Abstract
Schizophrenia is a disorder of still unknown aetiology characterized by positive, negative and cognitive symptoms. The first evident signs emerge at the end of adolescence and the beginning of adulthood as a psychotic episode. Patients are then treated with antipsychotics to ameliorate positive symptoms. However, this pharmacological approach is ineffective for negative and cognitive ones. Schizophrenia patients also exhibit metabolic and immune alterations, regardless of antipsychotic treatment. Clinical research in this field is challenging, as there is no way to identify people at risk before the first psychotic episode, and once it emerges, antipsychotic treatment is applied, worsening metabolic and immune profiles which may be detrimental for cognitive and negative symptoms. A faithful animal model of schizophrenia may be valuable to understand molecular events and brain regions involved in each of the symptoms, evaluate novel pharmacological compounds for unattended symptoms and explore objective diagnostic strategies. Here, we show that the selective dopamine D2 receptor deletion from parvalbumin interneurons, a mutation that results in schizophrenia-like phenotypes, causes intrinsic metabolic and immune defects in mice, in a similar way to what is described in schizophrenia patients. Mutant animals show dysglycaemia and dyslipidaemia, abnormal white blood cell counts, increased neutrophil-to-lymphocyte ratio, CD4/CD8 ratio imbalances, increased circulating C-reactive protein levels and reactive microglia. Therefore, selective dopamine D2 receptor deletion causes a wide spectrum of phenotypes resembling those described in patients. This animal line may be a useful research tool to expand our knowledge on the aetiology of schizophrenia.
Similar content being viewed by others
Introduction
Despite intense research, schizophrenia remains a disorder of unknown etiology that ranks 18th among the top 25 causes of years lived with disability (YLD)1. It is characterized by three types of signs and symptoms. Positive symptoms are the most well-known and characteristic, including psychomotor agitation, hallucinations and delusions. These symptoms generally become evident at the end of adolescence or the beginning of adulthood. However, there is a prodromal stage where other subtle symptoms may also be present. Negative symptoms include reduced social interaction and motivation, poor personal care and anhedonia, among other features. Finally, cognitive symptoms include deficits in working memory and diminished executive function capabilities2 and are core features of schizophrenia3. Pharmacological treatment is based on the administration of antipsychotics, which mainly acts as dopamine D2 receptor (DRD2) antagonists4, reducing the excessive dopamine neurotransmission in subcortical regions5. However, antipsychotics are only effective to manage positive symptoms of the disease. Although clozapine has shown partial efficacy in addressing negative symptoms, albeit with limitations, new pharmacological developments are required to improve both negative and cognitive symptoms and further research is therefore needed to better understand the mechanisms underlying each of these symptom dimensions.
In addition to the positive, negative and cognitive symptoms, patients also exhibit metabolic impairments. Despite weight gain, dyslipidaemia and diabetes mellitus being adverse effects of antipsychotics, there are reports indicating that metabolic imbalances are present even before the initiation of pharmacological treatment with these drugs6. Metabolic abnormalities exhibited by diagnosed patients include alterations in fatty acids, such as increased triglyceride levels and decreased cholesterol levels, including HDL and LDL7,8,9. Patients display poor blood glucose level control, marked by elevated post-prandial glucose levels and insulin resistance10. One consequence of these metabolic abnormalities is that patients with schizophrenia have a reduced life expectancy of around 15 to 20 years, primarily attributed to an increased risk of cardiovascular disease11.
Immune alterations were also described in patients with schizophrenia. An elevated low-grade peripheral inflammatory tone, characterized by an increased level of proinflammatory cytokines, such as IL-6 or C-reactive protein (CRP)12,13,14,15, increased microglial reactivity16, alterations in white blood cell number17, increased CD4+/CD8+ T lymphocyte ratio18, and increased neutrophil-to-lymphocyte ratio19 has been found in patients. Maternal infection during pregnancy has been identified as a risk factor for the development of schizophrenia later in life. Such infections can affect the dopaminergic, GABAergic and glutamatergic neurotransmission20.
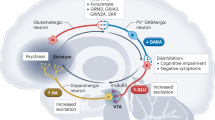
The etiology of schizophrenia is explained by different hypotheses. Among them, the dopaminergic hypothesis, one of the most enduring in the field, postulates an imbalance in dopamine neurotransmission21, although there is no conclusive evidence of dopaminergic system dysfunction itself and therefore, research efforts are focused on the regulation of dopamine release from midbrain neurons22. On the other hand, the GABAergic hypothesis is supported by evidence pointing to an abnormal inhibitory neuron function, particularly those expressing the neuropeptide parvalbumin (Pvalb)23. Moreover, DRD2 is expressed in parvalbumin interneurons in the prefrontal cortex (PFC) along the transition between adolescence and adulthood, where dopamine enhances the inhibitory activity of these interneurons24,25. Based on both hypotheses, we generated a mouse line through the selective deletion of the DRD2 exclusively from inhibitory interneurons expressing parvalbumin (PvalbΔDRD2)26,27. The selective ablation of the D2 receptor from parvalbumin interneurons results in a collection of molecular, cellular, physiological, and behavioral phenotypes reminiscent of those observed in patients26,27,28. These phenotypes include reduced dendritic spine density, reduced GSK3β expression, reduced mRNA expression levels of DRD1, DRD2, GAD67, Pvalb and the NR2B subunit of the NMDA receptor. Additionally, there is a reduced dopamine release in the PFC, increased dopamine metabolites in the striatum, hypersensitivity to amphetamine, abnormal excitatory/inhibitory balance, lower PFC metabolic glucose consumption, impaired presynaptic dopaminergic functioning and adult onset behavioral phenotypes, among other characteristics26,27. However, a detailed analysis of metabolic and immune profiles has not been performed in this genetically modified mouse line. Therefore, in this work, we aimed to analyze the presence of intrinsic metabolic and immune abnormalities as a result of specific DRD2 deletion from parvalbumin interneurons.
