Abstract
Cellular networks realize their functions by integrating intricate information embedded within local structures such as regulatory paths and feedback loops. However, the precise mechanisms of how local topologies determine global network dynamics and induce bifurcations remain unidentified. A critical step in unraveling the integration is to identify the governing principles, which underlie the mechanisms of information flow. Here, we develop the cumulative linearized approximation (CLA) algorithm to address this issue. Based on perturbation analysis and network decomposition, we theoretically demonstrate how perturbations affect the equilibrium variations through the integration of all regulatory paths and how stability of the equilibria is determined by distinct feedback loops. Two illustrative examples, i.e., a three-variable bistable system and a more intricate epithelial-mesenchymal transition (EMT) network, are chosen to validate the feasibility of this approach. These results establish a solid foundation for understanding information flow across cellular networks, highlighting the critical roles of local topologies in determining global network dynamics and the emergence of bifurcations within these networks. This work introduces a novel framework for investigating the general relationship between local topologies and global dynamics of cellular networks under perturbations.
Similar content being viewed by others
Introduction
Cellular networks are inherently characterised by tremendous complexity, including numerous interacting molecules, i.e., nodes, and intricate regulations, i.e., edges, between them1,2,3. Furthermore, these nodes and edges intertwine to form diverse paths and feedback loops4,5,6. Together, these nodes, edges, paths, and feedback loops constitute local structures of cellular networks, commonly referred to as local topologies7,8,9. Ultimately, it is the interplay of all these local topologies that determines the global network dynamics, e.g., the stability of equilibria and the occurrence of bifurcations10,11,12.
Most biological processes associated with cellular networks are intricately regulated by an array of exogenous and endogenous stimuli such as ligands, inhibitors, growth factors, and even drugs13,14,15,16, and these processes often exhibit multiple states. Slight variations in these exogenous or endogenous molecules may significantly influence the network dynamics, such as input-output relations and qualitative fate transitions induced by various bifurcations17,18,19. Furthermore, the critical state, as well as the critical transition can be effectively identified20,21,22. It is crucial to achieve insight into how biological processes react to perturbations and how such perturbations propagate within networks, ultimately resulting in cell fate transitions23,24. Fundamentally, an accurate understanding of the effects of these perturbations on cellular networks is crucial for predicting alterations in cell fates and achieving intentional control over these processes, ultimately contributing to the elucidation of network dynamics.
To achieve meaningful insights into the dynamics of a cellular network, relying solely on isolated information regarding individual local structures is insufficient. Actually, network dynamics arise not just from individual nodes but are also intricately intertwined with complex dynamical regulations between them25,26,27,28. Specifically, information on individual nodes or edges is excessively local and insufficient, while the dynamics of a cellular network is global and complex29,30. There exists a notable gap between local topologies and network dynamics.
In addition, for small perturbations to a cellular network, the quantitative variation in an equilibrium can be approximately captured by the linear stability approach31,32,33. However, the linearized approach is invalid for large perturbations. Therefore, how to approximately calculate the quantitative equilibrium variations under large perturbations is less clear and needs to be further investigated. Especially, when a bifurcation occurs, a slight perturbation may cause a large equilibrium variation, inducing qualitative differences in cell states, i.e., cell fate transitions.
Local topologies determine global network dynamics in cellular networks34,35, however, the intricate mechanisms of how local topologies such as paths and feedback loops function are still in need of further exploration. Especially, when perturbations arise, it is worth studying how the perturbations propagate within the networks, how local topologies such as paths and feedback loops operate and determine network dynamics, and ultimately, how local topologies collectively contribute to the emergence of bifurcations under perturbations36. Therefore, the relationship between local topologies and network dynamics remains a fundamental yet unresolved problem in cellular networks.
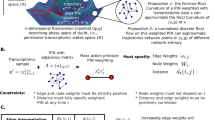
In this study, based on perturbation analysis and network decomposition, we propose a theoretical approach, termed the cumulative linearized approximation (CLA) algorithm, which allows us to investigate the intricate relationship between local network topologies, global network dynamics, and the emergence of bifurcations in cellular networks. To elucidate the general relationship between local network topologies34, global network dynamics35, and occurrence of bifurcations36 in cellular networks, we propose a theoretical approach, which integrates perturbation analysis and network decomposition. More exactly, the network dynamics can be determined by integrating information related to local topologies under perturbations. The methodology facilitates the identification of how paths affect quantitative equilibrium variations and how feedback loops determine the occurrence of bifurcations. We have confirmed the feasibility of the approach in a three-variable model of early T cell development, as well as in a more intricate epithelial-mesenchymal transition (EMT) network. In both models, the CLA algorithm successfully determines the variations in equilibrium under perturbations and accurately identifies the crucial roles played by local topologies, specifically the dominant pathways and feedback loops, in shaping network dynamics. This framework provides valuable insights into the analysis of critical local topologies for cell fate transitions in cellular networks and guide the selection of effective systematic perturbation strategies.
