fixation stability task with distracter mode (r = 0.63), and PNASS positive scores had a positive correlation with average velocity in fixation stability task with distracter mode (r = 0.58) (all q < 0.05).
Negative correlations were found between fixation stability task and MCCB scores, especially attention (r range: −0.36 to −0.42), visual learning (r range: −0.35 to −0.39), and processing speed (r range: −0.36 to −0.41) when including all subjects (all q < 0.05). When considering each group, significant negative correlations were found between saccadic amplitude (r = −0.55) and average velocity (r = −0.57) in fixation stability task with distracter condition and attention in CHR, and between saccadic amplitude in fixation stability task with distracter condition (r = −0.56) and working memory in HCs (all q < 0.05; Fig. S2).
Positive correlations were observed between ReHo of left STG and numbers of fixations (r = 0.43, p = 0.032) and saccades (r = 0.49, p = 0.013) in HS4X task in CHR, and between ReHo of right MCC and average velocity (r = 0.46, p = 0.021) in LS4 task in FSZ. However, only correlation between left STG and saccadic numbers in HS4X was surveyed after Bonferroni correction (q < 0.05) (Fig. S3). These results suggest potential links between neuronal activity patterns in specific brain regions and different eye movement behaviors.
Classification analysis
The eye movement features obtained using different feature selection methods are presented in Table S5 and Fig. S4. SPEM tasks were effective in distinguishing between FSZ and CHR, though with moderate accuracy. Free-viewing and fixation stability tasks showed greater efficacy in differentiating between CHR and HCs with accuracy of 86%. Combining features from free-viewing, fixation stability, and SPEM tasks resulted in a high accuracy of 94% for distinguishing between FSZ and HCs (Table S6 and Fig. S5).
Moreover, a combination of ReHo in the right MCC, superior OFG, and left ITG could be used as features to discriminate FSZ from CHR with a high accuracy of 92.3%. A combination in the right STP and MCC could discriminate FSZ from HCs with an accuracy of 90.0%; and a combination of ReHo in the left MTG and STG displayed accuracy of 88.7%, which could discriminate CHR from HCs. Other combinations and their accuracies can be seen in Table S7 and Figure S6.
Genes related to ReHo value
After FDR-BH correction (q < 0.05), we found 1639 genes (732 PLS1+ and 907 PLS1- genes) in FSZ and 1127 genes (736 PLS1+ and 391 PLS1-) in CHR were significantly associated to ReHo alteration (Supplementary Material 2). CHR has 123 PLS1+ genes and 112 PLS1- genes overlapping with FSZ. Spatial autocorrelation test showed a significant difference from random after 5000 permutations (p < 0.001).
Gene enrichment and specific expression analysis
Supplementary Material 3 reflects all significant results of gene functional enrichment analysis. We found genes in FSZ and CHR have some of the identical enrichment categories, which were involved in the structure and functions of synapses (i.e., synapse membrane, organization, assembly, and trans-synaptic signaling regulation) and signaling pathways (i.e., calcium signaling pathway, oxytocin signaling pathway) (Figure S7-S8).
TSEA showed that all PLS1 genes were significantly enriched for the cerebral cortex, especially PLS1+ genes in CHR (Fig. 2A). CSEA demonstrated the PLS1 genes in CHR and PLS1- genes in FSZ were specifically expressed in multiple types of immune cells and neurons (e.g., Astrocytes, Glt25d2, and Ntsr+ neurons) (Fig. 2B). Temporal-specific analysis showed that these significant genes were expressed at almost all developmental periods and different brain regions have their characteristics of gene expression profiles at different periods. (Fig. 2C). More details can be attached in Supplementary Material 4.
A Specific gene expression of various tissues in TSEA. B Specific gene expression of various cell types in CSEA. C Temporal-specific gene expression in different regions and developmental stages. ReHo regional homogeneity, FSZ first-episode schizophrenia, CHR clinical high-risk, TSEA Tissue-specific expression analysis, CSEA Cell type-specific expression analysis, RetR.Rods Rod cells, RetC.Cones Cone cells, Hyp Hypothalamus, BF Basal forebrain, BS Brain stem, Cb Cerebellum, Cpu Striatum(Caudate and Putamen), Ctx Cortex, Epi Habenula (epithalamus), Spc Spinal cord, Hcrt Hypocretinergic neurons, Chat Cholinergic neurons, Slc6a4 Serotonergic neurons, Septin4 Bergman glia and mature oligodendrocytes, Pcp2 Purkinje cells, Neurod1: Granule cells, Lypd6 Stellate and basket cells, Grp Unipolar brush cells and Bergman glia, Grm2 Golgi neurons, Fthfd Astroglia, Cnp Myelinating Oligodendrocyte, D2 Drd2+ medium spiny neurons, D1 Drd1+ medium spiny neurons, Pdgfrajd340 Oligodendrocyte Progenitor Cells, Etv1_ts88 Immune Cells, Pnoc Pnoc+ neurons, Ntsr Ntsr+ neurons, Glt25d2 Glt25d2 neurons, Cort Cort+ neurons.
