Abstract
Streptococcus suis infection represents a major challenge in pig farming and public health due to its zoonotic potential and diverse serotypes, while existing vaccines lack effective cross-protection. This study employed reverse vaccinology and immunoinformatics to identify 8 conserved proteins across 11 prevalent serotypes of S. suis. 16 candidate epitopes were selected to design three multi-epitope antigens against S. suis (designated as MEASs), which fused with a dendritic cell-targeting peptide to improve antigen presentation in host. Purified MEASs displayed favorable cross-reactogenicity against 29 serotype-specific antiserums. Robust humoral and cellular immune responses can be induced by MEAS 1 and MEAS 3 in a mouse model, which provided substantial protection against virulent strains from two different serotypes. In particular, their immune serums exhibited positive opsonization effects within bloodstream and macrophage phagocytosis. Taken together, we identified two promising MEASs with excellent cross-protection, offering potential in preventing S. suis infections in a mouse model.
Similar content being viewed by others
Introduction
Streptococcus suis, a common zoonotic pathogen1, predominantly affects swine by colonizing the respiratory, digestive, and genital areas, with a strong presence in the tonsils and nasal passages2. Transmission to swine occurs through wounds, respiratory secretions, contact with piglets, and possibly aerosols3,4. In swine, infections can cause severe conditions, including arthritis, sepsis, and meningitis5. In humans, exposure to infected swine or contaminated pork products may be infected, and serious cases may develop into and streptococcal toxic shock-like syndrome (STSLS)6 or bacterial meningitis. S. suis has 29 recognized serotypes, marked by the capsular polysaccharide (CPS), with 11 known to infect both swine and humans, including 1, 2, 4, 5, 7, 9, 14, 16, 21, 24 and 317,8,9. Worldwide, reports of S. suis infections have been documented, with serotypes 2, 3, 5, 7, 9, and 31 (SS2, 3, 5, 7, 9, 31) being prevalent10.While in China, notable outbreaks in 1998 (Jiangsu) and 2005 (Sichuan) were caused by SS2, resulting in STSLS with high mortality rates6. Since 2006, Guangxi Zhuang Autonomous Region has reported the most human infections, primarily involving SS2 and SS1411.
Vaccination was crucial for preventing S. suis infections, despite challenges presented by the genetic, phenotypic, and geographical variability among its 29 serotypes12,13. Existing vaccines often struggled to provide effective control against diverse strains within a certain serotype14. Consequently, there was a growing focus on developing new vaccines, particularly subunit vaccines, which have garnered attention due to their potential to induce robust immune responses15,16,17. Numerous research efforts have predominantly explored surface-associated and secreted proteins18,19,20,21, targeting them to elicit strong immune responses during pathogen-host interactions. Enolase (Eno) serving as a successful example due to its protective effect against SS2, which was widely present on the surface of S. suis22. A vaccine used recombinant attenuated Salmonella Choleraesuis vector had shown promise in providing protection against multiple serotypes including SS2, 7, and 923. Enhancing vaccine efficacy, studies have demonstrated that vaccines incorporating multiple antigens24, such as Muramidase-released protein (MRP) and Extracellular Factor (EF), have proven to be effective against SS2 infections25. Furthermore, innovative strategies like the co-delivery of surface-associated antigens, such as SaoA and Eno, with recombinant Salmonella Choleraesuis vectors, have shown to offer cross-protection across SS2, 7, 9 and 1/226. However, with >16 widely spread serotypes and at least 11 causing human infections, the current vaccines remain inadequate, offering protection to only half or less than 20% of these serotypes. This emphasizes the urgent need for continued efforts to enhance vaccine efficacy against a broader spectrum of S. suis serotypes.
The advancement of reverse vaccinology has led to the identification of candidate genes and the screening for immune-protective antigens at the genomic level. This strategy hinged on the utilization of genetic engineering and recombinant expression techniques for the crafting of vaccine molecules27. By adopting this method, conservative surface proteins with high species specificity and immunogenicity could be identified using immune informatics28. Furthermore, large-scale production has achieved through genetic recombination expression techniques, which were actively applied in production29. Multi-epitope vaccines were developed by strategically designing immunogens based on antigenic epitope sequences from single or multiple target antigens, which was crucial for effective antigen presentation across different genetic backgrounds. Epitope-based vaccines have been explored for various pathogens including tumors, bacteria, parasites, and viruses30,31,32,33. Amalgamation of multiple epitopes into an antigen resulting cross-protection effect has been investigated for pathogens like Staphylococcus aureus34, Escherichia coli35, Streptococcus agalactiae36, and Streptococcus pneumoniae37. In S. suis, the development of multi-epitope vaccines has been a focused area of study. However, the challenge persisted in identifying antigens with superior immunogenic properties amid the multitude of serotypes. Zhang et al. has led to the creation of a promising universal multi-epitope vaccine–MVHP6 against S. suis by immunoinformatics, showing promising immune responses in simulations but lacking verified in vivo protective efficacy38. Similarly, Liang et al. developed MVSS, an epitope-based vaccine for S. suis through reverse vaccinology, which reduced histopathological damage and bacterial loads in mice but did not confer significant protective effects39. Therefore, it is crucial to develop epitope-based vaccines using optimized schemes that provide effective protection against S. suis.
In this study, we harnessed the synergy between computer simulations and phenomic data to craft multi-epitope antigens to design multi-epitope antigens against S. suis (designated as MEASs). We meticulously evaluated the humoral and cellular immune responses elicited by MEASs in vitro and ascertained their cross-protective efficacy through rigorous immunoprotection assays in vivo. The intricate design and evaluative procedures of MEASs were elucidated within Fig. 1. (1) Recognizing the critical shortfall in current S. suis vaccines, which fail to tackle the multitude of resistant strains across varied genetic backgrounds, we meticulously curated genomic sequences of S. suis pathogenic strains from the GenBank database. This step was crucial for informed selection based on epidemiological data sourced from China (Fig. 1a). (2) Leveraging a synergistic approach that fuses reverse vaccinology, immunoinformatics, and pan-genomics, we analyzed a comprehensive collection of 100 pathogenic strains emblematic of both prevalent and zoonotic serotypes in China. Computer-assisted screening identified 8 conserved antigens which contain 16 candidate epitopes, leading to the construction of three sets of MEASs (Fig. 1b). (3) To ascertain the immunogenic potency of the MEASs, we adopted an animal model to simulate the immune response, monitoring both humoral and cellular immunity. The subsequent step involved in vivo challenge tests to quantify their protective efficacy, meticulously measuring bacterial clearance and opsonization by antibodies to validate the enhancement of immune mechanisms (Fig. 1c). The outcomes of these comprehensive evaluations have rendered MEASs 1 and MEAS 3 as promising antigens for a broad-spectrum vaccine against S. suis, underscoring their potential for development and practical application.