Results
Here, we use the three-variable model in early T cell development and EMT network as illustrative examples to demonstrate the feasibility of the CLA algorithm. Using the CLA algorithm, which integrates linear stability analysis with perturbation iteration, we are able to quantitatively assess the equilibrium variations in a system subjected to a large perturbation. Furthermore, this approach allows us to determine the specific contributions of different pathways to the overall equilibrium variations. For more details on the CLA algorithm, see section “Methods.” The detailed information about all ordinary differential equations (ODEs) and parameters employed, and related information on all paths and feedback loops in the two examples are presented in Supplementary Materials.
Three-variable model in early T cell development
The first example is the three-variable model related to early T cell development37. T cells, which are white blood cells with disease-fighting capabilities, originate from hematopoietic stem cells and undergo a complex and intricate development process within the thymus. During this process, multipotent progenitor cells of T cells differentiate and ultimately mature into functional T cells38,39,40. The development and differentiation of T cells, which involves multiple cellular states, are tightly regulated by a diverse array of transcription factors and environmental signals41,42.
In our example, the network composed of three nodes TCF-1 (T), PU.1 (P), and GATA3 (G) is shown in Fig. 1. Here, the perturbation is imposed on TCF-1, and the output node is PU.1. We use the basal production rate of TCF-1, denoted as k0T, to signify the perturbation to TCF-1. There exist two paths from TCF-1 to PU.1. One is the direct path from TCF-1 to PU.1. The other is the indirect path from TCF-1 to PU.1 through GATA3, which includes two regulation edges: the regulation from TCF-1 to GATA3 and the regulation from GATA3 to PU.1.
With the perturbation to k0T, the bifurcation diagrams for the three-variable model, derived from ODEs and the CLA algorithm, are depicted in Fig. 2. Obviously, there are two stable equilibrium (SE) curves: C1 and C2. Fate transitions between the two cell states occur through two saddle-node bifurcation: SN1 and SN2. The equilibrium curves obtained using the two approaches exhibit a near-perfect overlap, with minimal deviations only observed in the vicinity of the bifurcation points, thus demonstrating good approximation capabilities of the CLA algorithm.
The expression levels of TCF-1 A, PU.1 B, and GATA3 C as functions of k0T. The solid and circled equilibrium curves are derived from the ordinary differential equations (ODEs) and cumulative linearized approximation (CLA) algorithm, respectively. Purple and blue (dark or light) curves denote stable and unstable equilibria, respectively.
When the bifurcation parameter k0T approaches the bifurcation points, the CLA algorithm encounters difficulties due to the presence of sufficiently small Jacobian determinants in Eq. (13) or Eq. (15), resulting in its inability to accurately compute the equilibria. The total equilibrium variation in PU.1 is induced by two paths. The variation induced by the indirect path from TCF-1 to PU.1 through GATA3 takes the form
While the variation induced by the direct path from TCF-1 to PU.1 is
The total equilibrium variation in PU.1 is
The equilibrium variation in PU.1 induced by the perturbation imposed on TCF-1 through two paths defined by Eqs. (1)-(2) is shown in Figs. 3A and 4A. When the perturbed parameter, i.e., \({k}_{0T}={\bar{k}}_{0T}+\Delta {k}_{0T}\), is smaller than the saddle-node bifurcation, i.e., \({k}_{0T,S{N}_{1}}\) at the C1 branch, the equilibrium variations in PU.1 are mainly induced by the indirect path from TCF-1 to PU.1 through GATA3. Here, \({\bar{k}}_{0T}\) is the basal parameter value and Δk0T is the perturbation to it. The contribution degrees of the two paths are shown in Fig. 3B. The perturbation to TCF-1 leads to the equilibrium variations in PU.1, which are mainly attributed to the indirect path, i.e., the perturbation is mainly transmitted from TCF-1 to PU.1 via GATA3.