PPI network analysis
PLS1 genes in FSZ could construct PPI networks with 614 edges (p < 0.001). The most important genes were CTNNB1, DVL2, GNAQ, and CACNA1C. Similarly, PLS1 genes in CHR could form PPI networks with 489 edges (p < 0.001). The most important genes were CAMK2A, PPP1CC, FOXO1, and YWHAG (Fig. 3).
A FSZ. B CHR. Nodes indicate significant genes, and lines indicate the relationship between their interactions. The size and color of the nodes indicate their importance by the betweenness centrality method. PPI Protein-protein interaction, ReHo regional homogeneity, FSZ first-episode schizophrenia, CHR clinical high-risk.
Discussion
The predominant findings support our hypothesis that abnormal eye movements and ReHo exist across three groups. FSZ exhibited varying degrees of abnormalities in free-viewing, SPEM, and fixation stability tasks, some of which were also observed in CHR. Performance on fixation stability task in CHR correlates with attention scores. Abnormal ReHo alterations were found in orbitofrontal gyrus, temporal gyrus, and cingulum among three groups, and these alterations were associated with specific eye movement features. Transcriptome-neuroimaging analysis identified ReHo-related genes in FSZ and CHR, enriched in various biological functions and pathways. Using SVM, we differentiated the three groups based on these eye movement and ReHo features, suggesting that these alterations may have potential as biomarkers. However, given the small sample size and the specific characteristics of CHR, these findings should be interpreted with larger sample sizes and more diverse cohorts.
Consistent with previous researches43,44, CHR individuals have mild to moderate impairments between FSZ and HCs in certain cognitive dimensions. The level of attention and vigilance observed in CHR was higher than in FSZ and HC groups, suggesting that CHR individuals were in an increased state of attention and vigilance to new stimuli in the environment45. Moreover, no differences were found in problem-solving ability and working memory between CHR and HC, or between FSZ and CHR, which aligns with the findings of previous meta-analyses44, suggesting that these cognitive impairments may emerge in the later stages of the disease.
Fixation reflects attentional flexibility and information processing speed, while saccades indicate the ability to execute and control eye motion. During free-viewing task, the reduced saccadic amplitude and velocity in FSZ suggest a narrower and lower search for information, while the smaller scan path indicates overall abnormalities in information acquisition, showing a slow and more limited search mode compared to HCs. Similar results were reported by Bestelmeyer et al., who observed longer fixation durations, slower saccadic velocities, and more restricted eye movement patterns in schizophrenia across different picture types46. These abnormalities in schizophrenia are likely to lead to incomplete acquisition of environmental information, contributing to abnormal perceptual processes47. CHR exhibits eye movement abnormalities similar to FSZ, characterized by reduced saccadic amplitude and scan path, suggesting that these deficits may emerge early in the disease and are comparable in severity to those seen in FSZ.
SPEM tasks included HS and LS modes at different frequencies, with or without background interference, and a color change counting task to assess cognitive load. Levy et al. pointed out that when a participant’s eye movement speed is slower than target speed, they compensate for the distance gap by generating catch-up saccades, which leads to increased peak velocity48. In this study, FSZ showed decreased accuracy in tracking targets compared to CHR and HCs, as evidenced by increased saccadic peak velocity and amplitude, and prolonged saccadic duration. Additionally, FSZ had more severely impaired eye movements in LS task than in HS task, suggesting that these abnormalities become more pronounced as task difficulty increases. While eye movement metrics in CHR and HC were generally similar, CHR exhibited lower peak velocity compared to HC, indicating mild impairments in CHR, and did not yet exhibit the compensatory catch-up saccades seen in FSZ. Additionally, negative correlations were found between saccadic amplitude and velocity and SIPS scores in CHR, suggesting that slower and narrower tracking may represent more serious symptoms.
In fixation stability task, FSZ displayed increased saccadic duration, amplitude, and scan path in distracter mode, consistent with previous findings of impaired fixation stability in schizophrenia8. These results suggest potential deficits in motor switching, visuospatial cognitive and control functioning in schizophrenia49. CHR existed increased saccadic duration and peak velocity in distracter mode, and performance falling between FSZ and HCs, indicating these deficits may emerge early in the disease. Furthermore, negative correlations were found between fixation stability and MCCB scores, especially in attention, visual learning, and processing speed, indicating that fixation stability requires the involvement of these functions and could serve as a reliable indicator for cognitive function. Fixation stability in FSZ was positively correlated with PANSS scores, suggesting fixation stability is influenced by the disease severity and may be a symptom-related indicator.