a Based on the antigenicity of CPS, S. suis is classified into 29 serotypes. Among them, 11 serotypes are capable of infecting both pigs and human9. b Depicted the design process of three MEASs based on reverse vaccinology. Leveraging a synergistic approach that fuses reverse vaccinology, immunoinformatics, and pan-genomics, we analyzed a comprehensive collection of 100 pathogenic strains emblematic of both prevalent and zoonotic serotypes in China. Computer-assisted screening identified 8 conserved antigens which contain 16 candidate epitopes, leading to the construction of three sets of MEASs. A prevalence study was carried out, consistent with prior reports on S. suis in over 1500 tonsil samples from pig herds, regardless of disease status. Following the prevalence study, 106 samples were randomly selected for strain isolation (covered > 20 serotypes) and genetic epidemiological analysis, revealed that eight candidate proteins were widely distributed across the clinical S. suis strains. c Illustrated the assessment of three MEASs immune protective effect in vitro and in vivo. The subsequent step involved in vivo challenge tests to quantify their protective efficacy, meticulously measuring bacterial clearance and opsonization by antibodies to validate the enhancement of immune mechanisms. Figure 1a created in BioRender. KAKA, L. (2024) BioRender.com/e15l225.
Results
Screening the conserved antigens of multiple serotypes of S. suis
S. suis, an important zoonotic pathogen, was classified into numerous serotypes based on the capsular types, with at least 11 serotypes have been reported to cause human infections9 (Fig. 1a). The most severe case of S. suis infection can lead to toxic shock syndrome and potentially fatal outcomes. Although a multitude of virulence factors have been identified in current studies, their presences were often limited to specific serotypes. Identifying conserved virulence factors or antigens across different serotypes remained a challenge, which hampered the development of universally protective vaccines. In this study, we have curated a collection of 100 S. suis genomes from GenBank database, exclusively sourced from China, to identify conserved antigens spanning multiple serotypes, in line with the procedures illustrated in Fig. 1b. These genomes represented 11 main prevalent serotypes, including 1, 2, 1/2, 3, 5, 7, 8, 9, 14, 31 and chz serotypes. Following the pan-genome phylogenetic analysis, we identified 826 conserved proteins which were based on a matrix of gene presence and absence screened from core genome analysis (Supplementary Fig. 1). From this pool, 21 proteins that were localized in the cell walls and extracellular space. The selection process utilized bioinformatic such as TMHMM, CCTOP, PSORTb online servers. To enhance the immunoprotection potential of fusion antigens, candidate proteins were further refined by referencing transcriptome data40 and proteomics data41. The antigenicity of these proteins was scrutinized using the VaxiJen server, leading to the identification of eight highly antigenic proteins: polysaccharide Deacetylase family protein (DAC); putative 5’-Nucleotidase (pS5nA); 5’-Nucleotidase (S5nA); Manganese-dependent superoxide dismutase (Mdsd); Subtilisin-like serine protease (Slsp); Pneumococcal-type histidine triad protein (HtpS); CdaR family protein (CdaR); Serine protease Do-like HtrA (HtrA) (Table 1). The primary route of S. suis infection were contracted by individuals working within the swine farming and pork processing industries, with the infection vector being both infected pigs and carrier pigs, as they are considered the main transmitters10. Consequently, a prevalence study was carried out by this study, on S. suis in over 1500 tonsil samples from pig herds, regardless of disease status, across multiple provinces in China. The findings showed different positive rates: Sichuan (63%), Inner Mongolia (23%), Hebei (67%), Guangxi Zhuang Autonomous Region (19.66%), and Guizhou (42%) (Supplementary Fig. 2a). Following the prevalence study, 106 samples were randomly selected for strain isolation and genetic epidemiological analysis, with the aim of assessing the distribution of candidate antigens. Based on the positive rates of eight antigens have shown that except for S5nA, which had a positive rate of 55.6%, the rates of the other proteins were all above 80% in the S. suis isolates from healthy pig tonsils. The results revealed that eight candidate antigens were widely distributed across the clinical S. suis strains (Supplementary Fig. 2b).
Designing and optimizing the scheme of multi-epitope antigens against S. suis
Based on the eight candidate antigens, we engineered multiple epitope antigens targeting the major prevalent and potential zoonotic serotypes of S. suis as the procedures showed in Fig. 1b. To predict B cell and T cell epitopes, we employed ABCpred, IEDB TEPITOP, and other tools. In particular, the epitopes that could be recognized by both B cells and T cells were screened to evaluate their antigenicity, sequence composition and physicochemical properties. As shown in Supplementary Table 4, there were 16 candidate epitopes selected based on the scores, retrieved their hydrophobicity indices by Expasy server. We developed three schemes, each containing 8 or 10 epitopes, connected by a linker (GPGPGLRMKLPKS) to increase hydrophilicity from the center outward. We appended a dendritic cell-targeting peptide (FYPSYHSTPQRP) to the end of these schemes to boost antigen presentation efficiency. The arrangements of three MEAS were shown in Fig. 2a, which were recombinant proteins of 287aa, 352aa, and 363aa, respectively. The antigenicity values of MEASs were 1.1367, 1.1390, and 1.0841, indicating strong immunogenicity. Moreover, it was determined that the MEASs were not allergenic and toxic. The sequences and secondary structures of the MEASs were predicted using the PSIPRED website (Supplementary Fig. 3a), while their tertiary structures were analyzed through the I-TASSER (Supplementary Fig. 3b) and Galaxy Refine servers (Supplementary Fig. 3c), with visualization provided by Pymol (Supplementary Fig. 2b).