Conversely to the perturbation effect on the C1 branch, when the perturbed parameter k0T is larger than the left saddle-node bifurcation, i.e., \({k}_{0T,S{N}_{2}}\) at the C2 branch, the equilibrium variations in PU.1 are mainly induced by the direct path from TCF-1 to PU.1, as shown in Fig. 4A. The contribution degrees of the two paths are shown in Fig. 4B. These results suggest that the same path may play distinct roles in different cell states.
Feedback loops determine stability
It has been shown that each determinant composes a feedback loop or several independent feedback loops43. More exactly, for a given matrix M, the matrix determinant takes the form
where Mis are all sub-matrices.
Still considering the three-variable model. Its Jacobian matrix takes the form
where Jij = ∂fi/∂xj at \({\bf{x}}=\bar{{\bf{x}}}\) with xi (i = 1, 2, 3) representing TCF-1, PU.1, and GATA3, respectively.
The network shown in Fig. 1 can be decomposed into three sub-networks, as shown in Fig. 5. Each sub-network corresponds to a feedback loop and is related sub-matrix. Three feedback loops are the self-activation feedback loop of TCF-1, the feedback loop between TCF-1 and PU.1, and the feedback loop connecting TCF-1, PU.1, and GATA3, as shown in Fig. 5. Denote the sub-matrices which represent these three feedback loops as J1, J2, and J3, respectively. For example, the sub-matrix corresponding to the largest feedback loop is
It is easy to know that the determinant of matrix J takes the form
Actually, these three matrices exactly correspond to the three feedback loops shown in Figs. 1 and 5. Decomposing a network into distinct feedback loops, i.e., decomposing the Jacobian matrix of a nonlinear dynamical system into all its sub-matrices, can be used to determine the role of each feedback loop in determining the stability of equilibria and the occurrence of local bifurcations. When a local bifurcation with at least one zero eigenvalue occurs, the determinant of the Jacobian matrix J is also zero. Therefore, the emergence of a local bifurcation under a perturbation can be determined by analyzing the alterations in the determinants of the sub-matrices, which correspond to respective feedback loops.
The bifurcation diagram with k0T as a control parameter and the determinants of the Jacobian matrix and all sub-matrices are shown in Fig. 6. With continuous increase in k0T, SE curve C1 with higher PU.1 jumps to SE curve C2 with lower PU.1 through a saddle-node bifurcation SN1. As k0T increases, the equilibrium and therefore the determinant of the Jacobian matrix vary. At saddle-node bifurcation SN1, Jacobian matrix has a zero eigenvalue and therefore \(\det (J)=0\). The occurrence of the bifurcation SN1 is mainly induced by the feedback loop connecting all the three nodes corresponding to the sub-matrix J3. As k0T approaches \({k}_{0T,S{N}_{1}}\), \(\det (J)\) approaches zero quickly, which is mainly induced by the steep variation in \(\det ({J}_{3})\) because the changes in \(\det ({J}_{1})\) and \(\det ({J}_{2})\) are quite gradual, as shown in Fig. 6A. Similarly, the occurrence of the bifurcation SN2 is also mainly induced by the same feedback loop, corresponding to the sub-matrix J3, as shown in Fig. 6B.
EMT network
To assess the suitability of our approach for more intricate biological networks, we applied the CLA algorithm to the EMT network44. EMT plays critical roles in various biological processes, including tissue repair and pathological conditions such as normal embryonic development, wound healing, and cancer metastasis45,46,47. EMT is a biological process where cells undergo a morphological transformation from epithelial (E) to mesenchymal (M) phenotypes, with a hybrid (H) phenotype that exhibits characteristics of both E and M present during this transition. During EMT, epithelial cells can lose their polarity and adhesion, transforming into mesenchymal cells with enhanced motility and invasive capabilities48,49,50.