Results
Metabolic characterization of PvalbΔDRD2 animals
DRD2 selective deletion in parvalbumin interneurons was accomplished by a parvalbumin-Cre line29 mated to a floxed DRD2 line30 in a C57BL/6 J background. Littermates with DRD2flox/flox or Pvalb-Cre::DRD2flox/flox (PvalbΔDRD2) genotype were used as control or mutant groups, respectively. The PvalbΔDRD2 mouse line consistently express schizophrenia-like behaviors, as previously reported26. Behavioral experiments showed that mutant animals have increased locomotor activity and reduced interest in nest building. They also showed abnormal performance in the Y maze and in the novel object recognition test, both associated with cognitive deficits. Additionally, mutant animals exhibited abnormal anxiety levels in the elevated plus maze (Supplementary Fig. 1 and Supplementary Table 1). Metabolic alterations are present in schizophrenia patients even before the administration of antipsychotics6. To study if adult PvalbΔDRD2 animals show an abnormal lipid profile reminiscent of those reported in patients, we quantified circulating triglycerides, cholesterol, HDL and LDL levels in control and mutant animals. Our results showed that adult PvalbΔDRD2 animals have a significantly increased concentration of triglycerides in circulating blood (Ctl: 1.158 ± 0.063 g/l, n = 17; Mut: 1.349 ± 0.065 g/l, n = 12; p = 0.025, t = 2.048, df:27; Fig. 1A) but a reduced concentration of cholesterol (Ctl: 1.126 ± 0.068 g/l, n = 17; Mut: 0.907 ± 0.051 g/l, n = 13; p = 0.024, t = 2.381, df:28), HDL (Ctl: 0.201 ± 0.010 g/l, n = 17; Mut: 0.163 ± 0.014 g/l, n = 13; p = 0.013, t = 2.643, df:28) and LDL (Ctl: 0.924 ± 0.059 g/l, n = 17; Mut: 0.743 ± 0.046 g/l, n = 13; p = 0.030, t = 2.276, df:28; Fig. 1B−D).
Circulating levels of (a) triglycerides, b total cholesterol, c high density lipoprotein-cholesterol (HDL-C) and d low density lipoprotein-cholesterol (LDL-C) in control (gray) and mutant (red) animals. n = 17 for control and 12–13 for mutant group. Animals between 180 and 300 days of age. Data is presented as mean ± s.e.m. *p < 0.05. Unpaired t test.
Impaired glucose tolerance is recurrent in patients10, therefore, we evaluated the ability of control and mutant PvalbΔDRD2 animals to manage blood glucose levels in a glucose tolerance test (GTT). Our findings showed that adult mutant animals have impaired glucose control, as they exhibited increased glucose levels at 30 and 60 min after glucose administration (RM-ANOVA, Interaction: p < 0.0001; F = 7.73; Fig. 2A and supplementary Table 1). Accordingly, the area under the curve (AUC) was increased in the mutant group compared to controls (Ctl: 18550 ± 2146, n = 11; Mut: 25,420 ± 1702, n = 14; p = 0.0182, t = 2.542, df:23; Fig. 2B). Interestingly, although mutant animals showed metabolic abnormalities, they had similar average weight compared to control animals (Ctl: 34.23 ± 0.789 g, n = 20; Mut: 33.56 ± 0.748 g, n = 28; Mann Whitney test p = 0.32; Fig. 2C).
a Mutant animals required more time to reach control circulating glucose levels in a glucose tolerant test (GTT). b Area under the curve (AUC) in the GTT and c total weight for control (gray) and mutant (red) animals. n = 11 and 14 for control and mutant groups, respectively in the GTT. Animals 180 days of age. n = 20 for control and 28 for mutant group in the weight test. Data are presented as mean ± s.e.m. a Two-way ANOVA with Bonferroni as post-test. b Unpaired t test. c Mann Whitney test. *p < 0.05 and **p < 0.01.
