Abstract
The study explores the effect of pre-fermentation heat treatment (PFHT) on the flavor and metabolomic profiles of kiwi wine (KW) derived from three kiwifruit cultivars. Six KW groups were involved, namely with/without PFHT for green (GWH/GW), yellow (YWH/YW), and red (RWH/RW) kiwifruit. E-tongue analysis effectively distinguished the taste profiles across these KW groups, identifying significant variations. A total of 97 volatile components were characterized using GC-MS and GC-IMS, 12 of them were identified as key volatile compounds based on a combination of t-tests (p < 0.05) and variable importance in projection (VIP) scores. GC-MS and GC-IMS results demonstrated that PFHT significantly altered volatile profiles, specifically decreasing ester content while increasing aldehyde levels in comparison to untreated samples. Furthermore, 71 non-volatile compounds were identified by 1H-NMR, with 10 key metabolites (p < 0.05, VIP > 1) contributing to the observed differences. PFHT notably influenced metabolomic profiles, particularly in carbohydrate and organic acid levels, displaying cultivar-specific differences. Green kiwifruit-derived KW showed the most pronounced sensitivity to PFHT, as reflected in both flavor and metabolic profiles. These findings offer valuable insights for optimizing KW production processes and scaling up industrial production.
Similar content being viewed by others
Introduction
Kiwifruit, originating from Yangtze River Valley of China1, stands as a highly nutritious fruit with a distinctive flavor, cultivated commercially across various nations. As the foremost producer of kiwifruit, China has witnessed a significant surge in kiwifruit production in recent years, resulting in an oversupply of fresh kiwifruit2. Consequently, processed kiwifruit products such as vinegar, jam, preserves, and wine, have gained prominence. Kiwi wine (KW), a fermented beverage crafted from kiwifruit, stands out among various kiwi products for its elevated polyphenols and vitamin C contents3. Developing innovative production technologies for KW is key to improving its quality and in turn increasing its popularity among consumers.
Pre-fermentation heat treatment (PFHT) was first applied to red wine production in France, consisting in a heat treatment of the grapes before fermentation for durations ranging from 30 min to 24 h4. This treatment facilitates the extraction of phenolic compounds (anthocyanins and tannins) and polysaccharides from grapes, contributing to the wine’s fullness5,6, which in turn influences the color, phenolic composition and volatile composition of the resulting red wine7. Particularly, PFHT significantly enhanced the formation of ethyl esters, acetates, and fatty acids in red wine4, indicating a notable modification in the wine’s flavor profile. Therefore, PFHT holds potential as a production technology for KW to improve its quality, particularly its flavor.
Flavor, encompassing taste, aroma, and chemical composition, is a pivotal characteristic that aptly mirrors the quality of wine. The aroma profile of wine consists of various elements including esters, aldehydes, alcohols, phenols, acids, ketones, and terpenes, which are widely acknowledged as the primary contributors to the delightful aroma of wine8. Given the complexity of wine flavor, employing multiple analytical techniques is essential to obtain a comprehensive view of its flavor profile9. Moreover, non-volatile metabolites within the wine matrix can influence organoleptic qualities by interacting with volatile compounds10. Therefore, investigating the flavor profile of kiwi wine requires a comprehensive approach that considers both volatile and non-volatile compounds.
To the best of our knowledge, while the application of PFHT has been extensively studied in red wine, its application and impact of PFHT on the quality of KW, particularly its flavor and metabolomic profiles, represent a novel exploration in this field. To achieve a thorough understanding of the flavor and metabolomic profiles of KW made from green, yellow, and red kiwifruits induced by PFHT, intelligent sensors (E-tongue), multi-Omics approaches (GC-MS, GC-IMS, 1H-NMR) and multivariate data analysis were employed. This study could provide valuable insights into the intricate influence of PFHT on the flavor attributes and metabolomic changes of KW, offering guidance for optimizing KW processing and supporting KW industry-scale production.
Results
E-tongue analysis
The physicochemical properties of KW samples were quantitatively analyzed, with detailed results presented in Supplementary Table 1. To comprehensively investigate the flavor characteristics, an E-tongue analysis was initially employed to compare the taste differences among KW produced from green, yellow, and red kiwifruits, with and without pre-fermentation heat treatment (PFHT). As shown in Fig. 1a, the response values of SCS, which is sensitive to bitterness, were relatively high across all samples. Following PFHT, the response values of AHS, associated with sourness, decreased compared to untreated samples.
Radar chart (a) and an rPCA model based on the response values of E-tongue sensors showing significant differences between KW with/without PFHT for green (GWH/GW), yellow (YWH/YW), and red (RWH/RW) kiwifruit. In score plot (b), a wide, empty circle represents the median for each group, with superscript capital and lowercase letters indicating the significance of the sample along PC 1 and PC 2, respectively. The correlations between response values and their significance along PC 1 is depicted in the loading plots (c). Dark gray bars indicate significant correlations (p < 0.05).
To evaluate the overall taste differences among the three types of KW samples with/without PFHT, an rPCA was performed on the dataset. As depicted in Fig. 1b, PC1 represented 83.3% of the variance, effectively capturing the comprehensive information of the samples. This suggests a successful differentiation among the six groups of KW based on PC1. Significant differences (p < 0.05) were observed among unheated KW samples (RW, GW, YW) both along PC1 and PC2. Additionally, comparison between heated and unheated samples within the same kiwifruit cultivar revealed significant differences (p < 0.05) along PC1 across all cultivars. Notably, PC1 scores increased uniformly for all KW samples following PFHT, with the most pronounced increase observed in green KW. In detail, KW treated with PFHT exhibited higher response values for CTS (saltiness), PKS, CPS, and NMS (umami), and lower response values for AHS (sourness), compared to their untreated counterparts, as shown in Fig. 1c.