The core EMT network is shown in Fig. 7. In the network, there are nine molecules: a main inducer transforming growth factor-β (TGF-β), two cell fate microRNA regulators miR34 and miR200, two core transcription factors SNAIL1 and ZEB, two mRNA snail1 and zeb, and the epithelial state maker E-cadherin as well as the mesenchymal state maker N-cadherin. The perturbation is imposed on TGF-β, i.e., k0T, and the output node selected is E-cadherin or N-cadherin. The transition between different cell states occurs as the parameter k0T varies. There are three distinct states characterized by different levels of E-cadherin and N-cadherin expression: (i) high E-cadherin and low N-cadherin, representing the epithelial (E) cell state; (ii) low E-cadherin and high N-cadherin, representing the mesenchymal (M) cell state; and (iii) medium levels of both E-cadherin and N-cadherin, representing the hybrid (H) cell state. For a more intuitive analysis of state transitions between E, H, and M states, the equilibrium curves of E-cadherin and N-cadherin in response to k0T derived from ODEs and the CLA algorithm, are shown in Fig. 8. The equilibrium curves derived from both approaches demonstrate a nearly perfect overlap, exhibiting only negligible deviations in the proximity of the bifurcation points.
The expression levels of E-cadherin A and N-cadherin B as functions of k0T. The solid and circled equilibrium curves are derived by the ordinary differential equations (ODEs) and cumulative linearized approximation (CLA) algorithm, respectively. Purple and blue (dark or light) curves denote stable and unstable equilibria, respectively.
The bifurcation diagrams illustrate that there exist transitions between three cell states. Four saddle-node bifurcations occur when k0T varies, i.e., SN1, SN2, SN3, and SN4. The system stays in the monostable E state when k0T is small. As k0T gradually increases, forward transitions from E to H through SN1 and then from H to M through SN3 occur. Conversely, backward transition from M to E through SN4 for cells at M state or from H to E through SN2 for cells at H state occurs as k0T decreases. More exactly, with the change of k0T, the system may undergo EMT, i.e., E to M indirectly through H or mesenchymal-epithelial transition (MET), i.e., M to E directly. While if k0T decreases before it goes up to SN3, backward transition from H to E via SN2 occurs. Therefore, four different types of fate transitions, i.e., E to H, H to M, H to E, and M to E, may occur.
Next, we analyze how different paths affect equilibrium variations in the cell fate makers E-cadherin and N-cadherin. The variations in E-cadherin are affected by the perturbation to \({\bar{k}}_{0T}\) via four distinct paths. However, among these paths, only the path TGF-β → snail1 → SNAIL1 ⊣ E-cadherin, i.e., ΔPE,2, exerts a significant influence on the variations, as shown in Figs. 9A-B. Therefore, with the perturbation increase, the decrease in E-cadherin preceding the cell fate transition from E to H is primarily induced by this path, which contributes almost entirely to the variations observed in E-cadherin, as shown in Fig. 9A. As the perturbation decreases, the same path also plays a significant role in the accumulation of E-cadherin prior to the fate transition from H to E, as shown in Fig. 9B.
The equilibrium variations in N-cadherin are also influenced by the perturbation to \({\bar{k}}_{0T}\) through four distinct paths. Among these paths, the path TGF-β → snail1 → SNAIL1 → zeb → ZEB → N-cadherin, i.e., ΔPN,4, exerts the most significant impact on the fate transition from M to E, as shown in Fig. 9C. While the path TGF-β → snail1 → SNAIL1 → N-cadherin, i.e., ΔPN,2, plays the most significant role in the accumulation of N-cadherin and thus the fate transition from H to M, as shown in Fig. 9D.
With a continuous increase in k0T, cell fate transition from E to H occurs through the saddle-node bifurcation SN1, as shown in Fig. 8. As k0T increases, the equilibrium and, consequently, the determinant of the Jacobian matrix vary. At the saddle-node bifurcation SN1, the Jacobian matrix possesses a zero eigenvalue, resulting in a zero determinant, namely \(\det (J)=0\). Based on the decomposition of Jacobian matrix, the occurrence of the saddle-node bifurcation SN1 is primarily induced by the feedback loop between SNAIL1 and miR34, which corresponds to the sub-matrix J3, as shown in Fig. 10A. The determinant \(\det (J)\) tends to zero as k0T approaches the bifurcation SN1, during which only \(\det ({J}_{3})\) increases significantly, while the determinants of all other sub-matrices remain nearly constant.
Conversely, as k0T continuously decreases, the cell fate transition from H to E occurs through SN2, as shown in Fig. 8. The emergence of bifurcation SN2 is still primarily attributed to the feedback loop between SNAIL1 and miR34, corresponding to the sub-matrix J3, as shown in Fig. 10B. Therefore, the cell fate transition between E and H is mainly driven by the feedback loop between SNAIL1 and miR34.