Compared to HCs, increased ReHo was observed in the right MCC and STP in FSZ and left STG and MTG in CHR, reflecting alterations specific to schizophrenia. The MCC, a central node within auditory and salience networks, is involved in reward-based decision-making and intentional motor control50,51. Previous findings have associated thinner cortices of right MCC with aggression and positive symptoms in schizophrenia52. Notably, our study revealed higher ReHo in the right MCC in FSZ compared to CHR and HCs, implying potential cingulate hyperactivation in FSZ individuals, which may correlate with increasing persistence of positive symptoms during disease progression. The temporal lobe mediates language processing, visual and auditory short-term memory53, and social cognition54, associated with positive symptoms, particularly auditory hallucination, in schizophrenia55. Studies have indicated a negative correlation between bilateral temporal pole thickness and age in schizophrenia, suggesting underlying progressive cortical thinning in this population56. Structural imaging studies consistently reported reductions in gray matter thickness or volume, notably in the frontal and temporal cortices in CHR57,58, indicating the primary involvement of temporal lobe in development of schizophrenia. Moreover, increased ReHo in the temporal lobe was observed in the left hemisphere in CHR, whereas it was confined to the right hemisphere in FSZ. Left-lateralized abnormalities are suggested to play an important role in escalating psychosis risk59,60. Increased ReHo is mostly interpreted as temporal consistency of neuronal activity, suggesting functional compensation in brain tissue for damage61. Thus, we hypothesize the left temporal lobe impairment may be integral to the early disease stages, reflecting as increased ReHo in compensation, and then these deficits develop toward the right temporal lobe as psychosis progresses.
Compared with CHR, FSZ displayed increased ReHo in the right MCC and decreased ReHo in the right superior OFG and left ITG, highlighting potential features for distinguishing FSZ from CHR. The OFG, involved in decision-making and emotional processing, has been associated with schizophrenic thought disorder62. Studies have indicated that CHR who later transitioned to psychosis exhibited significant reductions in the right OFG sulcogyral pattern63. Longitudinal brain trajectory analyses have observed greater reductions in GMV, cortical surface areas and thickness over time, particularly in the temporal and frontal cortex among individuals who remained symptomatic or progressed to psychosis compared to non-converters57. Thus, these ReHo alterations may reflect intermediate transitional changes from left to right in CHR to FSZ transition process based on our above hypothesis.
In this study, the correlation analysis revealed that the temporal lobe may be involved in SPEM process. MT and MST areas play primary roles in processing visuomotor information and receiving motion-related inputs from visual cortex, allowing for adjustment of saccade when tracking moving targets64. Ninthin et al. found that SPEM impairment in schizophrenia is primarily due to the interference of abnormal electrical signals in the functional connectivity between the anterior (FEF areas) and posterior (MT, MST) regions65. Interestingly, correlation between eye movements and ReHo was observed only in SPEM tasks, but not fixation stability tasks, probably because fixation stability tasks reflect attentional inhibition, requiring subjects to refrain from eye motions and inhibit activation of saccadic-related temporal cortex. Moreover, activity of MT and MST neurons is related to speed of visual stimulus13. The correlation we observed was specific to HS4X and LS4 tasks, suggesting that a tracking frequency of 0.4 Hz may be an appropriate target for investigating brain functional activity of saccades in SPEM tasks. Additionally, we found that right MCC may be associated with SPEM tasks. Previous studies found increased activation of the anterior cingulate in schizophrenia during SPEM tasks, suggesting its involvement in more complex challenging tasks compared to simple motor or attention tasks66. Posterior cingulate activity has been linked to attentional modulation during motion processing67, while anterior cingulate activity is involved in tasks requiring inhibition68. Our data suggest that FSZ may rely on these mechanisms to a greater extent than HCs.
In this study, LR, LASSO, RF, and SVM-RFE were used to screen eye movement features, with the best results obtained using LASSO and SVM-RFE methods for classification. LASSO model for discriminating schizophrenia from HCs achieved a high accuracy of 94.0%, which is close to 98.3% reported by Benson et al., who used a similar paradigm. The lower accuracy in this study than Benson et al. may be related to the different ethnicity8. Notably, we found that fixation stability and free-viewing tasks proved more effective in distinguishing CHR and HCs, while SPEM tasks were better for differentiating FSZ and CHR, suggesting that eye movement abnormalities in fixation stability and free-viewing tasks manifest early in the disease and persist during disease progression. As psychosis progresses, patients develop more severe abnormalities in SPEM.