GC–MS analysis
To explore the flavor profile of KW, GC-MS was initially utilized to analyze the volatile compounds present in KW samples. A total of 59 VOCs were identified through GC-MS analysis. However, the number of VOCs varied among the six groups, with 36, 45, 34, 39, 35, and 35 volatile compounds identified in GW, GWH, RW, RWH,YW, and YWH, respectively. These identified volatiles comprised 18 esters, 7 alcohols, 10 aldehydes, 8 acids, 2 ketones, and 14 miscellaneous compounds, as listed in Supplementary Table 2, and their concentrations listed in Supplementary Table 3.
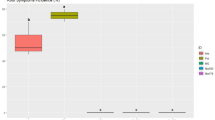
Figure 2a, b present the numbers and proportions of volatile compound categories across the six KW groups. Heated samples exhibited a higher number and proportion of aldehydes compared to unheated samples, whereas the proportion of esters was lower in heated samples. To elucidate the impact of PFHT on the volatile compounds in KW, PCA models were constructed using compounds exhibiting significant differences in binary comparisons between KW with and without PFHT, shown in Supplementary Fig. 1. The PCA model demonstrated clear separation among different types of KW from all considered cultivars. PLS-DA models further exhibited robust predictive capability, and the variable important in projection (VIP) values were obtained for detailed analysis. The VIP plot of PLS-DA was employed to assess the contribution of individual variables to the classification11. As depicted in Fig. 2, cineole, ethyl caprate, and ethyl tetradecanoate, with VIP values greater than 1 in KW from three cultivars, were identified as key differential compounds affected by PFHT.
The number (a) and relative amount (b) of VOCs from different categories identified by GC–MS in KW without (RW, YW, GW) and with (RWH, YWH, GWH) PFHT. The labels of volatile compounds are listed in Supplementary Table 1. PLS-DA based on volatile compounds concentrations for KW with/without PFHT produced by green flesh (c), yellow flesh (e), and red flesh (g) kiwifruits. VIP scores (d, f, h) illustrating the key volatile compounds contributing to the significant differences between KW with and without PFHT across all three kiwifruit cultivars. Two bars closed to VIP scores indicate the concentration of molecules between the two groups.
GC-IMS analysis
To comprehensively understand alterations in the flavor profiles of KW fermented from three kiwifruit cultivars induced by PFHT, GC-IMS was additionally employed to detect VOCs in KW samples. A total of 38 VOCs were jointly identified across six samples, including 10 alcohols, 6 aldehydes, 18 esters, 2 ketones, 1 acid and 1 miscellaneous compound, as listed in Supplementary Table 4. Additionally, single volatile compounds such as butyl isovalerate, isoamyl acetate, ethyl acetate, ethyl hexanoate, ethyl caprylate, butyraldehyde, 1-hexanol, 3-methyl-1-butanol, and pentanol exhibited multiple spots or signals. These were detected as monomers (M) and dimers (D) in the fingerprint region, displaying varying concentrations.
Figure 3 summarizes the processing pipeline of GC-IMS data for the VOCs in KW fermented from three cultivars, with/without PFHT. As presented in Fig. 3a, the fingerprint analysis revealed a notable distinction among the six groups of KW samples, with varying signal intensities for each group. The corresponding heatmap (Supplementary Fig. 2) corroborated these findings, further illustrating the main trend and cluster analysis in the VOCs of six groups of KW. Notable differences in peak intensities were observed when comparing VOCs in KW from different cultivars. Comparison of the VOCs in KW produced from the same cultivar revealed significant variations in peak levels between PFHT treated and those untreated samples. The greatest Euclidean distance between treated and untreated samples was observed in KW produced from green kiwifruit. Moreover, as depicted in Fig. 3b, PFHT treatment resulted in a higher proportion of acids and a lower proportion of alcohols compared to unheated samples across three types of KW.
Fingerprint plot (a) and bar chart (b) representing the variation of volatile compounds concentrations for each group. PLS-DA based on volatile compounds concentrations for KW with/without PFHT produced by green flesh (c), yellow flesh (e), and red flesh (g) kiwifruits. VIP scores (d, f, h) of PLA-DA. Venn plot (i) of the number of compounds related types in KW by three cultivars. The key volatile compounds in KW (j).
To effectively highlight and differentiate the variations among KW samples subjected with and without PFHT, PCA models were applied to the VOCs detected by GC-IMS for KW from three kiwifruit cultivars, shown in Supplementary Fig. 3. The cumulative variance explained by PC1 and PC2 was 93.2%, 81.7%, and 90.9% for KW made from green, red, and yellow kiwifruits, respectively, indicating a robust summarization of the overall sample characteristics. PCA score plots shows clear separations between the clusters of unheated and heated KW samples fermented from the same cultivar. To further identify key volatile compounds, PLS-DA models were utilized, as shown in Fig. 3c–h. Based on the GC-IMS data, nine key differential compounds were identified across the three types of kiwifruit wines, each exhibiting VIP values greater than 1 and p-values below 0.05, as shown in Fig. 3i. These compounds included ethyl hexanoate-D, ethyl butanoate, ethyl isobutyrate, hexanal, isoamyl acetate-D, isobutyraldehyde, pentan-2-one, propyl acetate, and tetrahydrofuran, as shown in Fig. 3j.