The cell fate transition from M to E via the saddle-node bifurcation SN4 or the transition from H to M through SN3 is mainly induced by the feedback loop between ZEB and miR200, which corresponds to the sub-matrix J2, as shown in Figs. 10C-D. In addition, the feedback loop TGF-β → snail1 → SNAIL1 → zeb → ZEB ⊣ miR200 ⊣ TGF-β, corresponding to the sub-matrix J7, also plays a crucial role in the fate transition from M to E via the saddle-node bifurcation SN4, as shown in Fig. 10C.
Discussion
As modern biomedical science advances rapidly, the threat to human health has gradually shifted from diseases caused by isolated pathogenic factors to complex diseases. Diseases often originate from intricate regulations among numerous molecules and their surrounding environments, making it highly challenging to analyze them from isolated factors. A perturbation to a single molecule can trigger a cascading series of downstream effects via intricate regulatory networks. Consequently, focusing solely on the individual pathogenic effects of disease-causing genes is inadequate to accurately uncover the emergence of complex diseases. Instead, our research emphasizes a thorough exploration of the intricate interplay among the components within the cellular networks.
To achieve a profound understanding of intricate regulatory mechanisms of cellular networks, it is necessary to investigate the correlations between local network topologies, global network dynamics, and the occurrence of bifurcations. This exploration encompasses multiple dimensions, including the analysis of individual nodes, edges, paths, and feedback loops. Through this research, we can comprehensively and precisely illuminate the underlying causes of various dynamics, thereby offering novel insights and methodologies for dynamics control and therapeutic interventions.
Theoretical analysis and numerical simulations have already validated the feasibility of this approach, for instance, in the context of early T cell development and EMT networks. The network-based approach integrates perturbation analysis and network decomposition. It offers a theoretical framework to reveal how perturbations determine equilibrium variations by integrating distinct regulatory paths. In addition, the crucial roles of feedback loops in determining the stability of equilibrium and the occurrence of bifurcations can be identified. The relationship between local topologies and network dynamics uncovers the significance of nodes, edges, paths, and feedback loops in response to perturbations.
Although the focus is on perturbations to scalar parameters, the synergistic effects of combinatorial perturbations can also be extensively examined. The network decomposition approach introduced offers a comprehensive framework for conducting network dynamics and perturbation analysis in both mathematical and biological sciences. This framework not only aids in discovering crucial relationships between network components, but also has the potential to be applied to various other regulatory systems, such as embryonic stem cell differentiation51, cancer metastasis52, the developmental process of B cell18, and other networks related to cell fate transition53,54. This approach offers an efficient protocol for clinical treatment of certain diseases through the use of specific medications. However, a limitation of our methodology lies in its inability to analyze global bifurcations, restricting its application solely to local bifurcations with zero eigenvalues. Furthermore, the selection of the perturbation step size can significantly impact the accuracy of our calculations, particularly when the perturbation is in proximity to bifurcation points. Therefore, our future research will focus on identifying the essential information associated with both local critical states and global bifurcations.
Methods
By integrating network decomposition with perturbation analysis, we introduce the CLA algorithm under large perturbations. This approach enables us to ascertain how such perturbations influence variations in equilibria and ultimately result in the occurrence of bifurcations.
Consider a dynamical system
The vector \({\bf{x}}\in {{\mathbb{R}}}^{n}\) denotes the state of a cellular network at time t, \({\bf{p}}\in {{\mathbb{R}}}^{m}\) denotes the vector parameter, and the vector function f(x; p) represents the constraints, which determine the dynamics of the network.
Equilibrium variations under a small perturbation
For simplicity, we consider the case of scalar parameter. The case of vector parameter can be similarly investigated. At the basal parameter value \(\bar{p}\), the equilibrium \(\bar{{\bf{x}}}\) satisfies
In response to a small perturbation to the parameter: \(\bar{p}\to \bar{p}+\Delta p\), the equilibrium varies from \(\bar{{\bf{x}}}\) to \(\bar{{\bf{x}}}+\Delta {\bf{x}}\).
