Abstract
Treatment resistance affects up to one in four individuals with psychosis in the first few years of illness. However, there is limited information about the brain changes associated with treatment resistance, restricting our ability to develop effective prognostic biomarkers or new treatments. Using resting-state functional MRI, we examined striatocortical connectivity in 87 patients who presented a non-affective first-episode of psychosis and 118 healthy controls, with follow-up imaging on more than half of the participants in the next 6 years, totaling 361 images. Crucially, we identified 30 patients who presented treatment-resistant psychosis in this follow-up period. Thus, we examined baseline (at first episode) and longitudinal striatocortical differences within psychosis subgroups (treatment-responsive and treatment-resistant psychosis), and between patients subgroups and healthy controls. Compared to healthy controls, participants with treatment-responsive psychosis presented baseline differences in functional connectivity of ventral striatal systems, without changes over time; whereas patients with treatment-resistant psychosis showed both baseline and longitudinal differences in ventral striatal systems, compared to healthy controls. Treatment-responsive and treatment-resistant psychosis groups differed in longitudinal changes in connectivity between ventral striatal and temporal cortical regions. This is one of the circuits which has been previously related to symptom improvements in patients with first-episode of psychosis. No baseline differences were observed between the two psychosis groups. Overall, treatment-resistant psychosis is characterized by longitudinal changes in striatal systems in early psychosis, which might be used as the basis of future prognostic biomarkers.

Similar content being viewed by others
Introduction
Approximately one in four individuals who develop psychosis will not benefit from conventional antipsychotic medications in the first few years of illness, and are described as presenting treatment resistance1. However, it frequently takes years before treatment resistance is recognized, particularly since the only existing method to establish it is based on the careful clinical documentation of the failure to respond to at least two courses of treatment2. Such delay in diagnosis is also associated with a poorer future response to effective treatments, such as clozapine3. Understanding the underlying biological changes associated with these heterogeneous clinical progressions after the onset of psychosis could inform new interventions and prognostic markers.
A critical system in psychosis examined using resting-state functional MRI focuses on striatocortical interactions4. Striatocortical circuits are involved in motor, cognitive, and emotional functions, and distinct patterns of functional connectivity are associated with these different neural circuits in healthy subjects5. Several lines of evidence suggest that these systems are reorganized in psychosis. For example, a study described a dorsal-to-ventral gradient of hypoconnectivity to hyperconnectivity between striatal and prefrontal regions in first-episode psychosis4, with ventral hyperconnectivity also being exacerbated by ketamine6 and observed in high-risk groups defined genetically7. A reversed gradient has also been proposed for posterior cortical areas and striatal regions in psychosis, with increased connectivity in dorsal systems and reduced connectivity in ventral striatal regions4. Also, longitudinal changes in striatocortical systems have been associated with treatment response in the first episode, with improvements in symptoms associated with an increase in dorsal striatal connectivity with frontal areas, and ventral striatal with limbic regions, as well as a decrease in connectivity between ventral striatal and parietal regions8. Some patients may experience a delayed response, or respond only after switching to a second antipsychotic medication. However, a clinically important group requiring early identification are those who continue to show no response, a condition referred to as treatment-resistant schizophrenia. These patients may suffer from a neurobiologically distinct form of psychosis9. One study examined cross-sectional differences between 16 patients with established treatment-resistant schizophrenia and 22 treatment-responders, suggesting these systems are differentially implicated in response to treatment, showing hyperconnectivity between dorsal striatal and frontal regions in treatment-resistant patients10. Furthermore, a combined index from 91 connections in striatal systems has been shown to predict response to treatment in first episode11, suggesting that some of these changes might be picked up at an early stage. In summary, the available evidence, coming mostly from small cross-sectional studies, suggests that functional MRI-based striatocortical connectivity is sensitive to case-control differences in psychosis and, more interestingly, to differences related to treatment response.