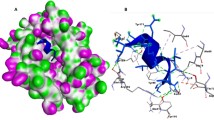
a The schematic designs of the three MEAS arrangements. b 3D structural analysis of MEAS 1/2/3. c Diagram of the docking model of MEAS1-SLA1 complex and MEAS1-TLR2 complex; Other docking model of MEAS-MHC I complex, MEAS-MHC II complex, MEAS TLR4 complex were shown in Supplementary Fig. 4. The dashed box indicates the docking region of the complex, while the right side shows the specific docking amino acid residues and the interactions between each amino acid.
In assessing the designed multi-epitope antigens’ potential for immune receptor recognition, we conducted molecular docking simulations with human major histocompatibility complex (MHC I, MHC II, TLR2, TLR4) and swine leukocyte antigen (SLA-1) by the ClusPro 2.0 server, considering the zoonotic nature of diverse S. suis serotypes. We also examined interaction residues using the PDBsum server. These results demonstrated that all three MEAS exhibited good affinity for both human and porcine immune receptors (Fig. 2c and Supplementary Fig. 4). Utilizing the C-IMMSIM simulation for the immunization feature representation of the MEASs, we observed a broad activation of the immune system post-injection, characterized by a significant increase in antibody titers (Supplementary Fig. 5a). Immune cell populations, including B cells and T cells, also displayed substantial growth (Supplementary Fig. 5b, c and d). The simulated cytokine responses indicated strong immunological reactions to the antigen (Supplementary Fig. 5e), suggesting that the designed three MEASs had the potential to elicit strong immune responses in silico.
Preparation of MEASs for assessing their reactogenicity against 29 serotype-specific antiserums
After codon preference optimization, the coding sequences of three MEASs were cloned into vector pET 28a (+), resulting in the recombinant plasmids: pET 28a-meas 1, pET 28a-meas 2, and pET 28a-meas 3 (Fig. 3a), respectively. These recombinant plasmids were then transformed into the E. coli BL21 cells for following protein expression and purification. SDS-PAGE analysis confirmed that MEAS 1/2/3 were overexpressed and successfully purified (Fig. 3b). Meanwhile, the specific bands at around 35.3 kDa, 42.3 kDa and 43.9 kDa were detected by Western Blot analysis using the anti-his McAb (Fig. 3c), which completely correspond to the purified bands of the SDS-PAGE gel, verifying the efficient purification of MEAS 1/2/3. Reactogenicity of proteins plays a crucial role in vaccine development. To evaluate their reactogenicity, indirect ELISA tests were performed using serum samples from 29 distinct serotypes of S. suis provided by WOAH Reference Lab for Swine Streptococcosis. The purified MEASs were immobilized on porous polystyrene plates and incubated with the antiserums. A negative serum was used as control. The results indicated that majority antiserums could be able to bind to the plate-coated with MEASs. Notably, MEAS 1 was recognized by the most antiserums. Although anti-SS5 and anti-SS31 serums showed lower recognition rates, the three MEASs were collectively recognized by over 20 serotype-specific antiserums. These findings demonstrated that MEASs displayed favorable cross-reactivity with various S. suis serotype-specific antiserums, highlighting their potential to invoke a comprehensive immune response in vaccine formulations.
a pET28a meas1, pET28a meas2, pET28a meas3 were chemically transformed into BL21 cells. The universal primers T7 F/R were used for microbial amplification of DNA, followed by DNA electrophoresis for identification. b SDS-PAGE showed the MEAS 1/2/3 expression and purification. c The reactivity of anti-his tag antibody with MEAS1/2/3 were tested in Western Blot. d The reactivity of each MEAS with 29 classical serotype-specific antiserums of S. suis.
Evaluation of MEASs immune effect in vitro
The utilization of the mouse model has been a cornerstone in the study of numerous human infectious diseases and serves a critical role in the preliminary phases of vaccine development. It has also been widely employed in both of immunization and challenge experiments focused on S. suis42. In this study, the immunization procedure for mice was illustrated in Fig. 4a. We measured the specific IgG antibody titers against the corresponding MEASs in the immune serum through ELISA following each immunization series. The results demonstrated that, compared to the preimmune serum, the antibody titer stepwisely increased with the three immunizations. After the third immunization, the antibody titer reached over 25,600 for MEAS 2, and over 102,400 for MEAS 1 and MEAS 3 (Fig. 4a). Western Blot with immunized mouse serum was consistent with SDS-PAGE, indicating that polyclonal antibodies against the corresponding MEASs were produced after triple immunizations (Supplementary Fig. 6), and also making the ELISA results more convincible.
a The mouse immunization procedure and blood collection timeline, with PBS+adjuvant serving as the control. The red box below highlights the determination of antibody titers in serum after three immunizations with MEASs. b The expression of IgG1, IgG2a and IgG2b in serum of each group after three times of immunization through ELISA. c Isolated spleen lymphocytes after three immunizations, and lymphocyte proliferation was measured using CCK 8 after incubation with MEAS 1, MEAS 2 and MEAS 3 for 48 h. d The expression of IFN-γ and IL-4 in the supernatant of lymphocytes incubated with MEAS 1, MEAS 2 and MEAS 3 by ELISA. e The proportion of CD3+CD4+ and CD3+CD8+ T lymphocytes was determined by flow cytometry. f The ratio of CD3+CD4+ / CD3+CD8+ in different groups. Significance (P)-value summary analysed by Unpaired two-tailed Student’s t-test (*P < 0.05; **P < 0.01; ***P < 0.001; ns no significance). Figure 4a mouse model was created in BioRender. KAKA, L. (2024) BioRender.com/e31e489.