Metabolomic analysis
In this study, 1H-NMR was applied to evaluate the impact of PFHT on the metabolomic profiles of KW samples. A total of 71 metabolites were identified and quantified (Supplementary Table 5), encompassing amino acids and amines (17), alcohols (7), carbohydrates (7), nucleotides (2), organic acids (20), and other classes of compounds (18).
To elucidate the changes in the metabolome composition, an UpSet plot (Fig. 4a) was generated to visualize the distribution of unique metabolites across the six groups of samples. A total of 44 molecules were detected in all groups using 1H-NMR. It is noteworthy that two metabolites were exclusively detected in RW and RWH, while four metabolites were only detected in GW and GWH. Additionally, certain metabolites were absent in KW samples with PFHT.
UpSet plot (a) of metabolites in KW with/without PFHT produced by green (GWH/ GW), yellow (YWH/YW), and red (RWH/RW) kiwifruit. Proportion (b) of metabolites of different categories in the six groups of KW. AL, alcohols; OC organic acids, CA carbohydrates, AA amino acids, NL nucleotides, ML miscellaneous. PLS-DA based on volatile compounds concentrations for KW with/without PFHT produced by green flesh (c), yellow flesh (e), and red flesh (g) kiwifruits. VIP scores (d, f, h) of PLA-DA.
Figure 4b presents the proportions of metabolite categories across the six groups, with alcohols exhibiting the highest relative abundance. A comparative analysis between KW samples with and without PFHT from the same cultivar revealed various changes. In particular, compared with GW, the proportion of alcohols in GWH increased from 71.2% to 87.6%, while carbohydrates decreased from 21.6% to 4.1%. An opposite trend in alcohols and carbohydrates proportions was observed in KW produced from red and yellow kiwifruit.
To effectively illustrate and distinguish the differences in the nonvolatile profiles among KW treated with/without PFHT, PCA models were calculated based on their concentrations in KW by the three cultivars, as depicted in Supplementary Fig. 4. In PCA models, clear separations between the clusters of unheated and heated KW samples fermented from the same cultivar. Furthermore, as in the case of non-volatile compounds, t-tests and VIP values were utilized to screen for significant differential compounds, as shown in Fig. 4c–h. 10 non-volatile compounds were identified as significantly different based on VIP > 1 and p < 0.05 across all three types of KW, including alcohols and polyols (1), amino acids, peptides, and analogues (3), organic acids and derivatives (4), and others (2).
Correlation between E-tongue and key non-volatile compounds
To investigate the correlations between specific compounds and taste attributes, as well as between non-volatile compounds, canonical correspondence analysis (CCA) and Mantel tests were conducted using the E-tongue response values and the key non-volatile compounds identified by ¹H-NMR, as shown in Fig. 5. The CCA results demonstrated that 54.33% and 11.25% of the variation in the KW metabolic profile could be explained by the CCA1 and CCA2 axes, respectively. The red arrows in the CCA biplot represent the contribution of E-tongue sensors. Notably, the SCS sensor, which is sensitive to bitterness, was the most influential across all KW samples, as shown in Fig. 5a. The relationship between key metabolites and E-tongue sensor responses can be illustrated by the vertical projection of metabolite points onto the sensor arrows. The coordinate positions of these projections reflect the positive or negative correlations between metabolites and sensor responses. For example, the SCS sensor exhibited a positive correlation with most key metabolites.
Canonical correspondence analysis (CCA) (a) and Mantel tests (b) of key non-volatile compounds quantified by 1H-NMR and E-tongue sensor responses. A negative and positive correlation between key non-volatile compounds was indicated by the blue and red colors in the upper triangular heat map. The color and type of lines indicate the r and p value of Mantel correlations between molecules’ levels and E-tongue sensor responses, respectively.
As illustrated in Fig. 5b, the SCS sensor showed a significant (p < 0.05) positive correlation with the majority of non-volatile compounds, highlighting the complexity of the taste profile in KW. Additionally, quinic acid and 2-hydroxyisobutyrate exhibited a significant correlation with the AHS sensor, which is particularly responsive to sourness.
Discussion
In recent years, there has been an increasing demand for newly developed low-alcohol fruit wines, driven by the promotion of healthier dietary choices12. KW, a fermented fruit wine derived from kiwifruit13, aligns with this trend due to its low alcohol content, making it an attractive option for consumers seeking wines with reduced alcohol content14. However, the immature technology leading to insufficient flavor and unsatisfactory sensory attributes continues to hinder the development of this fruit wine.
PFHT, a technique adapted from grape wine production, has demonstrated potential in enhancing wine flavors, particularly those linked to ethyl esters, acetates, and fatty acids4. Its application in KW production is particularly intriguing, especially regarding its impact on KW flavor and metabolomic profiles, which profoundly influences consumer perception and acceptance of wine. To attain a thorough understanding, the study aims to investigate the impact of PFHT on the flavor and metabolomic profiles of KW derived from various cultivars using a combination of analytical techniques, including GC-MS, GC-IMS, 1H-NMR, and E-tongue.