Immune characterization of PvalbΔDRD2 animals
To perform an immune profile of mutant PvalbΔDRD2 animals we first determined the proportion of lymphocytes and neutrophils by blood smear quantifications. Our results showed that mutant animals have a reduced proportion of lymphocytes (Ctl: 77.07 ± 1.24, n = 32; Mut: 72.62 ± 1.43, n = 26; p = 0.022, t = 2.353, df:56) and an increased proportion of neutrophils in peripheral blood (Ctl: 17.31 ± 0.94%, n = 32; Mut: 21.16 ± 1.202, n = 26; p = 0.013, t = 2.556,df:56) (Fig. 3A, B). We then performed an absolute count of white blood cells and found that control and mutant animals showed non-significant differences in total leukocyte (Ctl: 20160 ± 764.6, n = 17; Mut: 21100 ± 1141, n = 13; p = 0.483, t = 0.7102, df:28) and lymphocyte (Ctl: 16180 ± 747.9, n = 16; Mut: 16340 ± 1063, n = 13; p = 0.898, t = 0.1447, df:27) counts (Fig. 3C, D). However, we observed an increase in total neutrophil population (Ctl: 2830 ± 220.2, n = 16; Mut: 3643 ± 278.4, n = 13; p = 0.0284, t = 2.298, df:27; Fig. 3E). Accordingly, neutrophil to lymphocyte ratio (NLR) showed an increase in mutant animals (Ctl: 0.174 ± 0.015, n = 16; Mut: 0.231 ± 0.022, n = 13; p = 0.039, t = 2.165, df:27; Fig. 3F). Given this abnormal leukocyte count in mutant animals, we further characterized white blood cell profiles using flow cytometry. Total lymphoid CD3+ cells showed non-significant differences between both groups (Ctl: 15.19 ± 1.689%, n = 6; Mut: 17.53 ± 0.676%, n = 10, p = 0.153, t = 1.510, df:14; Fig. 4A). However, we observed an increase in mutant CD4+ population (Ctl: 54.20 ± 1.172%, n = 6; Mut: 59.94 ± 0.328%, n = 10; p < 0.0001, t = 5.829, df:14) and a reduction in the CD8+ cell population of the same genotype (Ctl: 39.33 ± 1.923%, n = 6; Mut: 35.08 ± 0.605%, n = 10; p = 0.022, t = 2.829, df:14; Fig. 4B, C). Accordingly, the CD4+/CD8+ ratio was increased in mutant animals (Ctl: 1.398 ± 0.092, n = 6; Mut: 1.714 ± 0.034, n = 10; p = 0.0021; t = 3.771, df:14 Fig. 4D). Regarding myeloid linage analysis, CD11b+ population showed non-significant differences in control and mutant groups (Ctl: 25.98 ± 1.434%, n = 6; Mut: 27.06 ± 1.175%, n = 10; p = 0.576, t = 0.5723, df14; Fig. 4E). However, a segmented analysis in CD11b+/Ly6Ghigh/Ly6Cmid-level, the prospective neutrophil population, showed an increase in the mutant group (Ctl: 43.43 ± 1.036%, n = 6; Mut: 48.73 ± 1.218%, n = 10; p = 0.0099, t = 2.982, df:14; Fig. 4F) but not in the CD11b+/Ly6Glow/Ly6Clow-level, the prospective monocytic population (Ctl: 17.05 ± 0.555, n = 6; Mut: 16.59 ± 0.783, n = 10; p = 0.684, t = 0.415, df:14; Fig. 4G). As we identified blood cell profiles alterations in PvalbΔDRD2 animals, we quantified the plasma levels of an inflammatory molecule reportedly increased in schizophrenia patients, C-reactive protein (CRP), and found that mutant animals showed an increase in circulating CRP compared to control animals (Ctl: 573.8 ± 28.58, n = 13; Mut: 664.5 ± 29.21, n = 13; p = 0.0362, t = 2.219, df:24; Fig. 4H).
a Percentage of lymphocytes and b neutrophils, c absolute leukocyte count, d lymphocyte count and e neutrophil count in control (gray) and mutant (red) animals. f neutrophil-to-lymphocyte ratio (NLR) from absolute values in control and mutant animals. Animals are between 71 and 73 days of age. Data are presented as mean ± s.e.m. Unpaired t test. *p < 0.05.
a–d Lymphoid and e–g myeloid flow cytometry analysis. a CD3+, b CD4+ and c CD8+ cell proportion in control (gray) and mutant (red) animals. d CD4+/CD8+ ratio. e CD11b+ cell proportion, f CD11b+, Ly6G high, Ly6C mid cell proportion corresponding to the neutrophil population and g CD11b+ Ly6G low and Ly6C low cell proportion corresponding to the monocyte population. h C-reactive protein content in circulating blood. Animals between 96 and 116 days of age. Data is presented as mean ± s.e.m. Unpaired t test. *p < 0.05 and **p < 0.01.
An increased microglial reactivity was found in post-mortem brain studies from patients31, although inconsistencies are common32. To analyze if mutant PvalbΔDRD2 animals showed increased microglial reactivity, we performed an immunofluorescence study with the anti-Ionized calcium-binding adapter molecule 1 (IBA1) antibody and quantified fluorescence intensity and morphological changes of labeled cells in prefrontal cortex. Microglial cells of mutant animals showed increased reactivity compared to controls (Fig. 5A, B), as they have an increased cell body Iba1 immunoreactivity intensity (Ctl: 24498 ± 603.4, n = 268; Mut: 28643 ± 706.4, n = 271; p < 0.0001; Fig. 5C), but reduced number of end points (Ctl: 119.8 ± 12.66, n = 7; Mut: 88.97 ± 4.14, n = 7; p = 0.0175) and branches (Ctl: 310.2 ± 25.08, n = 7; Mut: 228.8 ± 12.18, n = 7; p = 0.0129, t = 2.918, df:12) compared to control animals (Fig. 5D, E).