Currently, IgG can be divided into four subtypes, with IgG1 and IgG2 playing pivotal roles in the adaptive immune response to antigens. IgG1 has a pronounced affinity for soluble protein antigens and membrane proteins, often eliciting a vigorous antibody response. On the other hand, the immune response to bacterial capsular polysaccharide antigens is predominantly mediated by IgG2, highlighting the nuanced specificity and versatility of IgG subclasses in orchestrating an immune defense43,44. The levels of the antibody subtypes of immunized mice were measured using the ELISA method. As shown in Fig. 4b, the levels of IgG1, IgG2a, and IgG2b were significantly elevated after immunization. For IgG1, there was no significant difference among the groups immunized with the three different proteins. However, MEAS 2 eliciting lower levels of IgG2a and IgG2b antibodies than MEAS 1 and MEAS 3. The detection of cellular immunity levels can help determine the ability of vaccine immune response. The investigation into lymphopoiesis post-immunization demonstrated that mice vaccinated with MEASs exhibited a significant increase in the stimulation index (SI) for splenic lymphocytes as compared to the PBS+adjuvant control group (Fig. 4c). The secretion of IFN-γ and IL-4 after induction of MEASs significantly increased by ELISA method, this suggested that MEASs’ immunization could up-regulate the expression of Th1 and Th2 cytokines (Fig. 4d). The proportion of CD3+CD4+ and CD3+CD8+ T lymphocytes was determined by flow cytometry as shown in Fig. 4e. Immunization of MEAS 1 and MEAS 3 caused a significant increase in CD3+CD4+ T cell counts, while MEAS 2 exhibited no increase. And there were no significant changes in CD3+CD8+ T cell counts among the MEASs immunization compared with PBS+adjuvant control group (Supplementary Fig. 7). Revealing a higher ratio of CD3+CD4+ / CD3+CD8+ in MEAS 1 and MEAS 3 than PBS immunized group, MEAS 2 was also higher than PBS immunized group, although there was no statistic difference (Fig. 4f). In conclusion, both humoral and cellular immune responses were induced in the mice immunized with MEASs.
Immunoprotective analyses by survival curve measure and histopathologic examination in a mouse infection model
To assess the protective efficacy of three MEASs against S. suis infection, we employed highly virulent strains SS2 (ZY05719) and SS9 (GZ0565) as challenge agents. Immunized mice received intraperitoneal injections containing 5×108 CFU/mouse (ZY05719) and 3×108 CFU/mouse (GZ0565) for challenge purpose. As shown in Fig. 5a and b, the mortality rate in the PBS immunization group were 100%, regardless of infection with ZY05719 or GZ0565. Conversely, both MEAS 1 and MEAS 3 demonstrated protective percentages of 80% and 70% against SS9 (GZ0565) and SS2 (ZY05719), while MEAS 2 only provided 40% and 20% protection against SS9 and SS2 (ZY05719), respectively. These findings suggest that MEAS 1 and MEAS 3 provide comparably effective protection against S. suis infection, whereas MEAS 2 was less effective in comparison.
a The survival curve for each group (n = 10) challenged with 5×108 CFU/mouse of ZY05719. b. The survival curve for each group (n = 10) challenged with 3 × 108 CFU/mouse of GZ0565. c, d Histopathological analysis from different groups challenged with 3 × 108 CFU/mouse of ZY05719 and GZ0565. The green arrow indicated lymphocytes infiltration; the blue arrow indicated shrinking nucleus neurons hyperchromatic; the yellow arrow indicated hydropic degeneration of hepatocytes.
Histopathological analysis of the liver, spleen, and brain tissues from mice infected with S. suis strains ZY05719 and GZ0565 revealed notable differences between immunized and non-immunized groups. Compared to the uninfected control group (Supplementary Fig. 8), the PBS immunization group displayed visible lymphocyte infiltration in the meninges, along with numerous shrunken neurons with hyperchromatic nuclei and unclear boundaries between the nucleus and cytoplasm (Fig. 5c, d). While these features were not observed in the MEASs immunization groups. In the case of ZY05719 infection, liver tissue in the PBS immunization groups showed visible insoluble fiber proteins in the blood vessels and lymphatic cells, which was not observed in the MEAS 1 and MEAS 2 immunization groups. However, a small amount of lymphocytes were observed in the blood vessels of the MEAS 3 immunization group (Fig. 5c). Additionally, GZ0565 infection resulted in watery degeneration of a large number of liver cells, which was less pronounced in the MEASs immunization group (Fig. 5d). In addition, both ZY05719 and GZ0565 challenge led to severe splenic congestion in the unprotected groups, indicating abnormal splenic blood circulation (Fig. 5c, d). In stark contrast, this congestion was substantially ameliorated in mice vaccinated with MEAS proteins. These findings collectively suggesting that MEAS immunization could effectively protect mice from these pathological manifestations.
Immunoprotective analyses by measuring bacterial loads and inflammatory response levels of organs
The challenge experiments were modified with a lower inoculation dose at 3 × 108 CFU/mouse for strains ZY05719 and GZ0565 to assess the bacterial loads and inflammatory response levels of organs. Autopsies were performed on mice after 12 h, and the bacterial distribution in organs was quantified. Comparative analyses revealed that MEAS 1 and MEAS 3 immunization significantly lowered the bacterial burden of ZY05719 and GZ0565 in the bloodstream, brain, and spleen (Fig. 6a, b). Conversely, for the MEAS 2 group, bacterial loads reduction was comparable to the PBS group against ZY05719 (Fig. 6a), but MEAS 2 maintained efficacy in decreasing the levels of GZ0565 across various organs (Fig. 6b). These findings suggested that MEAS 1 and MEAS 3 were effective in conferring cross-protection against the infections by SS2 and SS9, while MEAS 2 offered subdued protection specifically against SS2.
Measured the bacterial loads and levels of inflammatory response in organs of each group (n = 3) challenged with 3 × 108 CFU/mouse of two strains. a, b Alive bacterial loads for tissues from different groups of challenged mice. c, d Cytokine transcription levels in Brain of mice from different groups. The gapdh was used as internal reference. Significance (P)-value summary analysed by Unpaired two-tailed Student’s t-test (*P < 0.05; **P < 0.01; ***P < 0.001; ns, no significance).
S. suis infection could lead to toxic shock syndrome, which was marked by an intense inflammatory response45. Therefore, it was crucial to modulate inflammation to effectively combat S. suis infection. To evaluate the inflammatory response in organs of immunized mice upon S. suis infection, we measured the transcription levels of inflammatory cytokines in brain tissue. The proinflammatory cytokines were firstly analyzed comparing to the group receiving PBS+adjuvant immunization, and most of them in MEAS 1 and MEAS 3 immunized groups exhibited a downward trend during S. suis infection (Fig. 6c, d), although individual data was partially deviated in GZ0565 infected group. In contrast, the MEAS 2 group presented the expression levels akin to those observed in the PBS+adjuvant-immunized group. Regarding anti-inflammatory factors, the immunization with MEAS 1 and MEAS 3 induced an increase in IL-4 transcription in the ZY05719 infection scenario (Fig. 6c), whereas in the GZ0565 infection instance, IL-4 transcription was notably lower in MEAS 3 than in the PBS-immunized group (Fig. 6d). This difference demonstrated that the immunization with MEAS 1 and MEAS 3 facilitated a swift immunological response to infection, enhancing the host’s ability to manage the inflammatory reaction effectively.