KW, renowned for its intricate aroma profile, contains a diverse array of VOCs, predominantly comprising alcohols, aldehydes, ketones, acids, esters and phenols15,16. The VOCs of KW originate from several sources, including the kiwifruit itself, yeast and bacterial metabolism, chemical reactions during storage, and enzymatic or non-volatile precursors during kiwifruit processing, winemaking, and/ or storage17. In this study, we identified a total of 97 VOCs combining GC-MS and GC-IMS. The primary disparity in VOCs among the six groups of KW lies in the variation of kiwifruit cultivars and the impact of PFHT, as evidenced by cluster analysis. Univariate and multivariate analyses based on GC-MS and GC-IMS showed that PFHT significantly influences the volatile composition of KW samples, resulting in major changes in the levels of aldehydes, alcohols, esters, and organic acids.
Aldehydes, significant contributors to the flavor profile of KW, can be produced via oxidation during sample preparation and fermentation processes18. Despite the low proportion of aldehydes detected in this study, considering their low flavor threshold and unique fatty aromas19, aldehydes constitute an important class of aroma compounds in KW. In addition, PFHT resulted in a higher proportion of aldehydes in KW, which might due to the oxidation of kiwifruit during heating and reduced production of alcohols by yeast metabolism of aldehydes. The high relative content of isobutyraldehyde in KW treated with PFHT could contribute to a slightly nutty flavor compared to the untreated groups20, along with low relative content of its correlated alcohol, isobutanol. In addition, a higher relative content of valeraldehyde in KW treated with PFHT could provide a stronger fruity and nutty note.
During wine fermentation, alcohols are primarily produced through yeast metabolism, playing a pivotal role in shaping wine aroma and complementing esters21. Higher alcohols, a group of aromatic compounds consisting of volatile molecules with more than two carbon atoms, are widely recognized as having a significant impact on wine aroma22. The concentrations of higher alcohols in wine primarily rely on yeast metabolism, wine type and chemical composition. Consistent with our study, Huang et al.23 found that the composition of alcohols in KW fermented from seven kiwifruit cultivars varied. Moreover, the effect of PFHT on alcohols levels in KW derived from three cultivars varied. For example, KW treated with PFHT contained a lower level of isobutanol, which mainly imparted a pungent odor to the kiwifruit wine24. However, higher contents of 3-hexen-1-ol and 1-hexanol were present in KW with PFHT, contributing to a pleasant flavor of wine25. The higher levels of 3-hexen-1-ol and 1-hexanol in GWH and YWH could be attributed to the fact that the high temperatures altered the chemical composition of kiwifruit juice, which in turn affected the alcoholic metabolism of yeast and the enzymic activities. It is essential to note that higher concentrations of higher alcohols do not automatically lead to a superior aroma profile in wine. When present at total concentrations below 300 mg/L, these compounds primarily enhance the overall complexity of wine aroma. Conversely, total higher alcohol concentrations exceeding 400 mg/L are believed to induce unpleasant sensory attributes that may overpower the wine’s aroma26.
Esters represent a pivotal class of aroma compounds, contributing substantially to the fruity aroma characteristic of wines and various other fermented beverages27. In this study, esters were the prevalent compounds identified by GC-MS and GC-IMS in KW, consistent with previous works21,28,29. Differences in the esters composition of KW derived from various kiwifruit cultivars were evident, in agreement with Huang et al.23. Focusing on KW made from green kiwifruits, ethyl nonanoate and methyl octylate emerge as primary esters, ethyl hexanoate and ethyl caprate dominate in KW made from red kiwifruit, while KW made from yellow kiwifruit exhibits higher levels of ethyl hexanoate and hexyl acetate. Additionally, isoamyl acetate-D, ethyl caprate, ethyl tetradecanoate, ethyl hexanoate-D, ethyl butanoate, and ethyl isobutyrate are identified as key esters across all kiwifruit varieties. These esters play a pivotal role in distinguishing volatile profiles among different groups, reflecting variations in processing methods.
The application of PFHT results in a relative decrease in the proportion of ester across all three kiwifruit wines, notably by 50% in both green and yellow kiwifruit fermentations. The reduction in relative content of esters might be attributed to the loss of some original esters, such as ethyl acetate and isoamyl acetate, during PFHT treatment20. The reduced formation of some esters, such as ethyl nonanoate and methyl octylate, via yeast alcohol metabolism. However, the effect of PFHT on the esters in KW is complex because of the various sources of esters in KW. In contrast to our findings, Geffroy et al.4 observed an increase in ester content in wine following PFHT. Apart from kiwifruit cultivars and PFHT, several other factors, such as yeast strains15,28 and fermentation methods30, significantly influence the final content of esters in KW.
Flavor of wine arises from a combination of odor and taste molecules, both volatile and nonvolatile31. Concerning nonvolatile composition, it is important in resulting wine flavor and primarily responsible for taste and tactile sensations, creating the psychological-sensory foundation of flavor on which the volatile build. 1H-NMR is perfectly tailored to the identification of nonvolatile compounds in wine, as its underlying physical principles render it inherently quantitative, irrespective of the molecular chemistry involved16. PFHT exerts a significant effect on the metabolic profile of KW samples, leading to alterations in the levels of amino acids, organic acids, and carbohydrates, contributing to specific taste characteristics of KW. These findings agreed with E-tongue analysis, showing that KW treated with PFHT exhibited the higher response values of CTS and NMS, which are mainly linked to saltiness and umami, respectively, along with a lower response value of AHS, lined to sourness.