Transcription-neuroimaging analysis showed connections and distinctions in gene expression profiles between CHR and FSZ. GO enrichment analysis indicated that ReHo-related genes were mainly enriched in synaptic structure and function. Neurodevelopmental hypothesis suggests that combined effects of genetics and environment during processes such as synaptic pruning in adolescence and early adulthood may contribute to neurological defects and increased risk of developing psychiatric symptoms58,69. Specifically, regional synaptic protein 2 A levels and synaptic density are reduced in schizophrenia in the hippocampus, cingulate, temporal and frontal cortices70,71. KEGG enrichment analysis highlighted associations with oxytocin and calcium signaling pathways. Early disturbances in oxytocin signaling potentially interfere with neuronal maturation, influencing processes like cytoskeletal rearrangements, axonal and dendritic elongation, and synapse formation72. Dysfunction within these pathways has been implicated in schizophrenia and represents promising targets for future therapeutic interventions73,74. TSEA analysis revealed that PLS1 genes were specifically expressed in the cerebral cortex, consistent with expectations. CSEA analysis found that PLS1+ genes in CHR were associated with Ntsr+ and Glt25d2 neurons. PLS1- genes in FSZ and CHR showed similar cell type expression, mainly in Bergmann glia, mature oligodendrocytes, and astroglia. Astrocytes are implicated in pathogenesis of schizophrenia by inhibiting synaptogenesis and impacting synaptic activity and plasticity75. Post-mortem tissue analysis in schizophrenia identified significant alterations in astrocyte morphology and density, along with deregulated expression of common astrocyte markers76,77. Temporal-specific expression analysis indicated that these genes were mostly expressed throughout all stages of the disease. PLS1+ genes may play a role in the thalamus at all development stages, while PLS1- genes show relevance in the hippocampus and striatum. These findings highlight the intricate and regional heterogeneity of transcriptional associations of schizophrenia. Using PPI analysis, we identified critical hub genes within PPI networks, which have been reported to be involved in schizophrenia and mental disorders through GWAS studies78,79. For example, CACNA1C, which encodes α-1C subunit of L-type voltage-gated calcium channel, was strongly associated with schizophrenia in the Han Chinese population80. Moreover, YWHAG involved in synaptic plasticity modulation and neuronal development, showed significantly higher baseline expression levels in patients who converted to psychosis81.
This study has several limitations worth noting. First, small sample size potentially impacts the reproducibility and reliability of our findings. Second, the cross-sectional design of this study limits our ability to capture the longitudinal transitions to psychosis in CHR individuals. Moreover, the CHR group comprises both those who convert to psychosis and non-converters and the possibility of two independent patterns of brain function between them could not be excluded. Thus, the findings in CHR should be interpreted with caution. Third, neuroimaging and gene expression data came from different subjects, leading to possibility of missing genes due to individual differences. Therefore, we applied a 50% DS threshold to obtain more conserved genes to minimize bias. Finally, while the AHBA dataset provides an excellent resource to investigate relationship between genes and neuroimaging, this gene expression information comes from only six individuals due to the scarcity of donor brains. Future work should rather be based on individual genes with corresponding brain imaging features.
Conclusions
Our study identifies distinct eye movement patterns and associated ReHo alteration in FSZ and CHR, suggesting the potential of these differences as early biomarkers. Furthermore, our findings establish spatial correlations between ReHo and gene expression profiles, shedding light on the genetic mechanisms underlying brain function in FSZ and CHR populations.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
References
Fusar-Poli, P. et al. The Psychosis High-Risk State. JAMA Psychiatry 70, https://doi.org/10.1001/jamapsychiatry.2013.269 (2013).
Probability of Transition to Psychosis in Individuals at Clinical High Risk https://doi.org/10.1001/jamapsychiatry.2021.0830.
Buckley, P. F. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Yearb. Psychiatry Appl. Ment. Health 2013, 111–112, https://doi.org/10.1016/j.ypsy.2012.07.042 (2013).
Fusar-Poli, P., Yung, A. R., McGorry, P. & van Os, J. Lessons learned from the psychosis high-risk state: towards a general staging model of prodromal intervention. Psychol. Med. 44, 17–24, https://doi.org/10.1017/s0033291713000184 (2013).
Caldani, S. et al. Saccadic eye movements as markers of schizophrenia spectrum: Exploration in at-risk mental states. Schizophr. Res. 181, 30–37, https://doi.org/10.1016/j.schres.2016.09.003 (2017).
Kojima, T. et al. Stability of exploratory eye movements as a marker of schizophrenia—a WHO multi-center study. Schizophr. Res. 52, 203–213, https://doi.org/10.1016/s0920-9964(00)00181-x (2001).