An immunofluorescence with Iba1 antibody was performed on PFC brain sections from (a) control or (b) mutant animals. (c) Cell body Iba1 intensity was determined along with (d) end-points per cell and (e) branches per cell. Animals between 93 and 96 days of age. Data is presented as mean ± s.e.m. c, d Mann Whitney test. e Unpaired t test. *p < 0.05 and *** p < 0.001. Scale bar: 10 µm.
Discussion
In this study, we analyzed metabolic and immune alterations produced after DRD2 specific deletion from parvalbumin interneurons in mice. We observed increased circulating triglyceride levels but reduced total cholesterol, HDL and LDL levels in mutant animals compared to controls. Mutant animals showed dysglycaemia and dyslipidaemia, even though both groups showed a non-significant difference in average weight. White blood cells profile analysis showed that mutant animals had an increased absolute neutrophil count but comparable lymphocyte absolute count with a concomitant increased neutrophil to lymphocyte ratio. Flow cytometry analysis showed that although the lymphoid CD3+ population was similar in both genotypes, the proportion of CD4+ cells in mutant animals was increased, while the CD8+ cell population was reduced. Total CD11b+ myeloid population was similar in both control and mutant groups. However, mutant animals showed an increased CD11b+/Ly6Ghigh/Ly6Cmid neutrophil population compared to control animals, in line with our previous blood count characterization. This immune cell population alteration was accompanied by an increased level of circulating proinflammatory molecule C-reactive protein in mutant animals and by prefrontal microglial reactivity, as we found an increased cell body Iba1 immunolabeling intensity and reduced end points and ramifications per cell in mutant animals.
We previously showed that DRD2 specific deletion from parvalbumin interneurons causes schizophrenia like phenotypes26,27. PvalbΔDRD2 mouse line show adult onset of behavioral phenotypes reminiscent of schizophrenia, including increased locomotor activity and anxiety but reduced motivation and cognitive performance (Supplementary Fig. 1). It also shows molecular, cellular and physiological impairments, including reduced Gsk3β protein levels in the PFC, reduced GAD1, Pvalb, DRD2, NR2B mRNA expression, reduced dendritic spine density in PFC, hippocampal hyperactivity, reduced expression of tyrosine hydroxylase in the ventral tegmental area, reduced metabolic glucose consumption in the PFC and nucleus reuniens, abnormal response to aripiprazole and hypersensitivity to amphetamine, among others. However, it has been reported that schizophrenia patients may also show metabolic and immunologic perturbations prior to antipsychotic administration, and therefore, it is expected that a faithful animal model of the disease should also reproduce these alterations.
Individuals with schizophrenia have a reduced life expectancy of about 15–20 years compared to the general population. One of the predominant causes of this precocious mortality rate, beyond suicide, accidents, and risk-taking behaviors, is associated with cardiovascular diseases11,33. Although antipsychotic treatment confers an increased risk of metabolic syndrome and cardiovascular diseases34, it has been shown that patients already show dyslipidaemia prior to antipsychotic administration7,9. We therefore analyzed circulating lipid levels in antipsychotic-free PvalbΔDRD2 animals and found significantly increased triglyceride levels, but reduced cholesterol, LDL, and HDL levels in mutant animals compared to control littermates, as reported for antipsychotic-free patients9. Antipsychotic treatment is also associated with hyperglycemia and, therefore, increased risk for type 2 diabetes mellitus. Meta-data studies on first-episode antipsychotic-free patients confirmed this abnormal glycaemic control8. To explore if the PvalbΔDRD2 animal line show a reminiscent glycaemic profile as antipsychotic-free schizophrenia patients, we performed a glucose tolerance test (GTT) and observed that, although mutant and control animals showed nonsignificant differences in basal levels of fasting glucose, mutant animals required significantly more time to reach control circulating glucose levels, a sign of glucose intolerance, in line with what has been described for schizophrenia patients8.
Peripheral blood circulating immune cells can reflect the general immune status and their potential to initiate or sustain an effective immune response. The neutrophil-to-lymphocyte ratio (NLR) is a biomarker that reflects two aspects of the immune system: acute and chronic inflammation associated with neutrophil count compared to adaptive immunity associated with lymphocyte count35. Recent analysis showed that schizophrenia patients have an increased NLR19. Absolute white blood cell counts from mutant and control animals showed that PvalbΔDRD2 animals had an increased absolute neutrophil count with comparable total lymphocyte and leukocyte counts. This increased neutrophil population causes a significantly elevated NLR in mutant animals, as reported in patients. A flow cytometry analysis using a panel of specific surface markers to identify myeloid and lymphoid derived cells showed that mutant animals have a significant increase in the CD11b+/Ly6G high/Ly6C mid neutrophil population36,37, in agreement with our previous observations, but showed similar proportions in the CD11b + /Ly6G low/Ly6C low antigen presenting cell (APC) population. A meta-analysis of T-cell profile showed an increased CD4/CD8 ratio in first episode psychosis patients with a significant increase in absolute CD4+ cells18. Lymphoid flow cytometry analysis in control and mutant animals showed a significantly increased proportion of CD4+ cells but a significantly reduced proportion of CD8+ cells, associated with a significant increase in the CD4/CD8 ratio, in line with clinical reports from patients18. Circulating C reactive protein (CRP) is an acute phase protein produced by hepatocytes that is regulated by interleukin-6, TNF-α and other inflammatory cytokines38. CRP levels correlate with metabolic syndrome, a collection of cardiovascular risk factors associated with the development of cardiovascular diseases39. Levels of this protein are increased in schizophrenia patients in the acute phase compared to controls and it was suggested that CRP could be also considered a state marker of the disease40. Here, we found that PvalbΔDRD2 animals also had increased circulating CRP levels compared to the control group.