Suppose that the perturbation is imposed on the node k, i.e., fk contains the perturbed parameter. Expanding Eq. (9) at the equilibrium \(\bar{{\bf{x}}}\), the linearized equation takes the form:
where J is the Jacobian matrix at the equilibrium \(\bar{{\bf{x}}}\) and
Suppose that the Jacobian matrix J is reversible. The equilibrium of Eq. (10) satisfies
For each node xj, the variation deviating from its original position \({\bar{x}}_{j}\), i.e., \(\Delta {\bar{x}}_{j}\), takes the form
where Ckj is the algebraic cofactor of element (k, j) in the Jacobian matrix J and \(\det (J)\) is the Jacobian determinant.
For each path Pi from the perturbed node k to the output node j, its contribution to the equilibrium variation is defined as
where
and τ(r1, ⋯ , rj−1, rj+1, ⋯ , rn) is the minimum number of pairwise exchanges to recover order 1, ⋯ , j − 1, j + 1, ⋯ , n. EP is the number of nodes minus one, i.e., the edges in the path Pi from the perturbed node k to the output node j and the edges of the loops, which are not connected to it, and Jpq represents the intensity of the edge from q to p in the path Pi, i.e.,
Therefore, the total equilibrium variation in node j induced by the perturbation to node k over all paths takes the form
where NP is total number of paths from node k to node j.
Similar to the tree diagram of chain rule, combining Eq. (14) and Eq. (18), we obtain
which means that we need multiply all the derivatives along each path and then add the products over all paths.
Due to the fact that the contribution of each path can be positive or negative, the contribution degree of each path Pi to the equilibrium variation in xj is defined as
Thus, we have
The equilibrium variation of xj attributed to each Pi is defined by Eq. (14). While the total equilibrium variation induced by all the paths, i.e., \(\Delta {\bar{x}}_{j}\), can be obtained by Eq. (19). The contribution degree of each path Pi to the total equilibrium variation in xj is defined by Eq. (20).
Equilibrium variations under a large perturbation
Under a small perturbation, the linearized Eq. (10) is used to calculate equilibrium variations. The linearized approach is invalid for large perturbations. For node xj, under a large perturbation Δp, we divide the interval \([\bar{p}\,\bar{p}+\Delta p]\) into s − 1 subintervals with equal width Δstep = Δp/(s − 1), and then compute each approximate \(\Delta {\bar{x}}_{j}\) under each small perturbation by
where h = 1, ⋯ , s − 1 and j = 1, ⋯ , n. Each \(\Delta {\bar{x}}_{j,h}\) can be calculated using the linearized approximation defined by Eq. (18), specifically for the case of small perturbations.
The vector form can be expressed as follows
Then, under a large perturbation, the equilibrium variation can be approximately calculated as follows
which includes the equilibrium \(\bar{{\bf{x}}}(\bar{p})\) and the equilibrium variation induced by the perturbation Δp, i.e., \(\mathop{\sum }\nolimits_{h = 1}^{s-1}\Delta {\bar{{\bf{x}}}}_{h}\). Therefore, we develop the CLA algorithm, i.e., Eq. (24), to explore the equilibrium variations of cellular network under large perturbations. Note that during the perturbation, if a local bifurcation occurs, the CLA algorithm may encounter difficulties due to the presence of a zero determinant for Jacobian J in Eq. (15).
Data availability
All data used and analysed during the current study are calculated in MATLAB.
Code availability
The code is compiled using MATLAB.
References
Barabási, A. L. & Albert, R. Emergence of scaling in random networks. Science 286, 509–512 (1999).
Girvan, M. & Newman, M. E. Community structure in social and biological networks. Proc. Natl Acad. Sci. Usa. 99, 7821–7826 (2002).
Li, C. & Wang, J. Quantifying cell fate decisions for differentiation and reprogramming of a human stem cell network: landscape and biological paths. PLoS Comput Biol. 9, e1003165 (2013).
Bhall, U. S. & Iyengar, R. Emergent properties of networks of biological signaling pathways. Science 283, 381–387 (1999).
Kobayashi, T., Chen, L. & Aihara, K. Modeling genetic switches with positive feedback loops. J. Theor. Biol. 221, 379–399 (2003).
Pett, J. P., Korenčič, A., Wesener, F., Kramer, A. & Herzel, H. Feedback loops of the mammalian circadian clock constitute repressilator. PLoS Comput Biol. 12, e1005266 (2016).
Kang, T., Moore, R., Li, Y., Sontag, E. & Bleris, L. Discriminating direct and indirect connectivities in biological networks. Proc. Natl Acad. Sci. Usa. 112, 12893–12898 (2015).