Assessment of the immune oposonization of MEASs’ antiserum
The humoral immune system, a cornerstone of the body’s defense mechanism against pathogens, encompasses antibodies, lysozymes, complement proteins, and others that were critical in neutralizing pathogens and amplifying immune responses. In this investigation, we scrutinized the impact of MEASs’ serum on the activity of immune cells and its potential to bolster the clearance of S. suis through immunoclearance mechanisms. To affirm the cross-protective nature of MEASs against a broad spectrum of S. suis serotypes, we extended our assessment beyond the SS2 strain ZY05719 and SS9 strain GZ0565, incorporating strains from SS5, SS31, SS chz: HN105, WUSS147, and CZ130302. Due to MEAS 2 showed relatively mediocre immune protection in vivo, we didn’t measure its related data in subsequent experiments. Upon treatment of these selected S. suis strains with corresponding MEASs’ antiserum, we assessed their survival in the presence of swine blood and RAW264.7 cells to measure live bacteria. When exposed to swine blood, the results indicating a significant decrease in the bacterial counts for all serotypes after being incubated with hyperimmune sera from both MEAS 1 and MEAS 3 immunizations, in comparison to the PBS immunization groups (Fig. 7a and Supplementary Fig. 9a). Following incubation with MEAS 1 and MEAS 3 hyperimmune serum, statistical analysis of macrophage activity revealed a significant increase in the phagocytosis and bactericidal activity against S. suis. The serum from MEAS 1 and MEAS 3 immunizations significantly improved RAW264.7 macrophage phagocytosis (Fig. 7b and Supplementary Fig. 9b), particularly improving the sterilization efficiency against strains WUSS147 and CZ130302 (Fig. 7c and Supplementary Fig. 9c). These findings highlighted the strong opsonizing properties of MEAS 1 and MEAS 3 immune serum, which effectively enhanced the phagocytic clearance of various S. suis serotypes.
a After incubating the MEAS 1 and MEAS 3 immune serum with HN105, WUSS147, and CZ130302, fresh swine blood was added. The live bacterial count was recorded at 1, 2, 4, and 6 h, calculated the survival rate and drew the survival curve. b The three MEAS immunized sera were incubated with HN105, WUSS147, and CZ130302, then added to RAW246.7 cells and incubated. The number of viable bacteria was counted at 0 h, and the phagocytosis rate was calculated. c From the time of phagocytosis, the number of viable bacteria was counted after 1, 2, and 3 h, and the survival rate was calculated, drew the survival curve. Significance (P)-value summary analysed by Unpaired two-tailed Student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001, ns no significance).
Discussion
S. suis disease represents a significant challenge in pig farming, with repercussions on both economic productivity and animal welfare. The zoonotic potential of S. suis further escalates its public health relevance2. To address this, our research employed reverse vaccinology and immunoinformatics techniques to screen conserved proteins across 11 S. suis serotypes. From these proteins, we identified 16 high-quality epitopes based on their physical and chemical properties. To optimize the expression and immunogenicity of multi-epitope recombinant proteins, we developed three fusion antigen constructs (MEAS 1/2/3) using a validated linker “GPGPGLRMKLPKS”36. These constructs were engineered to enhance their capacity to engage with immune system components. Furthermore, we attached a dendritic cell-targeting peptide “FYPSYHSTPQRP” to the C-terminal of the antigen constructs to improve their immune recognition and uptake by dendritic cells in both human and porcine hosts46,47,48. Preliminary assessments showed that these fusion antigens stimulate strong immune responses, including both humoral and cellular immunity. However, immunization of MEAS 2 was weaker than MEAS 1&3 in both humoral and cellular immune responses (Fig. 4). In murine models, vaccinations with MEAS 1 and MEAS 3 conferred significant protection against lethal doses of SS2 and SS9, rather than MEAS 2. This may be the reason why vaccination with MEAS 1 and MEAS 3, but not MEAS 2, resulted in significant protection against lethal doses of SS2 and SS9 in the mouse model. MEAS 1&3 also demonstrated enhancement in immune serum survival and phagocytic activity against other S. suis serotypes, such as SS5, SS31, and SS chz. In conclusion, following the immunization with MEAS, the host’s immune system experienced a cascade of responses. Antigen-presenting cells (APCs), T cells, and B cells were activated, orchestrating a targeted antibody production against S. suis. This immune activation also led to the recruitment and activation of macrophages and neutrophils, which significantly enhanced the phagocytic clearance of the pathogen during S. suis infection (Fig. 8). Both MEAS 1 and MEAS 3 exhibited strong potential as the foundation for an effective cross-protective vaccine against S. suis, which could significantly reduce the incidence of infection in vulnerable populations.
Following immunization with MEAS, dendritic cells became activated and promoted lymphocyte proliferation within the host. MEAS bound to MHC molecules, which were then recognized by T cell receptors, and promoted T cell differentiation. This leaded to an increased ratio of CD4+ T cells and the secretion of Th1 and Th2 cytokines. Concurrently, B cells were activated, differentiating into plasma cells that secreted specific IgG. During infection with S. suis, activated B and T cells rapidly, resulting in the secretion of specific antibodies and inflammatory cytokines. This accelerated antigen presentation by APCs facilitated increased antibody recognition and neutralization of S. suis. The process recruited macrophages and neutrophils, boosting phagocytic clearance of S. suis during infection. Figure 8 was created in BioRender. Kaka, L. (2024) BioRender.com/f35g115.