To shed light on brain changes associated with the emergence of treatment resistance in psychosis, we here used resting-state functional MRI to longitudinally examine the striatocortical connectivity of a large cohort of 87 patients who presented with a non-affective first episode of psychosis and were minimally medicated at first assessment. The sample included a total of 178 observations (up to 4 observations per participant) with follow-ups of up to 6.7 years from their baseline assessment at disease onset. Crucially, we identified 30 patients from this cohort who subsequently developed treatment resistance, being able to explore baseline differences (group effects) as well as differing longitudinal changes in striatocortical connectivity (group x time interactions). Considering that striatal systems undergo age-related changes12, and to help control for non-specific temporal changes after repeatedly visiting the scanner, we also included a cohort of 118 healthy participants (183 observations -up to 3 per participant-, with up to 4.4 years of follow-up). While recognizing that the existing evidence of the brain mechanisms underlying treatment resistance is limited, particularly with respect to longitudinal changes, we hypothesized that differences between the two subgroups of patients would be focused on circuits previously related to symptomatic improvements. According to the literature, treatment resistance could be associated with decreased connectivity between the dorsal striatum and frontal regions, decreased connectivity between the ventral striatum and temporal regions, and increased connectivity between the ventral striatum and parietal regions. Differences in baseline or longitudinal functional connectivity in these circuits could point to underlying biological differences between treatment-responsive and treatment-resistant patients.
Materials and Methods
Participants
We recruited 89 patients presenting with a first episode of non-affective psychosis according to the Mini International Neuropsychiatric Interview13 from the Early Intervention Service at the Instituto Psiquiátrico José Horwitz Barak in Santiago, Chile. This is a quaternary service providing inpatient care to young people all over Santiago and outpatient follow-up to those living in Northern Santiago (catchment area of around 1 million inhabitants). Patients with epilepsy, history of moderate to severe traumatic brain injury, mental retardation, drug dependence (not including tobacco or cannabis), or presenting neurological findings on MRI (such as cysts deforming the anatomical structure) were excluded. Two patients were excluded after MRI quality checks. Patients were scanned as soon as it was feasible for them to tolerate the scan according to their treating psychiatrist, with an overall median of 21 days after treatment initiation (Q1 = 14, Q3 = 32). Prescription of medication and other interventions was defined by their treating psychiatrist according to local guidelines. Medications used across patients can be found in Supplementary Fig. 2. 51 participants were successfully recontacted and consented to (one to four) further follow-up assessments and scanning sessions.
From this sample of 87 patients with first-episode psychosis, we identified 30 who subsequently developed treatment resistance. Patients were defined as treatment-resistant according to one of two criteria:
-
Patients who were started on clozapine at any point by their treating psychiatrist (N = 26). As has been previously shown, this is a highly specific criterion to identify cases but can have low sensitivity14.
-
Of the 61 patients never prescribed clozapine, 60.7% had at least one follow-up visit. In those cases, we clinically assessed treatment resistance according to Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) criteria2, defined as failure to adequately respond to at least two trials of different antipsychotics at appropriate doses and duration. With these criteria, 4 further patients were identified as treatment-resistant.
Controls had no personal or family (first-degree relative) history of psychosis, did not fulfill criteria for any other current psychiatric disorders, and tested negative for the prodromal syndrome according to the Scale of Prodromal Symptoms15.
All participants had the capacity to participate and consented at each clinical contact. The project was approved by the Ethical Committees of the Pontificia Universidad Católica de Chile (IDs 16-074, 190507001, and 190603005) and Servicio de Salud Metropolitano Norte.
Clinical Assessments
Participants had their symptoms rated according to PANSS in every clinical encounter16. Current medication was recorded and converted to chlorpromazine (CPZ) equivalents17.
MRI imaging and preprocessing
All participants were scanned using the same sequences in the 3 T Philips Ingenia scanner of the Red de Salud UC-Christus with a 16-channel brain coil. Resting-state functional MRI images were acquired with a single shot echo-planar imaging (EPI), total scan time 8.33 min, repetition time (TR) 2.5 s, time to echo (TE) 35 ms, flip angle of 82°, field-of-view (FOV) of 220 × 220 × 110 mm, and an isotropic spatial resolution of 2.75 mm. Subjects were asked to remain still and with their eyes opened. A structural T1-weighted image was also acquired with a voxel size of 1.0 mm³ isotropic, an inversion time delay of 965.2 ms, TE 3.6 ms, TR 7.7 ms, and a flip angle of 8°.