Amino acids have been extensively identified in KW in previous studies16,32. In this study, 14 amino acids were detected in KW using 1H-NMR, contributing to the bitterness, sweetness, and/ or umami taste of food33. However, since all amino acid concentrations were below their sensory thresholds, it was challenging to correlate perceived flavors with changes in amino acid content. Notably, some of these amino acids are aromatic, such as leucine, valine, isoleucine, phenylalanine, methionine, and tyrosine, serving as precursors for most VOCs during wine fermentation31, and as a nitrogen source for yeast growth and reproduction34. PLS-DA models showed that the higher concentrations of alanine, phenylalanine, and isoleucine presented in KW treated with PFHT across three types of KW. Agree with our findings, Geffroy et al.4 reported a two-hour heat treatment at 70 °C induced a significant increase in amino acid in red wine. The increase in amino acids might also have contributed to improved yields of the related aldehyde, higher alcohol, and volatile fatty acid31. During wine fermentation, valine could be converted to isobutyraldehyde, isobutanol, and isobutyric aicd via the Ehrlich pathway31.
Sugars, as primary energy sources for the growth and reproduction of yeast, are directly related to the sensory of wines. In this study, seven saccharides were characterized using 1H-NMR, with arabinose, fructose, fucose, and galactose detected in all KW samples. Consistent with the findings by Huang et al.13, the total sugar content varied among KW produced from different kiwifruit cultivars. The differences observed in the composition and content of sugars in the three types of KW treated with/without PFHT may be attributed to the varying fermentation substrates’ impact on the metabolic activity of yeasts and to the distinct sugar composition of kiwifruit. The concentration of glucose was the highest in KW derived from green kiwifruit, reaching 95.1 mmol/L, while it significantly decreased to 5.73 mmol/L in the KW subjected to PFHT (GWH) compared to GW. Consistent with the increase in alcohol contents of GWH, this decrease in carbohydrate content may be ascribed to the effect of PFHT on the glycolysis/gluconeogensis of yeast, leading to more alcohols production from carbohydrates.
Organic acids, important contributors to the sourness of wine, can originate from multiple sources, including yeast-mediated fatty acid metabolism and the tricarboxylic acid cycle (TCA cycle) during fermentation, as well as from the kiwifruit itself13. In this study, the predominant acids in all kiwi wines were quinic acid, malate, succinate, acetate, and galatonate, which aligns with the previous reports13,35. PFHT could alter these organic contents in KW, with changes varying depending on kiwifruit cultivars. Specifically, quinic acid, the main organic acid in kiwifruit, has a lower threshold to sour taste than citric acid or malic acid36, and has been suggested to be a precursor of chlorogenic acid and flavonoids37. Compare to the untreated KW, a significant increase (p < 0.05) on the levels of quinic acid was observed in KW treated with PFHT fermented from red kiwifruit, while a significant decrease (p < 0.05) was observed in KW treated with PFHT fermented from green kiwifruit and yellow kiwifruit. Interestingly, in KW produced from green kiwifruits, the concentrations of malate and fumarate in KW subjected to PFHT (GWH) were approximately 50% lower than those in unheated GW, while contrasting results were observed for acetate. Malic acid contributes to a strong and sharp taste sensation, although excessive levels can lead to a pungent taste38. The reduced concentration of malic acid may positively influence the taste of the wine. Acetate is one of the most important volatile acids produced by yeast, formed through the irreversible oxidation of acetaldehyde to acetic acid39. The disparity in acetate concentration among the different groups may be attributed to PFHT affecting yeast metabolism.
The present study provides a thorough understanding of the flavor and metabolomic profiles of KW derived from three kiwifruit cultivars, both with/without PFHT, utilizing a combination of E-tongue, GC-MS, GC-IMS, and 1H-NMR. The integration of these analytical techniques has demonstrated its effectiveness in providing a comprehensive flavor fingerprint, surpassing the capabilities of individual methods. E-tongue analyses further elucidate distinct flavor characteristics among different KW groups. A decrease in the proportion of ester and an increase in the proportion of aldehydes were observed in KW with PFHT, whereas modifications to the alcohols and acids induced by heating were complex and cultivar-dependent. 1H-NMR analysis revealed substantial modifications in carbohydrates and organic acids in KW resulting from the heating of kiwifruit pulp. PFHT induced varied effects on the flavor and metabolomic profiles of KW across cultivars, notably enhancing those derived from green kiwifruit more than others. This finding highlights PFHT’s potential to modify the flavor profile of kiwi wine made from green (XuXiang) kiwifruit. These insights are valuable for optimizing pretreatment methods in KW production and establishing protocols for processing and quality control. Further refinement of PFHT parameters, encompassing treatment durations, and temperatures, is necessary to optimize its efficacy in enhancing the quality of KW while minimizing any negative effects on taste and nutritional quality.
Methods
Materials and solvent
Three kiwifruit cultivars, Xuxiang (green flesh), Hongyang (red flesh), and Jinyan (yellow flesh), were harvested in September 2023, weighing about 100 grams each, from a plantation located in Pujiang, Sichuan, China. All fresh kiwifruits used in this study were highly mature40. Yeast (S. cerevisiae strain RW) was purchased from Angel Yeast CO., LTD (China). All chemicals used were of analytical grade.