In recent years, Southeast Asian populations have encountered an increased risk of S. suis infection, primarily through close contact with animals or consumption of raw pork products10,49. Additionally, S. suis has emerged as a significant occupational hazard in the pork industry in Western countries, not only affecting pigs and workers10. Human cases have shown a notable increase globally50,51. S. suis encompasses multiple serotypes, with 11 identified as infecting humans worldwide. SS2 and SS1/2, associated with clonal complex 1 (CC1), exhibited high virulence52. SS8 was another significant pathogenic serotype causing clinical diseases worldwide10,53,54. While SS chz, a novel variant, could cause meningitis in piglets55. Based on this, we screened conservative antigens that could potentially provide coverage against diverse high prevalent and virulent serotypes, including 1, 2, 1/2, 3, 5, 7, 8, 9, 14, 31, and chz. Due to S. suis early colonization of piglets, elimination through early weaning is impractical7, heightening the importance of vaccination as the most promising strategy for infection prevention2. Despite extensive research, current vaccines for S. suis have not achieved homologous protection, and there was a scarcity of studies evaluating cross-protection against other serotypes or heterologous strains14. At present, a universal vaccine to control multiple serotypes S. suis remains elusive. Multi-epitope vaccines are emerging as a novel and promising type due to their broad coverage and robust immune protection capabilities. To enhance cross-protection in epitope-based vaccines, it’s crucial to select conservative proteins that are present in different strains. Employing reverse vaccinology, we identified 8 highly conserved antigens which could potentially serve as targets for novel vaccine development in S. suis:
The DAC belongs to the polysaccharide deacetylase family, which can modify the cell wall to enhance biofilm formation, evasion of neutrophil phagocytosis, or resistance to lysozyme56,57,58. S5nA was an immunogenic surface 5’ nucleotidase, which has been shown to enhance the anti-phagocytosis, survival of in blood and contribute to immune evasion in diverse Streptococcal pathogen59,60,61, including S. suis62. Furthermore, numerous S. suis isolates encode another putative 5’ nuclease, designated as pS5nA, have been reported to associated with virulence in SS9 based on the comparative secretomics analysis of virulent and less virulent strains63. Superoxide dismutase (SOD) was a virulence factor found in many pathogens that reduces the oxidative burst of phagocytic cells. One such SOD, Mdsd, was conserved in S. suis64, and it likely helped S. suis resist oxidative stress by neutralizing ROS within macrophages to influence pathogen’s virulence65. SlsP was a subtilisin-like serine protease encoded on the cell surface, and have been identified as a virulence factor66. It could inhibit thrombin-mediated fibrin formation, contributing to its virulence mechanism67, while its immunization in a multicomponent vaccine did not demonstrate strong protective efficacy68. HtpS has been identified as an immunogenic surface protein in S. pneumoniae and S. suis69,70. And HtpS has been demonstrated to confer protection against SS2 infection70. CdaA, a highly conserved membrane protein in Gram-positive bacteria, interacted with membrane regulatory proteins like CdaR and influenced the steady-state formation of cell walls71,72. Extracellular HtrA serine proteases are exposed to the extracellular environment, promoting bacterial colonization and invasion of host tissues, disrupting epithelial barriers and causing substantial damage to host cells73. Inhibition of its proteolytic activity in vaccine development has been explored for its applications in antimicrobial therapy74,75.
Mice have proven to replicate typical clinical symptoms of S. suis infection, making them a convincing ideal model for studying infection and immunity characteristics related to this pathogen42. Our results showed that MEASs immunized mice produced robust Th1 and Th2 immune responses and strong antibody responses. Th1 and Th2 cell clones specifically stimulate antigen-specific B cells to secrete IgG2a and IgG1, respectively76. The differential levels of IgG subclasses induced by antigens reflect the host’s immune response pattern77,78. In our study, both of IgG1 and IgG2 levels significant increased, and higher IgG2 levels compared to IgG1 suggested dominance of a Th1 immune response following vaccination. This observation was supported by the expression of Th1 cytokines IFN-γ and Th2 cytokines IL-4. The Th1 type immune response played a crucial role in cell-mediated immune responses against intracellular pathogens79. CD4+ T cells identified MHC II classes of molecules on exogenous antigen peptide to differentiate into Th cells. Increased CD4+ T cell counts following vaccination indicate enhanced antigen recognition and cytokine secretion to aid in antibody production, as well as the activation of macrophages and other effector cells79. Indeed, similar phenotypes were also observed in this study, verifying the potential of MEASs as immune protective antigens. S. suis infection characterized a storm of inflammatory factors45. We also detected the level of inflammatory cytokines after S. suis infection, there was inconsistent in anti-inflammatory factor IL-4 between ZY05719 and GZ0565. ZY05719 infection progresses rapidly in mice and can cause signifcantly higher bacterial load and inflammatory response in infected organs comparing with GZ0565 infection, resulting in increased levels of anti-inflammatory factors than the control group (untreated mice) but lower than the PBS+adjuvant-immunized group. In contrast, GZ0565 infection progresses slowly with relatively lower bacterial load and inflammation in infected organs, thus the host need not to activate an excessive anti-inflammatory response. These results demonstrated that MEAS 1 and MEAS 3 could regulate the development of inflammation.
High titers of specific IgG were elicited by immunization with MEAS 1 and MEAS 3. S. suis infection was typically involved extracellular CPS that evaded neutrophil destruction and phagocytosis, making oposonization crucial for combating the pathogen in infected animals2,80. Therefore, we investigated whether serum from MEASs-immunized contained effective opsonization. Evaluated the survival of S. suis under these conditions by incubating immune serum treated bacteria with fresh swine blood and macrophages. The results demonstrated that compared to negative serum, immune serum from MEASs immunization significantly reduced the survival rate. And the opsonization demonstrated efficacy across various serotypes of S. suis, suggesting that MEAS 1 and MEAS 3 exhibited broad cross-protective capabilities.
In short, we developed multi-epitope antigens against S. suis and verified MEAS 1 and MEAS 3 as effective fusion antigens with robust cross-protection capabilities both in vitro and in vivo. This study highlighted MEAS 1 and MEAS 3 as promising candidate antigens for the development of S. suis vaccines, which could enrich the variety of vaccine components available for combatting this pathogen.