Ross, R. G., Olincy, A., Harris, J. G., Sullivan, B. & Radant, A. Smooth pursuit eye movements in schizophrenia and attentional dysfunction: adults with schizophrenia, ADHD, and a normal comparison group. Biol. Psychiatry 48, 197–203, https://doi.org/10.1016/s0006-3223(00)00825-8 (2000).
Benson, P. J. et al. Simple viewing tests can detect eye movement abnormalities that distinguish schizophrenia cases from controls with exceptional accuracy. Biol. Psychiatry 72, 716–724, https://doi.org/10.1016/j.biopsych.2012.04.019 (2012).
Reuter, B., Herzog, E. & Kathmann, N. Antisaccade performance of schizophrenia patients: Evidence of reduced task-set activation and impaired error detection. J. Psychiatr. Res. 40, 122–130, https://doi.org/10.1016/j.jpsychires.2005.02.007 (2006).
Arolt, V., Teichert, H.-M., Steege, D., Lencer, R. & Heide, W. Distinguishing schizophrenic patients from healthy controls by quantitative measurement of eye movement parameters. Biol. Psychiatry 44, 448–458, https://doi.org/10.1016/s0006-3223(97)00479-4 (1998).
Obyedkov, I. et al. Saccadic eye movements in different dimensions of schizophrenia and in clinical high-risk state for psychosis. BMC Psychiatry 19, https://doi.org/10.1186/s12888-019-2093-8 (2019).
Lisberger, S. G. Visual guidance of smooth pursuit eye movements. Annu. Rev. Vis. Sci. 1, 447–468, https://doi.org/10.1146/annurev-vision-082114-035349 (2015).
Ilg, U. J. & Thier, P. The neural basis of smooth pursuit eye movements in the rhesus monkey brain. Brain Cogn. 68, 229–240, https://doi.org/10.1016/j.bandc.2008.08.014 (2008).
Gottlieb, J. P., MacAvoy, M. G. & Bruce, C. J. Neural responses related to smooth-pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J. Neurophysiol. 72, 1634–1653, https://doi.org/10.1152/jn.1994.72.4.1634 (1994).
Lisberger, S. G. Visual guidance of smooth-pursuit eye movements: sensation, action, and what happens in between. Neuron 66, 477–491, https://doi.org/10.1016/j.neuron.2010.03.027 (2010).
Bois, C. et al. Hippocampal, amygdala and nucleus accumbens volume in first-episode schizophrenia patients and individuals at high familial risk: a cross-sectional comparison. Schizophr. Res. 165, 45–51, https://doi.org/10.1016/j.schres.2015.03.024 (2015).
Bartholomeusz, C. F. et al. Structural neuroimaging across early-stage psychosis: Aberrations in neurobiological trajectories and implications for the staging model. Aust. N.Z. J. Psychiatry 51, 455–476, https://doi.org/10.1177/0004867416670522 (2016).
Xie, Y. J. et al. Functional connectivity of cerebellar dentate nucleus and cognitive impairments in patients with drug-naive and first-episode schizophrenia. Psychiatry Res. 300, https://doi.org/10.1016/j.psychres.2021.113937 (2021).
Sasabayashi, D. et al. Resting state hyperconnectivity of the default mode network in schizophrenia and clinical high-risk state for psychosis. Cereb. Cortex 33, 8456–8464, https://doi.org/10.1093/cercor/bhad131 (2023).
de Zwarte, S. M. C. et al. The association between familial risk and brain abnormalities is disease specific: an ENIGMA-relatives study of schizophrenia and bipolar disorder. Biol. Psychiatry 86, 545–556, https://doi.org/10.1016/j.biopsych.2019.03.985 (2019).
Zuo, X.-N. & Xing, X.-X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neurosci. Biobehav. Rev. 45, 100–118, https://doi.org/10.1016/j.neubiorev.2014.05.009 (2014).
Zuo, X.-N. et al. Toward reliable characterization of functional homogeneity in the human brain: Preprocessing, scan duration, imaging resolution and computational space. NeuroImage 65, 374–386, https://doi.org/10.1016/j.neuroimage.2012.10.017 (2013).
Huang, Y. et al. Altered regional homogeneity and cognitive impairments in first-episode schizophrenia: a resting-state fMRI study. Asian J. Psychiatry 71, https://doi.org/10.1016/j.ajp.2022.103055 (2022).
Zhao, X. et al. Abnormalities of regional homogeneity and its correlation with clinical symptoms in Naïve patients with first-episode schizophrenia. Brain Imaging Behav. 13, 503–513, https://doi.org/10.1007/s11682-018-9882-4 (2018).