Neuroinflammation, an inflammatory process, is characterized by the activation of microglial cells, permeability of the brain blood barrier, increased release of inflammatory cytokines and the infiltration of peripheral immune cells into the brain41, like CD4+ T cells. Neuroinflammation has been proposed to contribute to schizophrenia etiology, as patients show increased levels of inflammatory cytokines and reactive microglial cells16,31. As mutant animals exhibited an inflammatory phenotype, we characterized microglial cells in the prefrontal cortex and found an increased branching index and Iba1 immunostaining intensity, phenotypes associated with microglial reactivity and reminiscent of findings from patients.
Although parvalbumin is expressed in different regions of the central nervous system, it is also found in other tissues like the heart, gut and mesenterium. On the other hand, DRD2 receptor expression has been reported in the mesenteric nervous system42 and thus, peripheral contributions to the phenotypes described in this work cannot be excluded. In this work, male animals were used to perform all the experiments, but an experimental study with female PvalbΔDRD2 animals will be required to establish if metabolic and immune parameters resemble those shown in males. Therefore, conclusions from this work should not be extended to both genders. In this and previous work we characterized behavioral phenotypes showing that adult PvalbΔDRD2 mouse line exhibit behaviors reminiscent of positive, negative and cognitive symptoms26. An association between a particular behavioral phenotype and a specific immune profile would not be unexpected, given that such a correlation has been reported in patients19. In this work, our objective was to characterize the metabolic and immune profile of PvalbΔDRD2 animals. We did not aim at finding an association between metabolic and/or immune phenotypes with any specific behavior and therefore, further research will be needed to determine if such a correlation is present in this animal line. Each metabolic and immune phenotype described here has been characterized at a single point in time. A longitudinal analysis would be required to establish whether they fluctuate or remain stable. Our experiments were performed in antipsychotic-free mice, unveiling intrinsic metabolic and immune impairments, as reported in antipsychotic-free patients7,8,9,10,17,18. In a clinical setting, patients start to be treated with antipsychotics after the first psychotic episode and therefore, metabolic and immune profiles may be negatively affected. A chronic antipsychotic administration regime experiment in this animal line would be required to evaluate the impact at both metabolic and immune levels. In this work, microglial cell analysis was restricted to the prefrontal cortex, but the observed phenotype may not be affected to the same extent in other brain regions. Further research will be needed to establish if microglia is widely affected in this mutant line.
Animal models have been useful to shed light on the etiology of schizophrenia. These models range from pharmacological approaches43, prenatal interventions44,45 eventually combined with peripubertal stress46, brain lesions47 and genetic strategies48. Here, we used a mouse line with a specific genetic deletion of DRD2 from parvalbumin interneurons, showing that it resembles metabolic and immune alterations exhibited by antipsychotic free schizophrenia patients.
Our results reinforce the value of the PvalbΔDRD2 genetic mouse line as a valuable tool to study schizophrenia, offering a broad range of phenotypes that closely resemble the complex presentation of the disorder in patients. We believe that the use of the PvalbΔDRD2 mouse line may lead to a greater understanding of the disease´s etiology, ultimately advancing in the development of novel therapeutic strategies.
Methods
Animals
Adult male C57BL/6 J mice were used. Animals were housed in the corresponding facility at Instituto de Biología y Medicina Experimental (IBYME) and were maintained with food and water ad libitum in a temperature-controlled room (21 °C–23 °C) with light: dark (12:12 h, lights on 7:00 AM) cycle. To generate the selective DRD2 deletion only in parvalbumin interneurons, a Parvalbumin-Cre line29 was mated to a floxed DRD2 line30 in a C57BL/6 J background. Littermates with DRD2flox/flox or Pvalb-Cre::DRD2flox/flox genotype were used as control or mutant groups, respectively, for metabolic, immune or behavior experiments. At weaning time (postnatal day 20), animals were randomly assigned to cages in groups of five, without prior knowledge of their genotype. Sample sizes in animal experiments were chosen to match standards in the field. A formal power calculation prior to conducting the experiments was not performed. Genotyping of the Cre gene and the floxed allele were performed as previously described26, determining their study group allocation. Experiments were performed following the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health. The study was approved by the Institutional Animal Care and Use Committee of the Instituto de Biología y Medicina Experimental (IBYME, N019/2019 and 13/2024). This study is reported in accordance with ARRIVE guidelines.
Triglycerides, total cholesterol, high- and low-density lipoprotein cholesterol (HDL-C, LDL-C) determination
Triglycerides, total cholesterol and high-density lipoprotein-cholesterol (HDL-C) were determined in animals of 180 to 300 days of age using the following kits (Wiener Lab, Buenos Aires, Argentina) per manufacturer’s instructions: TG Color GPO/PAP AA, Colestat enzimático and HDL-Colesterol FT. Low density lipoprotein-cholesterol (LDL-C) concentration was calculated by subtracting the measured HDL-C form the total cholesterol levels.