Methods
Bioinformatic analysis for screening potential B and T cell epitopes
To screen for conserved protective antigens among prevalent and virulent serotypes of S. suis strains in China, 100 strains were selected from the NCBI database (https://www.ncbi.nlm.nih.gov/), which informations were shown in Supplementary Table 1. This selection comprised 39 complete genomes and 61 scaffold genomes, encompassing a total of 11 highly prevalent and pathogenic serotypes, including 1, 2, 1/2, 3, 5, 7, 8, 9, 14, 31, and chz. Through Prokka annotation tool81 and Roary software82, pan-genome analysis and visualization of results were performed, resulting in conserved proteins in the candidate genomes mentioned above.
We utilized the PSORTb server (version 3.0.2; https://www.psort.org/psortb/) for the conservative protein subcellular localization prediction, and through CCTOP online website (v1.1.0; https://cctop.ttk.hu/) and TMHMM server (https://services.healthtech.dtu.dk/services/TMHMM-2.0/) for integrated analysis of the transmembrane region, Proteins distributed on the cell wall surface and extracellular were screened for subsequent analysis.
In order to ensure whether the candidate proteins could induce immune response, the antigenicity of the above candidate proteins was analyzed by VaxiJen server (v.2.0; https://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html)83, and the proteins with good antigenicity were screened. Next, candidate antigens were then selected by analyzing the in vitro and in vivo transcriptome data40 and comparing the identified differential proteins of S. suis obtained from the blood of infected pigs41.
The candidate proteins were first predicted by linear epitope analysis through ABCpred server (https://webs.iiitd.edu.in/raghava/abcpred/ABC_submission.html)84,85, and then the epitopes with high affinity to MHC class I and II molecules in the candidate proteins were predicted by IEDB TepiTool (http://tools.iedb.org/tepitool/)86. Selection was based on the most frequent human MHC class II alleles (DRB1 * 01:01, DRB1 * 03:01, DRB1 *04:01, DRB1 * 07:01, DRB1 * 11:01, DRB1 * 13:01, DRB1 * 07:01, DRB1 * 11:01, DRB1 * 13:01, DRB1 * 15:01, DRB3*01:01, DRB3*02:02, DRB4*01:01 and DRB5*01:01) were screened for peptides with 50% allele coverage. The epitopes with high affinity to swine leukocyte antigen (SLA)87 (SLA-1* 0101, SLA-1* 0401, SLA-1* 0701, SLA-2* 0101, SLA-2* 0401) were evaluated. Peptides covering both B cell and T cell epitopes were screened out, and their antigenicity was evaluated by VaxiJen.
Prevalence study
In 2022, a total of 1500 tonsil samples were collected from five provinces in China: Guangxi Zhuang Autonomous Region, Sichuan Province, Hebei Province, Inner Mongolia Autonomous Region, and Guizhou Province. The positive rate of S. suis was detected using specific primers88. A molecular epidemic analysis of candidate antigens was conducted on 106 clinical isolates obtained from these samples, and the distribution of candidate proteins among the clinical isolates was calculated, specific primers were listed in the Supplementary Table 2.
Multiple epitope antigens design
The hydrophilic and hydrophobic indexes of the candidate epitopes were obtained by Expasy ProtParam tool (https://web.expasy.org/protparam/)89. According to the antigenicity, the number of epitopes covered and the recognition preference of T cells, three sets of fusion antigen schemes were constructed by linking the epitopes with “linker” (GPGPGLRMKLPKS)36. A dendritic cell-targeting peptide (FYPSYHSTPQRP) was added to the C terminus, which could target both human and porcine dendritic cells47,48,90. The designed MEAS sequences were uploaded to Expasy server to analyze the physicochemical properties of fusion antigens, then used AllergenFP (v.1.0, http://www.ddg-pharmfac.net/AllergenFP/method.html) to predict the sensitization and Toxin Pred 2 server (https://webs.iiitd.edu.in/raghava/toxinpred2/)91 to predict the toxicity of MEASs (Supplementary Table 5).
The MEAS sequences were uploaded to PSIPRED Workbench (PSIPRED 4.0, http://bioinf.cs.ucl.ac.uk/psipred/) to predict the secondary structure92,93, and the tertiary structure of the above antigens was predicted by I-TASSER (https://zhanggroup.org//I-TASSER/)94,95. Choose highest C-Score in the simulation model uploaded to GalaxyRefine server (https://galaxy.seoklab.org/)96, to obtain the optimized tertiary structure for subsequent analysis.
Molecular docking and simulation of immunity
To determine whether MEASs can effectively bind to immune receptors, molecular docking was performed using the Cluspro 2 server (https://cluspro.bu.edu/home.php)97,98 to predict interactions between MEAS 1/2/3 and human major histocompatibility complex MHC I (PDB ID: 4u6y), MHC II (PDB ID: 5jlz), Toll-like receptor 2 (TLR2) (PDB ID: 2z7x), Toll-like receptor 4 (TLR4) (PDB ID: 4g8a), and porcine leukocyte antigen SLA-1 (PDB ID: 3qq3). The docking results were visualized with Pymol, and the interacting amino acid residues were visualized using PDBsum99,100.
In order to assess the MEASs immune vaccine after the possibility of an immune response, using C-IMMSIM server (https://kraken.iac.rm.cnr.it/C-IMMSIM/)to simulate immune response101. The time steps of the three injections in the simulation phase were 1, 42, and 84, respectively. The standard time step of one injection was 8 h, corresponding to a 14 day gap between immunizations, with all other parameters set to their default values.
MEASs expression and purification
Based on the designed sequences, the recombinant plasmids pET28a-meas 1, pET 28a-meas 2 and pET28a-meas 3 were constructed and synthesized by Tsingke Biotechnology Company (Nanjing, China). The recombinant plasmids were then transformed into E. coli BL21(DE3) competent cells, construction of recombinant bacteria rMEAS 1, rMEAS 2 and rMEAS 3. Induction was carried out with IPTG at a final concentration of 500 μM, at 16 °C and 120 rpm for 16 h. Purification was performed using His-tag Ni-NTA affinity chromatography. The expression of MEAS after induction was identified by SDS-PAGE electrophoresis, and further confirmed by Western Blot analysis.