Preprocessing of the functional images followed previously published pipelines18 as used previously by our group19,20. This included (1) removal of the first 4 volumes; (2) slice-time correction; (3) 2-pass realignment of all volumes to the first volume and then to the mean volume; (4) coregistration of EPI data to the structural image; (5) application of the T1-weighted derived nonlinear transform to the EPI volume; (6) linear detrending of the BOLD time series; (7) intensity normalization; (8) spatial smoothing with a 6 mm FWHM Gaussian kernel; (9) bandpass-filtered between 0.008 and 0.08 Hz using a fast Fourier transform; and (10) residual movement managed using ICA-AROMA21 as well as regression of mean white matter and cerebrospinal fluid signals.
Regions of interest
Seeds for the connectivity analyses were based on previous studies5, and consisted of 6 bilateral striatal regions of interest (ROIs) based on 3.5 mm radius spheres.
For the caudate, 3 ROIs were seeded (with corresponding Montreal Neurological Institute coordinates):
DC: dorsal caudate (x = ±13, y = 15, z = 9);
sVC: superior ventral caudate (x = ±10, y = 15, z = 0), (also described in the literature as VSs);
iVC: inferior ventral caudate/nucleus accumbens (x = ±9, y = 9, z = −8), (also described as VSi).
As for the putamen, the 3 ROIs were:
DCP: dorsocaudal putamen (x = ±28, y = 1, z = 3),
DRP: dorsorostral putamen (x = ±25, y = 8, z = 6),
VRP: ventrorostral putamen (x = ±20, y = 12, z = −3).
The dorsal striatal system is composed of DC, DCP and DRP, whereas the ventral seeds are iVC, sVC, and VRP.
Statistical analyses
We performed a first-level analysis as in previous studies4,7: for each participant, changes in the BOLD signal across the cortical voxels were modeled using a linear model including the 6 striatal seeds:
For each seed, derived cortical gray matter t-maps were then passed to the following second-level linear mixed model:
Where: HC is a dummy coded variable with values of 1 for healthy controls and 0 for all patients; TR is a dummy coded variable with values of 1 for treatment-resistant patients and 0 for treatment-respondent patients or healthy controls; time refers to months transcurred since the first evaluation (t = 0 in the first MRI session); and (1 | ID) accounts for a random intercept for repeated measures per subject.
With this coding of the variables HC and TR, the reference in the model consisted of patients with treatment-responsive psychosis (HC = 0 and TR = 0). As such, the resistance term (TR) captured baseline differences between treatment-resistant and treatment-responsive patients, and its interaction with time (TR x time), any different longitudinal changes between these two groups. On the other hand, the healthy controls term (HC) identified baseline differences between controls and treatment-responsive psychosis; and its interaction with time (HC x time), longitudinal changes in controls compared to treatment-responsive psychosis. A contrast defined as HC + TR was also examined, to directly compare healthy controls with treatment-resistant patients. Confounders in the models included: sex; dose of antipsychotic medication in CPZ equivalents (APdose); and the mean value of framewise displacements of the functional MRI session (fdmean)22. To control for common developmental changes in striatocortical connectivity, we also included participants’ age at each session as a covariate. In this context, our variable of interest ‘time’ models only the inter-session changes controlled for maturational effects. As in previous studies10, we also controlled for the level of symptoms to identify neural substrates beyond the expected higher symptomatology in treatment-resistant cases, including the PANSS score for positive symptoms (TPPANSS) as a confounder. However, we also report on the Supplementary Information results without including symptoms as a confounder.
Statistical significance was determined using cluster-correction with the ‘3dClustSim’ algorithm from AFNI23, using the auto-correlation function method, with a voxel-wise p-value threshold of 0.01 and a per-cluster alpha value of 0.05.