Glucose tolerance test
For the glucose tolerance test (GTT) basal glycemia was measured in animals of 180 days of age. Animals were weighed before administering an intraperitoneal injection of glucose (20% in saline, 2 g/kg). Subsequent measurements were taken at 15, 30, 60, 90, and 120 min. Blood glucose levels were determined using a One Touch Select Plus Flex glucose meter (Sidus, Buenos Aires, Argentina) from tail blood after 10 h of fasting. The area under the curve (AUC) was determined for each animal.
Blood extraction
Peripheral blood extraction was performed in mice between 71 and 73 days of age. An incision of no more than 2 mm at the distal end of the tail using a blade was made, followed by gentle milking to collect approximately 20 µl of blood into EDTA pre-treated tubes to prevent coagulation. Additionally, a drop of blood was collected from the tail and placed onto a microscope slide for smear preparation. Finally, pressure was applied with gauze to achieve hemostasis, and the incision site was then cauterized.
Total white blood cell count
Blood samples were diluted 1:20 in Türk’s solution (3% of acetic acid and 0,1% methylene blue in distilled water). White blood cell count was performed using a Neubauer chamber.
Blood smear count
For blood smear analysis, the May-Grünwald Giemsa (MGG) staining method was employed. After spreading the sample on a microscope slide, it was air-dried at room temperature and fixed in absolute (100%) methanol for 3 min. MGG staining was then performed by applying 1 mL of May-Grünwald working solution (Biopack, Buenos Aires, Argentina) for 3 min, followed by a gentle rinse with tap water to remove excess solution. Then, 3 mL of a 1:10 dilution of Giemsa solution (Biopack, Buenos Aires, Argentina) in tap water was applied to each smear for 15 min, rinsed in water and air-dried. ~150 white blood cells were counted under the microscope to identify and calculate the percentage of leukocyte subtypes.
Flow cytometry and C-Reactive Protein (CRP) determination
Blood samples were obtained from animals aged 96–116 days and used for immunotyping by flow cytometry and CRP measurement. For flow cytometry, 25 µl of each sample were seeded into a well of a 96-well U-bottom multiwell plate. An equal volume of a mixture of fluorochrome-conjugated antibodies specific to the populations to be identified, at twice the final concentration to be used, was added and incubated for 25 min at room temperature. Then, the marked cells were fixed, and the red blood cells were lysed (Biolegend’s RBC Lysis/Fixation Solution) following the manufacturer’s instructions. Samples were acquired by the cytometer LSR Fortessa (Becton Dickinson). The data was analyzed using FlowJo software. All antibodies used for the cytometry determination were from Biolegend: Anti-mouse CD3 (clone 17A2), CD4 (RM4-5), CD8 (53-6.7), CD11b (M1/70), CD45 (30-F11), Ly6C (AL-21) and Ly6G (1A8). CRP was measured in mice serum using the Mouse C-Reactive Protein/CRP DuoSet ELISA Kit (Biotechne, Minneapolis, USA) per manufacturer’s instructions.
Tissue collection
Mice between 93 and 96 days of age were transcardially perfused with 4% paraformaldehyde (PFA) solution in PBS and the brain was removed and postfixed in the same fixative solution for 180 min at 4 °C. The tissue was cryoprotected sequentially in 10, 20, and 30% sucrose solution in phosphate buffer saline (PBS) and then cut serially in a cryostat in 40 μm thick coronal brain sections.
Immunofluorescence
Floating sections were rinsed, blocked with 1% bovine serum albumin (BSA)/0.3% Triton X-100/PBS for 2 h and incubated with primary antibody in blocking solution overnight at 4 °C (Rabbit anti-ionized calcium-binding adapter molecule 1 (Iba-1), 1:1000, Fujifilm Wako Chemicals, Cat. 019-19741). Next, sections were rinsed and incubated with the secondary antibody in blocking solution for 2 h at room temperature (Cy3 anti-rabbit IgG (H + L, 1:700, Jackson Immuno-Research Laboratories Inc., Cat. 711-165-152). Hoechst staining was used for cell nuclei detection (1:1000; Sigma-Aldrich). Finally, sections were rinsed and mounted in 0.1% gelatin (Sigma) in a 0.1 M tris buffered solution (pH 7.4), air-dried, dehydrated and mounted with DPX (Sigma). All rinses were performed in 0.3% Triton X-100/PBS three times for 5 min.
Image processing and quantification
Iba-1+ cells were analyzed in the prefrontal cortex, specifically in the cingulate, prelimbic and infralimbic regions. At least three brain sections per animal from three animals of each genotype were analyzed. Sections were selected within bregma +1.10 to +1.78. Images were acquired using a LSM 880 fluorescent confocal laser scanning microscope (Carl Zeiss, Germany) and data collection was performed with a 20X plan-apochromatic objective to ensure detailed visualization of microglial morphology and distribution. ImageJ software v1.54 (National Institutes of Health, US) was used for image analysis. To quantify the area and intensity of the entire Iba-1+ cells, the pseudo flat field was corrected. Next, the gamma mathematical process, the Gaussian blur filter and the unsharp mask filter were applied. Then, the convoluted background was subtracted and the parameters were measured from the binary images obtained after setting the most appropriate threshold method. To quantify the same parameters in cell bodies, a series of EDM binary operations were performed and particles smaller than 20 µm2 were excluded from the binary images. Then, Iba-1+ cell bodies binary images were subtracted from entire Iba-1+cells binary images to carry out the quantification corresponding to the branches. The analysis of the branches was performed from the skeleton of the entire Iba-1+cells using the Analyze Skeleton 2D/3D plugin. Iba+ cells were counted from Iba+ cell bodies binary images.