Li, X. et al. Abnormalities of regional brain activity in patients with schizophrenia: a longitudinal resting-state fMRI study. Schizophr. Bull. 49, 1336–1344, https://doi.org/10.1093/schbul/sbad054 (2023).
Yan, W. et al. Relationships between abnormal neural activities and cognitive impairments in patients with drug-naive first-episode schizophrenia. BMC Psychiatry 20, https://doi.org/10.1186/s12888-020-02692-z (2020).
Ma, X. et al. Decreased regional homogeneity and increased functional connectivity of default network correlated with neurocognitive deficits in subjects with genetic high-risk for schizophrenia: a resting-state fMRI study. Psychiatry Res. 281, https://doi.org/10.1016/j.psychres.2019.112603 (2019).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508, https://doi.org/10.1038/s41586-022-04434-5 (2022).
Hawrylycz, M. et al. Canonical genetic signatures of the adult human brain. Nat. Neurosci. 18, 1832–1844, https://doi.org/10.1038/nn.4171 (2015).
Liu, S., Abdellaoui, A., Verweij, K. J. H. & van Wingen, G. A. Gene expression has distinct associations with brain structure and function in major depressive disorder. Adv. Sci. 10, https://doi.org/10.1002/advs.202205486 (2023).
Li, Q. et al. Resting-state brain functional alterations and their genetic mechanisms in drug-naive first-episode psychosis. Schizophrenia 9, 13, https://doi.org/10.1038/s41537-023-00338-z (2023).
Dong, D. et al. Functional connectome hierarchy in schizotypy and its associations with expression of schizophrenia-related genes. Schizophr. Bull. https://doi.org/10.1093/schbul/sbad179 (2023).
Cao, H. et al. Cerebellar functional dysconnectivity in drug-naïve patients with first-episode schizophrenia. Schizophr. Bull. 49, 417–427, https://doi.org/10.1093/schbul/sbac121 (2023).
Morgan, S. E. et al. Cortical patterning of abnormal morphometric similarity in psychosis is associated with brain expression of schizophrenia-related genes. Proc. Natl. Acad. Sci. 116, 9604–9609, https://doi.org/10.1073/pnas.1820754116 (2019).
Han, Y. et al. Cortical anatomical variations, gene expression profiles, and clinical phenotypes in patients with schizophrenia. NeuroImage Clin. 39. https://doi.org/10.1016/j.nicl.2023.103451 (2023).
Ji, Y. et al. Genes associated with gray matter volume alterations in schizophrenia. NeuroImage 225, https://doi.org/10.1016/j.neuroimage.2020.117526 (2021).
Yan, C.-G., Wang, X.-D., Zuo, X.-N. & Zang, Y.-F. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14, 339–351, https://doi.org/10.1007/s12021-016-9299-4 (2016).
Arnatkevic̆iūtė, A., Fulcher, B. D. & Fornito, A. A practical guide to linking brain-wide gene expression and neuroimaging data. NeuroImage 189, 353–367, https://doi.org/10.1016/j.neuroimage.2019.01.011 (2019).
Markello, R. D. et al. Standardizing workflows in imaging transcriptomics with the Abagen toolbox. Elife 10, https://doi.org/10.7554/eLife.72129 (2021).
Jain, A., Tuteja, G. & Kelso, J. TissueEnrich: tissue-specific gene enrichment analysis. Bioinformatics 35, 1966–1967, https://doi.org/10.1093/bioinformatics/bty890 (2019).
Dougherty, J. D., Schmidt, E. F., Nakajima, M. & Heintz, N. Analytical approaches to RNA profiling data for the identification of genes enriched in specific cells. Nucleic Acids Res. 38, 4218–4230, https://doi.org/10.1093/nar/gkq130 (2010).
Shen, J. et al. Cell-type-specific gene modules related to the regional homogeneity of spontaneous brain activity and their associations with common brain disorders. Front. Neurosci. 15, https://doi.org/10.3389/fnins.2021.639527 (2021).
Hou, C.-L. et al. Cognitive functioning in individuals at ultra-high risk for psychosis, first-degree relatives of patients with psychosis and patients with first-episode schizophrenia. Schizophr. Res. 174, 71–76, https://doi.org/10.1016/j.schres.2016.04.034 (2016).
Hauser, M. et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and be most promising for predictive algorithms for conversion to psychosis. J. Clin. Psychiatry 78, e28–e40, https://doi.org/10.4088/JCP.15r10197 (2017).
Kim, W.-S. et al. Altered amygdala-based functional connectivity in individuals with attenuated psychosis syndrome and first-episode schizophrenia. Sci. Rep. 10, https://doi.org/10.1038/s41598-020-74771-w (2020).