KW samples
All kiwifruits collected for the study were promptly transported to the laboratory after being freshly harvested. The winemaking process was conducted on a laboratory scale, as depicted in Fig. 6. Briefly, GWH and GW represent the KW produced from green flesh kiwifruits with and without PFHT; YWH and YW represent the KW produced from yellow flesh kiwifruits with and without PFHT; RWH and RW represent the KW produced from red flesh kiwifruits with and without PFHT. Following fermentation, the KW samples were filtered through a triple layer of sterile gauze and stored at -80 °C until further analysis.
E-tongue analysis
An E-tongue (α-Astree; Alpha MOS, France) was employed in this study to assess the overall taste characteristic of KW samples. The taste analysis instrument consists primarily of two reference electrodes (PKS and CPS) and five liquid cross-sensitive electrodes (ANS, CTS, NMS, AHS, SCS), which respond to sweetness, saltiness, umami, sourness, and bitterness, respectively.
Following our previous study41, for each analysis, 80 mL of KW sample was transferred into a dedicated beaker designed for E-tongue analysis. Parameters including signal acquisition time, stirring rate, and analysis duration were configured to 120 s, 60 revolutions per min (rpm), and 3 min, respectively. After each analysis, the sensors underwent a thorough washing process with deionized water for 30 s. The output values were recorded between 100 and 120 s. To minimize errors, as suggested by ref. 42, each sample underwent eight replications, with five stable measurements chosen for subsequent data analysis.
GC-MS analysis
In accordance with our previous study43, five milliliters of KW samples were aliquoted into 20 mL headspace vials sealed with polyethylene and silicone septum caps. The samples were equilibrated at 50 °C for 30 min in a thermostatic bath. Subsequently, the headspace was accessed by piercing the septum with a needle containing a 50/30 µm DVB/CAR/PDMS fiber, which was then exposed to the headspace for an additional 30 min. The analytes extracted by the fiber were desorbed in the GC-MS system’s injection port without splitting, operating in Full-Scan mode ranging from 40 to 550 amu. Helium served as the carrier gas. Samples were separated on an HP-5 capillary column (30 m × 0.25 mm × 0.25 µm). The injector temperature was maintained at 230 °C, and the oven temperature was set to 40 °C for 3 min, ramping at 6 °C/min to 180 °C for 2 min, then increasing at 10 °C/min to 230 °C for 6 min. Volatile components were searched using the NIST 11 library and only molecular features exhibiting a matching degree exceeding 800 (with a maximum threshold of 1000) were considered for subsequent analysis. The relative abundance of each identified compound was quantified using an area normalization approach based on chromatographic peak integration.
GC-IMS analysis
GC-IMS analysis was conducted to determine the volatile organic compounds (VOCs) in all KW samples, following the methodology outlined by Zhang et al.16. The characterization of VOCs in KW samples was performed using GC-IMS (Flavorspec®, G.A.S. Instrument, Germany), equipped with an MXT-WAX capillary column (30 m × 0.53 mm × 1 μm) from Restek, United States. Briefly, each KW sample (1.5 mL) was introduced into a 20 mL headspace vial fitted with a magnetic screw-seal cover and incubated for 10 min at 60 °C. Subsequently, the sample was automatically injected into the injector (no split mode) using a heated syringe at 85 °C. The drift tube and column temperatures were maintained at 45 °C and 60 °C, respectively. The rate of drift gas flow was set to 150 mL/min. The GC column was programmed as follows: initial flow rate of 2 mL/min for 5 min, followed by an increase to 10 mL/min for 10 min, then to 15 mL/min for 5 min, further increased to 50 mL/min for 10 min, and finally to 100 mL/min for 10 min. Retention index (RI) calculations were performed using n-ketone C4-C9 as a reference compound. VOCs were identified by comparing their RI and ion drift times with those of standards in the GC-IMS library. Each sample underwent a single assay, and the relative quantification of each VOC was determined based on peak intensity. The Laboratory Analysis Viewer and Reporter provided by the GC-IMS instrument were utilized for three-dimensional (3D) topographic, two-dimensional (2D) discrepancy and fingerprint analyses.
1H-NMR analysis
The 1H-NMR analysis followed a previously established protocol41. Briefly, 0.5 mL of KW sample underwent centrifugation for 15 min at 18630 g and 4 °C. Subsequently, 0.35 mL of the supernatant, 0.35 mL of bi-distilled water, and 200 μL of 10 mM 3-(Trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt (TSP) were mixed and centrifuged under the same conditions. 1H-NMR spectra of the KW samples were acquired using a 600.13 MHz AVANCE III spectrometer (Bruker, Wuhan, China) at 298 K. The CPMG pulse sequence was employed with suppression of the solvent signal, and the following parameters were set: fid size: 32 k, number of dummy scans: 16, spectral width: 12 ppm, acquisition time: 2.28 s, delay d1: 5 s. The NMR spectra were pre-processed and adjusted using Topspin 3.1, with TSP serving as a reference for quantification and spectrum calibration.
For quantification, the concentration of each molecule was calculated based on the area of its corresponding signal, which was determined through global spectra deconvolution using MestReNova software (version 14.2.0-26256, Mestrelab Research S.L., Santiago De Compostela, Spain). A line broadening of 0.3 and baseline adjustment using the Whittaker Smoother procedure were applied during the analysis. Total ion counts (TIC) were utilized to quantify each molecule in every sample. Probabilistic quotient normalization (PQN) was employed to ensure proportionality between the area and concentration in each spectrum.