Results
87 patients with a first-episode were included in the analysis, alongside 118 healthy controls. 30 patients (34.5%) later developed treatment resistance. Table 1 describes the characteristics of the sample included and Fig. 1 the temporal distribution of longitudinal assessments in each group.
Analyses of striatocortical connectivity across groups (corrected for age and sex) revealed patterns consistent with previous reports5. Distinctive functional circuits were found for each striatal seed, with ventral caudate seeds being primarily associated with limbic areas (e.g., parahippocampal and orbitofrontal cortex), and dorsal caudate with cognition related regions (such as dorsolateral prefrontal cortex). A full depiction of these results is presented in Supplementary Fig. 1.
Treatment-responsive psychosis compared to healthy controls
In line with a previous study4, we found increased connectivity in patients compared to controls between the inferior ventral caudate and right temporal regions (cluster size = 1100 voxels, Fig. 2A), as well as reduced connectivity in patients between the ventrorostral putamen and occipital regions (cluster size = 2416 voxels, Fig. 2B). We did not find any statistically significant differences in the interaction term with time (HC x time), suggesting that changes observed at follow-ups were no different in those with treatment-responsive psychosis and healthy controls.
Blue (red) indicates increased (reduced) connectivity in patients relative to healthy controls. Cluster-corrected p < 0.05. iVC: inferior ventral caudate; VRP: ventrorostral putamen. A increased connectivity in patients compared to controls between the inferior ventral caudate and right temporal regions; B reduced connectivity in patients between the ventrorostral putamen and occipital regions.
Treatment-resistant compared to healthy controls
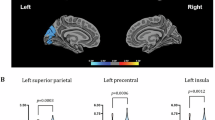
We found baseline increased connectivity in TR patients compared to controls between the ventrorostral putamen and right insular regions (cluster size = 685 voxels, Fig. 3A). We also found a significant interaction with time in the connectivity of the superior ventral caudate and a cluster spanning the postcentral and precentral gyrus and extending into the posterior area of the anterior cingulate gyrus (cluster size = 837 voxels, Fig. 3B). Patients with treatment resistance showed decreased connectivity with time in those connections, while the opposite was seen in healthy controls.
Blue indicates reduced connectivity in patients with treatment resistance relative to healthy controls. Cluster-corrected p < 0.05. VRP: ventrorostral putamen. sVC: superior ventral caudate. HC: healthy controls; TR: treatment resistant. A Reduced baseline connectivity in TR patients compared to controls between the ventrorostral putamen and right insular regions; B Time-related differences in striatocortical connectivity between TR patients and healthy controls. Reduced connectivity over time in TR patients between sVC and precentral gyrus expanding towards anterior cingulate cortex. In the interaction plot (right panel), each dot represents one observation, and observations from the same subject are connected with a dashed line. Shown ‘t-values’ are residuals from the model: t ~ age + sex + APdose + TPPANSS + fdmean + time + (1 | ID).
Treatment-resistant compared to treatment-responsive patients
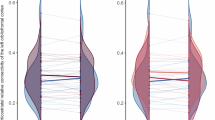
We found a significant interaction between treatment resistance and time (TR x time) in the connectivity of the superior ventral caudate and a cluster spanning the right superior and middle temporal gyrus (cluster size = 721 voxels, Fig. 4). Patients with treatment resistance showed a decreased connectivity with time in those connections, while the opposite was seen in treatment-responsive cases.
Blue indicates reduced connectivity over time in patients with treatment resistance. Cluster-corrected p < 0.05. NTR: non treatment-resistant (treatment-responsive); TR: treatment-resistant. sVC: superior ventral caudate. In the interaction plot (right panel), each dot represents one observation, and observations from the same subject are connected with a dashed line. Shown ‘t-values’ are residuals from the model: t ~ age + sex + APdose + TPPANSS + fdmean + HC + time + HC*time + (1 | ID).
There were no significant baseline (not interacted with time) differences associated with treatment resistance.