Behavior analysis
Behavioral experiments were conducted during the dark phase of the light/dark cycle between 6 pm and 11 pm and performed by an observer blind to genotype. All tests were videotaped using a computer-assisted data acquisition system (OBS Studio).
Open field: Spontaneous locomotor activity in an open field arena (50 × 50 cm) was measured for 30 min using ANY-maze Video Tracking System. Total distance traveled (horizontal activity) was analyzed.
Elevated plus maze: The elevated plus maze was used to study anxiety related behavior. The apparatus consists of a cross-shaped platform (four arms faced two to two with a length of 30 and 8 cm width each) elevated 50 cm from the floor. Two opposing arms are protected by walls (without a roof) and the other two are left unprotected. Because it is elevated from the ground, the open arms represent an aversive environment for the mouse. The proportion of time spent in open arms during 8 min is considered a measure of anxiety.
Y maze: Testing was carried out on a Y shaped maze (35 × 5 cm each arm). Mice were placed into the end of one arm and allowed to freely explore for 7 min. The sequence of arm entries was recorded. The spontaneous alternation behavior was calculated as the number of triads containing entries into all three arms divided by the total number of entries.
Novel Object Recognition Test: A mouse was placed in an empty arena and was allowed to explore it for 5 min. 24 h later, during the familiarization session, the mouse was presented with two identical objects and was allowed to freely explore them for 5 min. Three hours later, during the test session, one of the two objects was replaced by a new one and mice were allowed to explore for 3 min. The amount of time taken to explore the new object provides a discrimination index between known and novel objects and is associated with cognitive performance. Discrimination index was calculated as (Tn-Tf)/(Tn+Tf) where Tn: exploration time in novel object and Tf: exploration time in familiar object.
Nesting test: To study the motivation to build a nest, one piece of pressed cotton fiber (approximately 3 g each), was introduced in a cage in which a mouse was individually housed. Each piece of pressed cotton was weighed before the test. After the test, the unthreaded and identifiable pieces of nesting non-used material were weighed. Percentage of non-used cotton was calculated.
Statistics
Statistical analyses were conducted using GraphPad Prism Software. Two tailed unpaired t-tests were used to compare groups with normal distribution. Repeat measures (RM) ANOVA were used to compare blood glucose content. Normality test for Gaussian distribution was performed where t-test was used for analysis. Data that did not follow normal distribution were analyzed by Mann Whitney test. Data are expressed as mean ± SEM. Statistical data and a post hoc power analysis to evaluate the biological significance are included as a Supplementary Table 1.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Ferrari, A. J. et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2133–2161 (2024).
Kahn, R. S. et al. Schizophrenia. Nat. Rev. Dis. Prim. 1, 15067 (2015).
Kahn, R. S. & Keefe, R. S. E. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry 70, 1107–1112 (2013).
Remington, G. et al. Schizophrenia: antipsychotics and drug development. Behav. Brain Res. 414, 113507 (2021).
McCutcheon, R. A., Abi-Dargham, A. & Howes, O. D. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 42, 205–220 (2019).
Freyberg, Z., Aslanoglou, D., Shah, R. and Ballon, J. S. Intrinsic and antipsychotic drug-induced metabolic dysfunction in schizophrenia. Front. Neurosci. 11, 432 (2017).
Misiak, B., Stańczykiewicz, B., Łaczmański, Ł & Frydecka, D. Lipid profile disturbances in antipsychotic-naive patients with first-episode non-affective psychosis: a systematic review and meta-analysis. Schizophrenia Res. 190, 18–27 (2017).
Pillinger, T. et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry 74, 261–269 (2017).
Pillinger, T., Beck, K., Stubbs, B. & Howes, O. D. Cholesterol and triglyceride levels in first-episode psychosis: systematic review and meta-analysis. Br. J. Psychiatry 211, 339–349 (2017).
Perry, B. et al. The association between first-episode psychosis and abnormal glycaemic control: systematic review and meta-analysis. Lancet Psychiatry 3, 1049–1058 (2016).
Nielsen, R. E., Banner, J. & Jensen, S. E. Cardiovascular disease in patients with severe mental illness. Nat. Rev. Cardiol. 18, 136–145 (2021).
Kroken, R. A. et al. Constructing the immune signature of schizophrenia for clinical use and research; an integrative review translating descriptives into diagnostics. Frontiers in Psychiatry 9, 753 (2019).
Khandaker, G. M. et al. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2, 258–270 (2015).
Fond, G., Lançon, C., Auquier, P. and Boyer, L. C-Reactive protein as a peripheral biomarker in schizophrenia. an updated systematic review. Front. Psychiatry 9, 392 (2018).
Borovcanin, M. M. et al. Interleukin-6 in schizophrenia—is there a therapeutic relevance? Front. Psychiatry 8, 221 (2017).
Laskaris, L. E. et al. Microglial activation and progressive brain changes in schizophrenia. Br. J. Pharmacol. 173, 666–680 (2016).