Bestelmeyer, P. E. G. et al. Global visual scanning abnormalities in schizophrenia and bipolar disorder. Schizophr. Res. 87, 212–222, https://doi.org/10.1016/j.schres.2006.06.015 (2006).
Chu, A. O. K. et al. Comparison of cognitive functions between first-episode schizophrenia patients, their unaffected siblings and individuals at clinical high-risk for psychosis. Psychol. Med. 49, 1929–1936, https://doi.org/10.1017/s0033291718002726 (2018).
Levy, D. L., Sereno, A. B., Gooding, D. C. & O’Driscoll, G. A. Eye tracking dysfunction in schizophrenia: characterization and pathophysiology. Behav. Neurobiol. Schizophr. Treat. https://doi.org/10.1007/7854_2010_60 (2010).
Visual fixation and smooth pursuit eye movement abnormalities in patients with schizophrenia and their relatives. J. Neuropsychiatr. Clin. Neurosci. 7, 197-206, https://doi.org/10.1176/jnp.7.2.197 (1995).
Vogt, B. A. Midcingulate cortex: Structure, connections, homologies, functions and diseases. J. Chem. Neuroanat. 74, 28–46, https://doi.org/10.1016/j.jchemneu.2016.01.010 (2016).
Clark, D. L., Boutros, N. N. & Mendez, M. F. The brain and behavior: an introduction to behavioral neuroanatomy. Cambridge university press (2010).
Wong, T. Y. et al. An overlapping pattern of cerebral cortical thinning is associated with both positive symptoms and aggression in schizophrenia via the ENIGMA consortium. Psychological Med. 50, 2034–2045, https://doi.org/10.1017/s0033291719002149 (2019).
Leff, A. P. et al. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain 132, 3401–3410, https://doi.org/10.1093/brain/awp273 (2009).
Brunet, E., Sarfati, Y., Hardy-Baylé, M.-C. & Decety, J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia 41, 1574–1582, https://doi.org/10.1016/s0028-3932(03)00119-2 (2003).
Allen, P. et al. Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr. Bull. 38, 695–703, https://doi.org/10.1093/schbul/sbs066 (2012).
van Erp, T. G. M. et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol. Psychiatry 84, 644–654, https://doi.org/10.1016/j.biopsych.2018.04.023 (2018).
Merritt, K., Luque Laguna, P., Irfan, A. & David, A. S. Longitudinal structural MRI findings in individuals at genetic and clinical high risk for psychosis: a systematic review. Front. Psychiatry 12, https://doi.org/10.3389/fpsyt.2021.620401 (2021).
Job, D. E., Whalley, H. C., Johnstone, E. C. & Lawrie, S. M. Grey matter changes over time in high risk subjects developing schizophrenia. NeuroImage 25, 1023–1030, https://doi.org/10.1016/j.neuroimage.2005.01.006 (2005).
Mittal, V. A., Turner, J., Calhoun, V., Vargas, T. & Damme, K. S. F. Global and specific cortical volume asymmetries in individuals with psychosis risk syndrome and schizophrenia: a mixed cross-sectional and longitudinal perspective. Schizophr. Bull. 46, 713–721, https://doi.org/10.1093/schbul/sbz096 (2020).
Liloia, D. et al. Updating and characterizing neuroanatomical markers in high-risk subjects, recently diagnosed and chronic patients with schizophrenia: A revised coordinate-based meta-analysis. Neurosci. Biobehav. Rev. 123, 83–103, https://doi.org/10.1016/j.neubiorev.2021.01.010 (2021).
Baune, B. T. et al. Frequency dependent alterations in regional homogeneity of baseline brain activity in schizophrenia. PLoS ONE. 8, https://doi.org/10.1371/journal.pone.0057516 (2013).
Walton, M. E., Devlin, J. T. & Rushworth, M. F. S. Interactions between decision making and performance monitoring within prefrontal cortex. Nat. Neurosci. 7, 1259–1265, https://doi.org/10.1038/nn1339 (2004).
Lavoie, S. et al. Sulcogyral pattern and sulcal count of the orbitofrontal cortex in individuals at ultra high risk for psychosis. Schizophr. Res. 154, 93–99, https://doi.org/10.1016/j.schres.2014.02.008 (2014).
Grosbras, M. H., Laird, A. R. & Paus, T. Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum. Brain Mapp. 25, 140–154, https://doi.org/10.1002/hbm.20145 (2005).
Krishna, N., O’Neill, H., Sánchez-Morla, E. M. & Thaker, G. K. Long range frontal/posterior phase synchronization during remembered pursuit task is impaired in schizophrenia. Schizophr. Res. 157, 198–203, https://doi.org/10.1016/j.schres.2014.05.035 (2014).