Associations with symptoms
We did not find any significant clusters associated with PANSS score for positive symptoms in our analysis. A secondary analysis, not including this PANSS score in our model, showed similar results, particularly between patient subgroups and healthy controls (Supplementary Figs. 3 and 4). However, differences between TR and treatment-responsive patients in longitudinal connectivity between superior ventral caudate and the temporal lobe were no longer significant at the cluster level; instead, a baseline difference between dorsocaudal putamen and occipital regions was found (Supplementary Fig. 5).
Discussion
Understanding the biological changes occurring in early psychosis and the trajectories related to different clinical presentations may provide new perspectives into interventions or prognostic markers. We here used a large dataset, amounting to 361 total observations, providing a unique insight into longitudinal brain changes after a first-episode of psychosis. Importantly, this dataset included 61 images from a subgroup of 30 first-episode patients who were identified as treatment-resistant in the first few years of the illness, offering a distinctive view into the emergence of treatment resistance from the onset of psychosis.
Our main finding refers to significant differences in the progression in time of striatocortical connectivity between treatment-responsive and treatment-resistant cases in a network including the superior ventral caudate and temporal regions of the brain. Of note, this circuit is one of those implicated in treatment response to a first antipsychotic in first-episode patients8. Even when other circuits may also be implicated in treatment response, our results in this particular one suggest that changes in the temporal lobe may be the most related to treatment resistance. Relatedly, at least two previous studies in chronic treatment-resistant cases have shown widespread structural changes in the temporal lobe24,25. These findings are also in line with other studies suggesting that transcranial magnetic stimulation to temporal regions might help in reducing treatment-resistant auditory hallucinations26.
We did not find baseline differences between treatment-responsive and treatment-resistant cases of psychosis that could serve as the basis of an early prognostic marker. Previous studies had shown a potential predictive value of baseline striatal connectivity for treatment response11, suggesting that there are baseline differences in these systems between patients subgroups. However, that marker of response was based on the combination of multiple connections, and therefore our analysis might have been underpowered to detect differences in individual ones. Moreover, that study focused on early response, which does not necessarily imply that those patients would fulfill criteria for treatment resistance.
Our results show a distinctive longitudinal progression for treatment-resistant patients in ventral striatal systems. This is in contrast to current evidence from PET studies that has shifted the attention of the locus of a dopaminergic dysfunction central to schizophrenia from the ventral to the dorsal striatum27. However, there is also increasing evidence that dopaminergic dysfunction might be different in the subgroup of patients developing treatment resistance28. For example, a previous study reported larger differences in dopaminergic presynaptic synthesis in the ventral striatum compared to the dorsal striatum between treatment-resistant and treatment-responsive patients29. Our results rekindle the possibility that treatment resistance might be related to a progressive ventral striatal dysfunction30.
Regarding differences between first-episode of psychosis patients and healthy subjects, a previous study exploring cross-sectional differences reported broadly spread differences in striatocortical circuits4. However, case control differences have not been as clearly found in subsequent studies8. Our results looking at case-control differences in treatment-responsive psychosis showed less but consistent findings with those previous studies: hyperconnectivity in patients between the inferior ventral caudate and right temporal regions and hypoconnectivity between the ventrorostral putamen and occipital regions. Our analyses expand a period of 6 years of illness and take treatment resistance into account, so these may be the most stable changes in striatocortical connectivity for patients with treatment-responsive psychosis, and be present since their first-episode.
We did not find significant differences associated with longitudinal progression in treatment-responsive cases compared to controls. This stability in dopaminergic systems contrasts with the significant changes in treatment-resistant cases described previously, and is at odds with the clinical observation of an increased need for antipsychotic doses with chronicity31. However, those clinical findings stem mostly from studies that have not differentiated subgroups with different trajectories of response to treatment. On the other hand, we used a linear model, so we cannot rule out the existence of non-linear or even linear but smaller magnitude changes in treatment-responsive cases that were not picked up by the current study.