Statistical analysis
To achieve normality, data distribution was transformed using Box and Cox’s method44. Differences in metabolites between groups were assessed using t-test. For multivariate analysis, missing values exceeding 50% were excluded; remaining missing values were imputed with one-fifth of the minimum observed value, followed by PQN normalization and auto-scaling. Principal Component Analysis (PCA) and Partial Least Squares Discriminant Analysis (PLS-DA) were applied to explore metabolic trends using MetaboAnalyst 6.0 (https://www.metaboanalyst.ca). The performance of PLS-DA model is assessed by cross-validation method (5-fold cross-validation) and the method for performance measure (Q2). Compounds with a VIP value exceeding 1 are considered key differential compounds that contribute substantially to classification45. Robust PCA was proformed by R package, namely “rrcov”. Canonical Correspondence Analysis (CCA) and Mantel tests (Spearman correlation, mantel test) were performed using OmicStudio (https://www.omicstudio.cn/tool.) to investigate the correlations between taste attributes and non-volatile compounds.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Luh, B. S. & Wang, Z. Kiwifruit. Adv Food Res. 29, (1984).
Wang, B. & Peng, B. A feasibility study on monitoring residual sugar and alcohol strength in kiwi wine fermentation using a fiber-optic FT-NIR spectrometry and PLS regression. J. Food Sci. 82, 358–363 (2017).
Ma, T. et al. Comparison of the nutritional properties and biological activities of kiwifruit (Actinidia) and their different forms of products: towards making kiwifruit more nutritious and functional. Food Funct. 10, 1317–1329 (2019).
Geffroy, O. et al. Changes in analytical and volatile compositions of red wines induced by pre-fermentation heat treatment of grapes. Food Chem. 187, (2015).
Geffroy, O., Feilhès, C., Favarel, J.-L. & Lopez, R. Pre-fermentation heat treatment: a multitool technique to keep the pot boiling. IVES Technical Reviews, vine and wine https://doi.org/10.20870/ives-tr.2021.4779 (2021).
Wang, J., Huo, S., Zhang, Y., Liu, Y. & Fan, W. Effect of different pre-fermentation treatments on polyphenols, color, and volatile compounds of three wine varieties. Food Sci. Biotechnol. 25, 735–743 (2016).
Geffroy, O. et al. Modulating analytical characteristics of thermovinified Carignan musts and the volatile composition of the resulting wines through the heating temperature. Food Chem. 257, 7–14 (2018).
Tetik, M. A., Sevindik, O., Kelebek, H. & Selli, S. Screening of key odorants and anthocyanin compounds of cv. Okuzgozu (Vitis vinifera L.) red wines with a free run and pressed pomace using GC-MS-Olfactometry and LC-MS-MS. J. Mass Spectrometry 53, (2018).
Chen, L. et al. Effects of fermentation on flavor and antioxidant activity in ginkgo rice wine. Food Biosci. 53, (2023).
Sáenz-Navajas, M. P., Fernández-Zurbano, P. & Ferreira, V. Contribution of nonvolatile composition to wine flavor. Food Rev. Int. 28, 389–411 (2012).
Wang, S. et al. Investigating the effect of three phenolic fractions on the volatility of floral, fruity, and aged aromas by HS-SPME-GC-MS and NMR in model wine. Food Chem. X 13, 100281 (2022).
Mangindaan, D., Khoiruddin, K. & Wenten, I. G. Beverage dealcoholization processes: Past, present, and future. Trends Food Sci. 71, 36–45 (2018).
Huang, J. et al. Effect of inoculation method on the quality and nutritional characteristics of low-alcohol kiwi wine. LWT 156, (2022).
Li, S. et al. Enhancing antioxidant activity and fragrant profile of low-ethanol kiwi wine via sequential culture of indigenous Zygosaccharomyces rouxii and Saccharomyces cerevisiae. Food Biosci. 51, (2023).
Li, S. et al. Characterization of different non-Saccharomyces yeasts via mono-fermentation to produce polyphenol-enriched and fragrant kiwi wine. Food Microbiol. 103, 103867 (2022).
Zhang, Q. et al. Effects of S. cerevisiae strains on the sensory characteristics and flavor profile of kiwi wine based on E-tongue, GC-IMS and 1H-NMR. LWT 185, (2023).
Parker, M., Capone, D. L., Francis, I. L. & Herderich, M. J. Aroma precursors in grapes and wine: flavor release during wine production and consumption. J. Agric. Food Chem. 66, 2281–2286 (2018).
Vavoura, M. V., Badeka, A. V., Kontakos, S. & Kontominas, M. G. Characterization of four popular sweet cherry cultivars grown in greece by volatile compound and physicochemical data analysis and sensory evaluation. Molecules 20, 1922–1940 (2015).
Wang, F. et al. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography–ion mobility spectrometry (GC–IMS). Meat Sci. 175, (2021).
Lan, T. et al. Analysis of the aroma chemical composition of commonly planted Kiwifruit cultivars in China. Foods 10, 1645 (2021).
Lan, T. et al. Evaluation of the color and aroma characteristics of commercially available Chinese kiwi wines via intelligent sensory technologies and gas chromatography-mass spectrometry. Food Chem. X 15, (2022).