Huang, B. et al. Interrogating the topological robustness of gene regulatory circuits by randomization. PLoS. Comput. Biol. 13, e1005456 (2017).
Li, G. et al. Enabling controlling complex networks with local topological information. Sci. Rep. 8, 4593 (2018).
Wang, R. Q., Chen, L. N. & Aihara, K. Detection of cellular rhythms and global stability within interlocked feedback systems. Math. biosci. 209, 171–189 (2007).
Moon, J. Y., Lee, U., Blain-Moraes, S. & Mashour, G. A. General relationship of global topology, local dynamics, and directionality in large-scale brain networks. PLoS. Comput. Biol. 11, e1004225 (2015).
Rashid, M., Hari, K., Thampi, J., Santhosh, N. K. & Jolly, M. K. Network topology metrics explaining enrichment of hybrid epithelial/mesenchymal phenotypes in metastasis. PLoS. Comput. Biol. 18, e1010687 (2022).
Yen, H. Y. et al. Ligand binding to a G protein-coupled receptor captured in a mass spectrometer. Sci. Adv. 3, e1701016 (2017).
Wilson, A. J. Inhibition of protein-protein interactions using designed molecules. Chem. Soc. Rev. 38, 3289–3300 (2009).
Ren, X., Zhao, M., Lash, B., Martino, M. M. & Julier, Z. Growth factor engineering strategies for regenerative medicine applications. Front. Bioeng. Biotechnol. 7, 469 (2020).
Saminathan, A., Zajac, M., Anees, P. & Krishnan, Y. Organelle-level precision with next-generation targeting technologies. Nat. Rev. Mater. 7, 355–371 (2022).
Shinar, G., Milo, R. R., Martínze, M. R. & Alon, U. Input-output robustness in simple bacterial signaling systems. Proc. Natl Acad. Sci. Usa. 104, 19931–19935 (2007).
Martínez, M. R. et al. Quantitative modeling of the terminal differentiation of B cells and mechanisms of lymphomagenesis. Proc. Natl Acad. Sci. Usa. 109, 2672–2677 (2012).
Jiao, J. F., Luo, M. & Wang, R. Q. Feedback regulation in a stem cell model with acute myeloid leukaemia. BMC Syst. Biol. 12, 75–73 (2018).
Zhong, J., Han, C., Wang, Y., Chen, P. & Liu, R. Identifying the critical state of complex biological systems by the directed-network rank score method. Bioinformatics 38, 5398–5405 (2022).
Zhong, J. et al. Uncovering the pre-deterioration state during disease progression based on sample-specific causality network entropy (SCNE). Research 7, 0368 (2024).
Chen, L., Liu, R., Liu, Z. P., Li, M. & Aihara, K. Detecting early-warning signals for sudden deterioration of complex diseases by dynamical network biomarkers. Sci. Rep. 2, 342 (2012).
Yu, L. et al. Modeling the genetic regulation of cancer metabolism: interplay between glycolysis and oxidative phosphorylation. Cancer Res. 77, 1564–1574 (2017).
Akhtar, J. et al. Bistable insulin response: The win-win solution for glycemic control. Iscience 25, 105561 (2022).
Gross, T. & Blasius, B. Adaptive coevolutionary networks: a review. J. R. Soc. Interface 5, 259–271 (2008).
Fakhar, K. & Hilgetag, C. C. Systematic perturbation of an artificial neural network: A step towards quantifying causal contributions in the brain. PLoS Comput. Biol. 18, e1010250 (2022).
He, X., Tang, R., Lou, J. & Wang, R. Q. Identifying key factors in cell fate decisions by machine learning interpretable strategies. J. Biol. Phys. 49, 443–462 (2023).
Hu, Q., Luo, M. & Wang, R. Q. Identifying critical regulatory interactions in cell fate decision and transition by systematic perturbation analysis. J. Theor. Biol. 577, 111673 (2024).
Ullah, A. et al. Identifying vital nodes from local and global perspectives in complex networks. Expert. Syst. Appl. 186, 115778 (2021).
D’Souza, R. M., di, BernardoM. & Liu, Y. Y. Controlling complex networks with complex nodes. Nat. Rev. Phys. 5, 250–262 (2023).
Medio, A. & Lines, M. Nonlinear dynamics: A primer. (Cambridge University Press, 2001).