Our results comparing treatment-resistant patients to healthy controls showed reduced baseline connectivity in patients between the ventrorostral putamen and right insular regions. Previous studies have also pointed to the insula –together with the temporal poles and hippocampus– as one of the regions with aberrant functional connectivity preceding treatment resistance32. Cortical thinning of the insula and temporal lobes has also been identified as one of three subtypes of disease progression, showing significantly more progressed stages in treatment resistant patients33. We also found time-related differences between superior ventral caudate and precentral gyrus expanding towards anterior cingulate cortex. These results are in line with a recent study relating neuromelanin levels in the substantia nigra, a proposed MRI marker of dopaminergic functioning34, with the functional connectivity between the caudate and supplementary motor area connectivity in psychosis35. Moreover, previous MRI spectroscopy studies have reported higher glutamate levels in the anterior cingulate region (albeit a more anterior portion than our findings) in established treatment-resistant cases36,37, in cases resistant to clozapine38, and in those with a poor response at first-episode39. A potential explanation for why these baseline and longitudinal changes were not observed in our analyses comparing the two patient subgroups is that these markers of treatment resistance may exist along a continuum among individuals with psychosis, including those who respond to treatment. In that case, a larger sample would be needed to find significant differences.
The strengths of our study include having a larger sample size than previous reports of striatocortical connectivity in psychosis. Many cross-sectional case-report studies have been published in the field of psychosis. In contrast, our study is one of the relatively few with information about longer-term clinical outcomes of participants, with more than half of the participants reassessed and re-scanned at follow ups. Moreover, the recruited patients had minimal antipsychotic treatment at baseline. This design provided insights into the dynamic processes underlying early psychosis, and it allowed us to model subgroups of patients according to treatment response trajectories. A limitation in our study comes from the method used to identify subgroups of patients. We mainly defined treatment-resistant cases based on a highly specific but low-sensitivity criterion such as being started on clozapine14. However, to increase sensitivity, 60.7% of those who were never prescribed clozapine were clinically assessed and evaluated according to TRRIP criteria2. That left 39.3% of patients allocated to the treatment-responsive group only because they were not started on clozapine, which is likely to include a few patients who were treatment-resistant but had not started clozapine. If we assume the same rate of people with treatment-resistant psychosis who had never started clozapine that we encountered in those who were reassessed clinically, the impact of such misclassification would be low, relating to probably two misclassified participants. Moreover, such possible mislabeling would dilute any existing differences between the two groups, and therefore, if anything, would reduce the power of our analysis to find statistically significant differences. Another known limitation in our study is that we modeled medications based on chlorpromazine equivalents, as has been done previously in other longitudinal analyses examining changes in patients exposed to different treatments40. As such, our model did not consider the variations existing between different antipsychotics. Particularly, since most of our treatment-resistant sample comes from clozapine users, we cannot differentiate the effects of this particular medication on brain connectivity. Of note, a recent study found that clozapine treatment efficacy was associated with increased functional connectivity between the dorsal caudate and prefrontal regions within the frontoparietal network41.
In conclusion, our results show that treatment-resistant cases are a subgroup of patients with psychosis that present longitudinal changes in ventral striatal connectivity with cortical temporal regions. Since no baseline differences emerged between patient subgroups, leveraging striatocortical connectivity to predict progression to treatment resistance will require repeated functional MRI assessments during the early stages of the disease. However, changes in other systems in the brain, or even smaller effects in these same circuits, could still be present since the first episode of psychosis and should still be explored as potential earlier biomarkers of prognosis.
Data availability
The data used in this study are not publicly available, due to participants’ privacy, but the authors would consider requests for collaborative analyses on a case-by-case basis. Interested researchers may contact the corresponding author to discuss potential collaborations and access to the data, subject to institutional and ethical approvals.
Code availability
The custom code used to analyze data in this study is publicly available on GitHub https://github.com/angietep/Striatocortical-connectivity-FEPtrt/.
References
Siskind, D. et al. Rates of treatment-resistant schizophrenia from first-episode cohorts: systematic review and meta-analysis. Br. J. Psychiatry 220, 115–120 (2022).
Howes, O. D. et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) Working Group consensus guidelines on diagnosis and terminology. Am. J. Psychiatry 174, 216–229 (2017).