Tregellas, J. R., Tanabe, J. L., Miller, D. E. & Freedman, R. Monitoring eye movements during fMRI tasks with echo planar images. Hum. Brain Mapp. 17, 237–243, https://doi.org/10.1002/hbm.10070 (2002).
Antal, A., Baudewig, J., Paulus, W. & Dechent, P. The posterior cingulate cortex and planum temporale/parietal operculum are activated by coherent visual motion. Vis. Neurosci. 25, 17–26, https://doi.org/10.1017/s0952523808080024 (2008).
Jamadar, S. D., Fielding, J. & Egan, G. F. Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Front. Psychol. 4, https://doi.org/10.3389/fpsyg.2013.00749 (2013).
Pocklington, A. J., O’Donovan, M. & Owen, M. J. The synapse in schizophrenia. Eur. J. Neurosci. 39, 1059–1067, https://doi.org/10.1111/ejn.12489 (2014).
Osimo, E. F., Beck, K., Reis Marques, T. & Howes, O. D. Synaptic loss in schizophrenia: a meta-analysis and systematic review of synaptic protein and mRNA measures. Mol. Psychiatry 24, 549–561, https://doi.org/10.1038/s41380-018-0041-5 (2018).
Forsyth, J. K. & Lewis, D. A. Mapping the consequences of impaired synaptic plasticity in schizophrenia through development: an integrative model for diverse clinical features. Trends Cogn. Sci. 21, 760–778, https://doi.org/10.1016/j.tics.2017.06.006 (2017).
Bakos, J., Srancikova, A., Havranek, T. & Bacova, Z. Molecular mechanisms of oxytocin signaling at the synaptic connection. Neural Plast. 2018, 1–9, https://doi.org/10.1155/2018/4864107 (2018).
Goh, K. K., Chen, C.-H. & Lane, H.-Y. Oxytocin in schizophrenia: pathophysiology and implications for future treatment. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms22042146 (2021).
Glaser, T., Arnaud Sampaio, V. F., Lameu, C. & Ulrich, H. Calcium signalling: A common target in neurological disorders and neurogenesis. Semin. Cell Dev. Biol. 95, 25–33, https://doi.org/10.1016/j.semcdb.2018.12.002 (2019).
de Oliveira Figueiredo, E. C., Calì, C., Petrelli, F. & Bezzi, P. Emerging evidence for astrocyte dysfunction in schizophrenia. Glia 70, 1585–1604, https://doi.org/10.1002/glia.24221 (2022).
Tarasov, V. V. et al. Alterations of astrocytes in the context of schizophrenic dementia. Front. Pharmacol. 10, https://doi.org/10.3389/fphar.2019.01612 (2020).
Trépanier, M. O., Hopperton, K. E., Mizrahi, R., Mechawar, N. & Bazinet, R. P. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol. Psychiatry 21, 1009–1026, https://doi.org/10.1038/mp.2016.90 (2016).
Birnbaum, R. & Weinberger, D. R. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 18, 727–740, https://doi.org/10.1038/nrn.2017.125 (2017).
Dennison, C. A., Legge, S. E., Pardiñas, A. F. & Walters, J. T. R. Genome-wide association studies in schizophrenia: recent advances, challenges and future perspective. Schizophr. Res. 217, 4–12, https://doi.org/10.1016/j.schres.2019.10.048 (2020).
He, K. et al. CACNA1C, schizophrenia and major depressive disorder in the Han Chinese population. Br. J. Psychiatry 204, 36–39, https://doi.org/10.1192/bjp.bp.113.126979 (2018).
Demars, F. et al. Dysregulation of peripheral expression of the YWHA genes during conversion to psychosis. Sci. Rep. 10, https://doi.org/10.1038/s41598-020-66901-1 (2020).
Acknowledgements
The authors thank all the volunteers who took part in the study. This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 82171508 and 82071507), Natural Science Foundation of Tianjin (Grant No. 18JCQNJC10900) and Natural Science Foundation of Hunan (Grant No. 2023JJ30758).
Author information
Authors and Affiliations
Contributions
Zhaobin Chen and Yangpan Ou performed the data analysis and wrote the initial draft. Yudan Ding and Yong Liu conceptualized the study. Huabing Li and Feng Liu preprocessed the MRI data. Ping Li, Dongsheng Lv, Bing Lang, Jingping Zhao, Ying Wang, and Wenbin Guo developed the trial design and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Z., Ou, Y., Ding, Y. et al. Abnormal eye movement, brain regional homogeneity in schizophrenia and clinical high-risk individuals and their associated gene expression profiles. Schizophr 11, 64 (2025). https://doi.org/10.1038/s41537-025-00609-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41537-025-00609-x