Jackson, A. J. & Miller, B. J. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr. Scandinavica 142, 18–26 (2020).
Miller, B. J. et al. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry 73, 993–999 (2013).
Karageorgiou, V., Milas, G. P. and Michopoulos, I. Neutrophil-to-lymphocyte ratio in schizophrenia: a systematic review and meta-analysis. Schizophrenia Res. 206, 4–12 (2018).
Meyer, U. & Feldon, J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav. Brain Res. 204, 322–334 (2009).
Howes, O. D. & Kapur, S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophrenia Bull. 35, 549–562 (2009).
Grace, A. A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17, 524–532 (2016).
Lewis, D. A., Curley, A. A., Glausier, J. R. & Volk, D. W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35, 57–67 (2012).
Tseng, K.-Y. & O’Donnell, P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb. Cortex 17, 1235–1240 (2007).
Tseng, K. Y. & O’Donnell, P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse 61, 843–850 (2007).
Tomasella, E. et al. Deletion of dopamine D2 receptors from parvalbumin interneurons in mouse causes schizophrenia-like phenotypes. PNAS 115, 3476–3481 (2018).
Tomasella, E. et al. Impaired brain glucose metabolism and presynaptic dopaminergic functioning in a mouse model of schizophrenia. EJNMMI Res. 10, 39 (2020).
Bechelli, L. et al. Selective dopamine D2 receptor deletion from Nkx6.2 expressing cells causes impaired cognitive, motivation and anxiety phenotypes in mice. Sci. Rep. 13, 19473 (2023).
Hippenmeyer, S. et al. A developmental switch in the response of drg neurons to ETS transcription factor signaling. PLoS Biol. 3, e159 (2005).
Bello, E. P. et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci. 14, 1033–1038 (2011).
Gober, R. et al. Microglia activation in postmortem brains with schizophrenia demonstrates distinct morphological changes between brain regions. Brain Pathol. 32, e13003 (2022).
Schnieder, T. P. & Dwork, A. J. Searching for neuropathology: gliosis in schizophrenia. Biol. Psychiatry 69, 134–139 (2011).
Laursen, T. M., Nordentoft, M. & Mortensen, P. Bo. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 10, 425–448 (2014).
De Hert, M. et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 8, 114–126 (2012).
Song, M., Graubard, B. I., Rabkin, C. S. & Engels, E. A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 11, 464 (2021).
Zhang, X. et al. Rejuvenation of neutrophils and their extracellular vesicles is associated with enhanced aged fracture healing. Aging Cell 21, e13651 (2022).
Lee, P. Y. et al. Ly6 family proteins in neutrophil biology. J. Leukoc. Biol. 94, 585–594 (2013).
Pepys, M. B. & Hirschfield, G. M. C-reactive protein: a critical update. J. Clin. Investig. 111, 1805–1812 (2003).
den Engelsen, C. et al. High-sensitivity C-reactive protein to detect metabolic syndrome in a centrally obese population: a cross-sectional analysis. Cardiovasc. Diabetol. 11, 25 (2012).
Lestra, V. et al. Could CRP be a differential biomarker of illness stages in schizophrenia? A systematic review and meta-analysis. Schizophrenia Res. 246, 175–186 (2022).
González, H. & Pacheco, R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J. Neuroinflam.11, 201 (2014).
Clark, B. J. & Menninger, K. Peripheral dopamine receptors. Circul. Res. 46, 159–163 (1980).
Robinson, T. E. & Becker, J. B. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. Rev. 11, 157–198 (1986).
Flagstad, P. et al. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology 29, 2052–2064 (2004).
Meyer, U., Feldon, J., Schedlowski, M. & Yee, B. K. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci. Biobehav. Rev. 29, 913–947 (2005).
Du, Y. & Grace, A. A. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology 38, 1881–1888 (2013).
Lipska, B. K., Jaskiw, G. E. & Weinberger, D. R. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology 9, 67–75 (1993).
del Pino, I., Rico, B. & Marín, O. Neural circuit dysfunction in mouse models of neurodevelopmental disorders. Curr. Opin. Neurobiol. 48, 174–182 (2018).
Acknowledgements
We thank Fundación Rene Baron, Fundación Williams, Fundación Bunge y Born and Fundación Cherny for their contribution to the Institute. We also want to thank Maria Lucia Diaz Garcia from Sidus, Wiener Lab, Romina Girotti and to all the team of IBYME animal facility. This work was supported by a grant from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT, PICT 2020-2764 to D.M.G.), and Fundación Florencio Fiorini (D.M.G.). Fellowship to M.B. was granted from ANPCYT and to A.D.P. from National Research Council (CONICET).
Author information
Authors and Affiliations
Contributions
M.B., S.L.C., A.D.P. and F.V. conducted the experiments. M.B., S.L.C., A.D.P., L.P., F.V. and D.G. carried out the interpretation of the results. D.G. wrote the manuscript with support from M.B., S.L.C, L.P. and A.D.P. D.G. conceived the original idea and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Belmonte, M., Cardoso, S.L., Di Pietro, A.A. et al. Intrinsic metabolic and immune impairments in a genetic mouse model of schizophrenia. Schizophr 11, 100 (2025). https://doi.org/10.1038/s41537-025-00651-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41537-025-00651-9