Iruretagoyena, B. et al. Predictors of clozapine discontinuation at 2 years in treatment-resistant schizophrenia. Schizophr. Res. 235, 102–108 (2021).
Fornito, A. et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry 70, 1143–1151, https://doi.org/10.1001/jamapsychiatry.2013.1976 (2013).
Di Martino, A. et al. Functional connectivity of human striatum: a resting state fMRI study. Cerebral Cortex 18, 2735–2747, https://doi.org/10.1093/cercor/bhn041 (2008).
Dandash, O. et al. Selective augmentation of striatal functional connectivity following NMDA receptor antagonism: Implications for psychosis. Neuropsychopharmacology 40, 622–631, https://doi.org/10.1038/npp.2014.210 (2015).
Tepper, Á. et al. Functional Dysconnectivity in Ventral Striatocortical Systems in 22q11. 2 Deletion Syndrome. Schizophr. Bull. 48, 485–494 (2022).
Sarpal, D. K. et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 72, 5–13 (2015).
Potkin, S. G. et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr 6, 1 (2020).
White, T. P. et al. Dysfunctional Striatal Systems in Treatment-Resistant Schizophrenia. Neuropsychopharmacology 41, 1274–1285, https://doi.org/10.1038/npp.2015.277 (2016).
Sarpal, D. K. et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am. J. Psychiatry 173, 69–77 (2016).
Barber, A. D. et al. Age-normative pathways of striatal connectivity related to clinical symptoms in the general population. Biol. Psychiatry 85, 966–976, https://doi.org/10.1016/j.biopsych.2019.01.024 (2019).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. In: J. Clin. Psychiatry. pp 22–33 (1998).s
Ajnakina, O. et al. Validation of an algorithm-based definition of treatment resistance in patients with schizophrenia. Schizophr. Res. 197, 294–297 (2018).
Miller, T. J. et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 29, 703–715 (2003).
Kay, S. R., Fiszbein, A., Vital-Herne M & Fuentes, L. S. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. 13, 510-517 (1967).
Leucht, S., Samara, M., Heres, S. & Davis, J. M. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr. Bull. 42, S90–S94 (2016).
Parkes, L., Fulcher, B., Yücel, M. & Fornito, A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage 171, 415–436 (2018).
Ramirez-Mahaluf, J. P. et al. Dysconnectivity in schizophrenia revisited: abnormal temporal organization of dynamic functional connectivity in patients with a first episode of psychosis. Schizophr. Bull. 49, 706–716 (2023).
Tepper, Á. et al. Intra and inter-individual variability in functional connectomes of patients with First Episode of Psychosis. Neuroimage Clin 38, 103391 (2023).
Pruim, R. H. R. et al. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112, 267–277 (2015).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Cox, R. W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173, https://doi.org/10.1006/cbmr.1996.0014 (1996).
Zugman, A. et al. Reduced dorso-lateral prefrontal cortex in treatment resistant schizophrenia. Schizophr. Res. 148, 81–86 (2013).
Barry, E. F. et al. Mapping cortical surface features in treatment resistant schizophrenia with in vivo structural MRI. Psychiatry Res 274, 335–344, https://doi.org/10.1016/j.psychres.2019.02.028 (2019).
Otani, V. H. O., Shiozawa, P., Cordeiro, Q. & Uchida, R. R. A systematic review and meta-analysis of the use of repetitive transcranial magnetic stimulation for auditory hallucinations treatment in refractory schizophrenic patients. Int. J. Psychiatry Clin. Pract. 19, 228–232, https://doi.org/10.3109/13651501.2014.980830 (2015).
McCutcheon, R., Beck, K., Jauhar, S. & Howes, O. D. Defining the locus of dopaminergic dysfunction in schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr. Bull. 44, 1301–1311 (2018).
Demjaha, A., Murray, R. M., McGuire, P. K., Kapur, S. & Howes, O. D. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am. J. Psychiatry 169, 1203–1210 (2012).