Ruiz, J. et al. Effects on varietal aromas during wine making: a review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 103, 7425–7450 (2019).
Huang, J. et al. Evaluation of the quality of fermented kiwi wines made from different kiwifruit cultivars. Food Biosci. 42, (2021).
Longo, R., Carew, A., Sawyer, S., Kemp, B. & Kerslake, F. A review on the aroma composition of Vitis vinifera L. Pinot noir wines: origins and influencing factors. Crit Rev Food Sci Nutr. 61, 1589–1604 (2021).
Yu, H., Xie, T., Xie, J., Ai, L. & Tian, H. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 293, (2019).
Rapp, A. & Mandery, H. Wine aroma. Experientia 42, (1986).
Lilly, M., Lambrechts, M. G. & Pretorius, I. S. Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl. Environ. Microbiol. 66, 744–753 (2000).
Li, S. et al. Effect of sequential fermentation with four non-Saccharomyces and Saccharomyces cerevisiae on nutritional characteristics and flavor profiles of kiwi wines. J. Food Composition and Anal. 109, (2022).
Zhou, Y. et al. Cultivar difference characterization of kiwifruit wines on phenolic profiles, volatiles and antioxidant activity. Food Chem. X 18, 100691 (2023).
Yiman, Q., Miaomiao, L., Kun, Y. & Mingtao, F. Effect of skin maceration treatment on aroma profiles of kiwi wines elaborated with actinidia deliciosa “xuxiang” and a. Chinensis “hort16A”. J. AOAC Int. 102, 683–685 (2019).
Styger, G., Prior, B. & Bauer, F. F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 38, 1145 (2011).
Liu, D. et al. Effect of glutathione-enriched inactive dry yeast on color, phenolic compounds, and antioxidant activity of kiwi wine. J. Food Process. Preserv. 44, (2020).
Delompré, T., Guichard, E., Briand, L. & Salles, C. Taste perception of nutrients found in nutritional supplements: a review. Nutrients 11, 2050 (2019).
Zhang, G., Chen, W., Chen, W. & Chen, H. Improving the quality of matured coconut (Cocos nucifera Linn.) water by low alcoholic fermentation with Saccharomyces cerevisiae: antioxidant and volatile profiles. J. Food Sci. Technol. 55, 964–976 (2018).
Towantakavanit, K., Park, Y. S. & Gorinstein, S. Bioactivity of wine prepared from ripened and over-ripened kiwifruit. Cent. Eur. J. Biol. 6, (2011).
Marsh, K. et al. The use of fruit pulps to explore flavour in kiwifruit. in Acta Horticulturae 610 (2003).
Koskimäki, J. J. et al. Flavonoid biosynthesis and degradation play a role in early defence responses of bilberry (Vaccinium myrtillus) against biotic stress. Eur. J. Plant Pathol. 125, (2009).
Zhang, H. et al. Determination of organic acids evolution during apple cider fermentation using an improved HPLC analysis method. Eur. Food Res. Technol. 227, (2008).
Peng, W., Meng, D., Yue, T., Wang, Z. & Gao, Z. Effect of the apple cultivar on cloudy apple juice fermented by a mixture of Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus fermentum. Food Chem. 340, 127922 (2021).
Gao, J., Liu, J. & Pang, X. Characterization of the color, physicochemical properties, organic acids, and aroma profiles of kiwifruit wines by different fermentation patterns and fining stages. LWT 199, 116097 (2024).
Zhu, C. et al. Effects of Saccharomyces cerevisiae strains on the metabolomic profiles of Guangan honey pear cider. LWT 182, (2023).
Zhao, W. et al. Comparing the differences of physicochemical properties and volatiles in semi-dry Hakka rice wine and traditional sweet rice wine via HPLC, GC–MS and E-tongue analysis. Food Chem. X 20, 100898 (2023).
Zhou, B. et al. Comparison of aroma and taste profiles of kiwi wine fermented with/without peel by combining intelligent sensory, gas chromatography-mass spectrometry, and proton nuclear magnetic resonance. Foods 13, 1729 (2024).
Box, G. E. P. & Cox, D. R. An analysis of transformations. J. R. Stat. Soc.: Ser. B (Methodological) 26, (1964).
Galindo-Prieto, B., Eriksson, L. & Trygg, J. Variable influence on projection (VIP) for OPLS models and its applicability in multivariate time series analysis. Chemomet. Intell. Lab. Syst. 146, (2015).
Acknowledgements
This work was supported by the Natural Science Foundation of Sichuan Province (Grant No. 2024NSFSC0364), the WeiZhou Team Funds, Southwest Minzu University (Grant No. SMUWZ202409) and Luzhou Laojiao Postdoctoral Research Program.
Author information
Authors and Affiliations
Contributions
Conceptualization: C.Z. Data Curation: C.Z., L.L. Formal Analysis: Q.L., Z.Y. Funding Acquisition: C.Z., Y.L. Methodology: C.Z., L.L. Writing-Original Draft Preparation: Q.L., C.Z., L.L. Writing-Review & Editing: Q.L., Z.Y., L.L., C.S., J.T., Y.L., Z.A., S.Z., X.D., C.Z., L.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lan, Q., Yang, Z., Lin, L. et al. Sensory and Omics approaches reveal the impact of heat treatment before fermentation on kiwi wine. npj Sci Food 9, 88 (2025). https://doi.org/10.1038/s41538-025-00438-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00438-3