Ma, W., Trusina, A., El-Samad, H., Lim, W. A. & Tang, C. Defining network topologies that can achieve biochemical adaptation. Cell 138, 760–773 (2009).
Araujo, R. P. & Liotta, L. A. The topological requirements for robust perfect adaptation in networks of any size. Nat. Commun. 9, 1757 (2018).
Dumbser, M., Moschetta, J. M. & Gressier, J. A matrix stability analysis of the carbuncle phenomenon. J. Comput. Phys. 197, 647–670 (2004).
Strogatz, S. H. Exploring complex networks. Nature 410, 268–276 (2001).
Luongo, A. & D’Annibale, F. Linear stability analysis of multiparameter dynamical systems via a numerical-perturbation approach. AIAA J. 49, 2047–2056 (2011).
Ye, Y., Kang, X., Bailey, J., Li, C. & Hong, T. An enriched network motif family regulates multistep cell fate transitions with restricted reversibility. PLoS Comput. Biol. 15, e1006855 (2019).
Metcalf, D. On hematopoietic stem cell fate. Immunity 26, 669–73 (2007).
Rothenberg, E. V., Moore, J. E. & Yui, M. A. Launching the T-cell-lineage developmental programme. Nat. Rev. Immunol. 8, 9–21 (2008).
Ashby, K. M. & Hogquist, K. A. A guide to thymic selection of T cells. Nat. Rev. Immunol. 24, 103–117 (2024).
Zañudo, J. G. & Albert, R. Cell fate reprogramming by control of intracellular network dynamics. PLoS Comput. Biol. 7, e1004193 (2015).
Cacace, E., Collombet, S. & Thieffry, D. Logical modeling of cell fate specification-Application to T cell commitment. Curr. Top. Dev. Biol. 139, 205–238 (2020).
Zhao, Z. Z., Tang, R. Y. & Wang, R. Q. Matrix stability and bifurcation analysis by a network-based approach. Theory Biosci. 142, 401–410 (2023).
Tian, X. J., Zhang, H. & Xing, J. Coupled reversible and irreversible bistable switches underlying TGFβ-induced epithelial to mesenchymal transition. Biophys. J. 105, 1079–1089 (2013).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithlial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Ravikrishnan, A. et al. Regulation of Epithelial-to-Mesenchymal transition using biominmetic fibrous scaffolds. ACS Appl. Mater. Interfaces 8, 17915–17926 (2016).
Pei, D. Q., Shu, X. D., Gassama-Diagne, A. & Thiery, J. P. Mesenchymal-Epithelial transition in development and reprogramming. Nat. Cell Biol. 21, 44–53 (2019).
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Lu, M., Jolly, M. K., Levine, H., Onuchic, J. N. & Ben-Jacob, E. MircoRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc. Natl Acad. Sci. USA 110, 18144–18149 (2013).
Wang, H. Y., Zhang, X. P. & Wang, W. Regulation of epithelial-to-mesenchymal transition in hypoxia by the HIF-1α network. FEBS Lett. 596, 338–349 (2022).
Zhang, B. & Wolynes, P. G. Stem cell differentiation as a many-body problem. Proc. Natl Acad. Sci. US A. 111, 10185–90 (2014).
Huang, B. et al. Modeling the transitions between collective and solitary migration phenotypes in cancer metastasis. Sci. Rep. 5, 17379 (2015).
Li, C. & Balazsi, G. A landscape view on the interplay between EMT and cancer metastasis. NPJ Syst. Biol. Appl. 4, 1–9 (2018).
Yu, C., Liu, Q., Chen, C. & Wang, J. Quantification of the underlying mechanisms and relationships among cancer, metastasis, and differentiation and development. Front. Genet. 10, 1388 (2020).
Acknowledgements
This study was funded by the National Natural Science Foundation of China [Grants No. 12371497].
Author information
Authors and Affiliations
Contributions
Q.H., R.T. and X.H. performed the computational modeling, data analysis, and draft and revised the manuscript. R.W. conceived the study and draft and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, Q., Tang, R., He, X. et al. General relationship of local topologies, global dynamics, and bifurcation in cellular networks. npj Syst Biol Appl 10, 135 (2024). https://doi.org/10.1038/s41540-024-00470-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41540-024-00470-1
This article is cited by
-
Combinatorial perturbation analysis of CD4+ T cell regulatory networks based on bifurcation theory
Advances in Continuous and Discrete Models (2025)