Kim, E. et al. Presynaptic dopamine capacity in patients with treatment resistant schizophrenia taking clozapine: an [18F]DOPA PET study. Neuropsychopharmacology: 1–36 (2016).
Sheitman, B. B. & Lieberman, J. A. The natural history and pathophysiology of treatment resistant schizophrenia. J. Psychiatr. Res. 32, 143–150 (1998).
Oosthuizen, P., Emsley, R., Turner, H. J. & Keyter, N. A randomized, controlled comparison of the efficacy and tolerability of low and high doses of haloperidol in the treatment of first-episode psychosis. Int. J. Neuropsychopharmacol. 7, 125–131, https://doi.org/10.1017/S1461145704004262 (2004).
Skouras, S. et al. Aberrant connectivity in the hippocampus, bilateral insula and temporal poles precedes treatment resistance in first-episode psychosis: a prospective resting-state functional magnetic resonance imaging study with connectivity concordance mapping. Brain Commun. 6, fcae094 (2024).
Sone, D. et al. Disease progression patterns of brain morphology in schizophrenia: more progressed stages in treatment resistance. Schizophr. Bull. 50, 393–402, https://doi.org/10.1093/schbul/sbad164 (2024).
Cassidy, C. M. et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc. Natl. Acad. Sci. USA 116, 5108–5117 (2019).
Choi, S. et al. Fronto-striato-thalamic circuit connectivity and neuromelanin in schizophrenia: an fMRI and neuromelanin-MRI study. Schizophrenia 9, 81, https://doi.org/10.1038/s41537-023-00410-8 (2023).
Mouchlianitis, E. et al. Treatment-resistant schizophrenia patients show elevated anterior cingulate cortex glutamate compared to treatment-responsive. Schizophr Bull 42, 744–752, https://doi.org/10.1093/schbul/sbv151 (2016).
Egerton, A. et al. Dopamine and Glutamate in Antipsychotic-Responsive Compared with Antipsychotic-Nonresponsive Psychosis: A Multicenter Positron Emission Tomography and Magnetic Resonance Spectroscopy Study (STRATA). Schizophr Bull 47, 505–516 (2021).
Iwata, Y. et al. Glutamatergic neurometabolite levels in patients with ultra-treatment-resistant schizophrenia: a cross-sectional 3T proton magnetic resonance spectroscopy study. Biol. Psychiatry 85, 596–605 (2019).
Egerton, A. et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre 1 H-MRS study (OPTiMiSE). Mol. Psychiatry 23, 2145–2155 (2018).
Ho, B. C., Andreasen, N. C., Ziebell, S., Pierson, R. & Magnotta, V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry 68, 128–137 (2011).
Blazer, A. et al. Changes in corticostriatal connectivity and striatal tissue iron associated with efficacy of clozapine for treatment-resistant schizophrenia. Psychopharmacology (Berl) 239, 2503–2514, https://doi.org/10.1007/s00213-022-06138-0 (2022).
Acknowledgements
This work was funded by Agencia Nacional de Investigación y Desarrollo ANID Chile through FONDECYT regular 1240426 and 1200601 (to N.A.C.), and Anillo ACT1414 and ACT192064. The funding body had no role in the design of the study, collection and analysis of data and decision to publish.
Author information
Authors and Affiliations
Contributions
Conception and design (A.T., N.A.C.), acquisition of the data (J.V., C.D., J.P.R.-M., J.A., D.B., J.U., R.N., C.M., A.G.-V., N.A.C.), analysis (A.T., N.A.C.), interpretation of data for the work (A.T., D.A., R.A.M., P.M., A.G.-V., N.A.C.). All authors revised critically the manuscript and approved the final submission. N.A.C. and A.T. had full access to the data and are in agreement to be accountable for all aspects of this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tepper, A., Vásquez, J., Díaz Dellarossa, C. et al. Longitudinal changes in striatocortical connectivity in first-episode psychosis associated with the emergence of treatment resistance. Schizophr 11, 114 (2025). https://doi.org/10.1038/s41537-025-00653-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41537-025-00653-7