Immuneoreactivity detection
The concentration of purified MEASs was determined by BCA kit (Thermo, USA), then diluted to 10 µg/ml with sodium carbonate buffer (pH 9.6) and plated on microplate at 100 µL/well. The wells were blocked with PBST containing 0.5% BSA for 1 h at 37 °C at 200 µL/well. Adding different serotype source of S. suis rabbit antiserum (producted and saved in WOAH Reference Lab for Swine Streptococcosis, Nanjing, China) to incubation (1:2000) for 2 h at 37 °C, PBST washed three times, and horseradish peroxidase coupling goat anti rabbit IgG antibody (1:200) for 1 h at 37 °C. Then PBST washed three times. Used tetramethyl benzidine substrates (Vazyme, China) to detected antibody binding level. Absorbance was measured at 450 and 570 nm according to the manufacturer’s instructions. Calculated ratio of positive to negative serum. When the value was ≥ 2.1, defined positive.
Bacterial strains and culture
S. suis strains SS2 ZY05719, SS5 HN105, SS9 GZ0565, SS31 WUSS147, SS chz CZ130302, which were stored in our laboratory and were highly pathogenic. All S. suis cultures were grown at 37 °C at 5% CO2 in Todd Hewitt broth (THB) (Hopebio, Qingdao, China) or in THB agar plate supplemented with 5% (v/v) sheep blood. BL21 containing the recombinant vector were cultured in LB medium with kanamycin at 37 °C.
Immunization of mice and rabbits and detection of specific antibody levels
Animal studies adhered to ethical guidelines set forth by the Experimental Animal Welfare and Ethics Committee of Nanjing Agricultural University (Approval ID: SYXK(SU)2021-0086). All procedures followed strict regulations outlined by the relevant animal welfare agency. All animals were purchased from Comparative Medicine Center, Yangzhou University. MEAS and ISA 206 VG adjuvant (SEPPIC, France) mix ratio 1:1 by volume, and fully vortex emulsification, preparation of MEAS subunit vaccines. Five weeks of SPF female Blab/c mice were randomly divided into 4 groups (n = 40 each group) and marked carefully. Four-week-old New Zealand white rabbits were randomly divided into 4 groups and marked carefully, with 2 rabbits in each group. The first group of immune PBS+adjuvant was set to the control group; The remaining three groups were immunized with MEAS 1, MEAS 2 and MEAS 3, and the immunization doses were 50 μg/mouse and 500 μg/rabbit, respectively. Groups were the subcutaneous multipoint injection immune pathways, immune procedure was as follows: once every 14 days immune, dose for mice was 0.2 mL/mouse, dose for rabbit was 1 mL/rabbit, there were three times totally. On the 13th day after each immunization, three mice were randomly selected and were anesthetized rapidly with an isoflurane to collected blood samples from the orbital vein to separate serum. For rabbits, blood samples were collected from ear vein to separate serum after each immunization. On the 13th day after third immunization, the rabbits were anesthetized with intravenous Pentobarbital Sodium at a dosage of 30 mg/kg. Limb reflexes were assessed by squeezing the hind toes of the rabbits until no response, indicating successful anesthesia. Approximately 50 ml of blood was collected from the heart to separate serum and ensure euthanized by intravenous injection of Pentobarbital Sodium at a dosage of 100 mg/kg subsequently.
Purified MEASs was diluted to 10 µg/ml with sodium carbonate buffer (pH 9.6) and plated on microplate at 100 µL/well. The cells were blocked with PBST containing 0.5% BSA for 1 h at 37 °C at 200 µL/well. Adding different sources of MEAS 1–3 antiserum at 1:200 and doubling dilution to 1:204800, to incubation for 2 h at 37 °C, PBST washed three times, and horseradish peroxidase coupling goat anti mouse/rabbit IgG antibody (1:200) for 1 h at 37 °C. Then PBST washed three times. Used tetramethyl benzidine substrates (Vazyme, China) to detected antibody binding level. Absorbance was measured at 450 and 570 nm according to the manufacturer’s instructions. Calculated ratio of positive to negative serum. When the value was ≥2.1, defined positive. The last positive dilution rate was the serum titer.
Western blot
The reactivity of the immunized serum with MEASs were detected by Western Blot. Purified MEASs was separated on 12.5% SDS-PAGE gels and through the semi-dry transferred to the PVDF membrane. Blocking the membrane with 5% skimmed milk for 1 h at room temperature, and then incubated with a 1:2000 dilution of anti-MEAS 1/2/3 serum respectively for 2 h at room temperature. After PBST washed three times, change incubation to goat anti-mouse IgG conjugated to horseradish peroxidase (HRP). PBST washed three times again, then visualization by using the method of chemiluminescence detection results.
Subtype IgG level detection
Thirteen days after three times immunization, collected the serum. Serum was diluted by 1:100,000, subtype classes of serum were identified through a Mouse Monoclonal Antibody Isotype ELISA Kit (Proteintech, USA).
Mice challenge and bacterial load analysis of organs
Fourteen days after three times immunization of mice were administered by abdominal cavity infection SS2 strain ZY05719 (n = 10) and SS9 strain GZ0565 (n = 10), dose respectively 5 × 108 CFU/mouse and 3 × 108 CFU/mouse. Mice were observed daily after challenge, morbidity and mortality were recorded for 7 days. Two strains of bacteria doses were adjusted for 3 × 108 CFU/mouse (n = 15, respectively), after infection 12 h, the ethical and technical specifications for rapid cervical dislocation were strictly followed and the mice were guaranteed to be euthanized. Mice were autopsied, and different organs were homogenized with PBS as the ratio 1 g / 1 mL and then diluted to THB agar plate to calculate the number of bacteria (n = 5, each group).
Cytokine transcription level analysis
Tissue RNAs (n = 5, each group) were performed using FastPure cell/tissue Total RNA Isolation Kit V2 (Vazyme Biotech Co., Nanjing, China) according to the manufacturer’s instructions. RNA was reverse transcribed into cDNA using HiScript II Q RT SuperMix (Vazyme, Nanjing, China). Using ChamQ SYBR qPCR Master Mix (Vazyme China) joint QuantStudio 6 Flex instrument (Thermo the world’s science and technology, Shanghai, China) to validate the transcriptional level of cytokines il-1β, il-4, il-6, ifn-γ and tnf-α, and select gapdh as internal genes. The final data were counted using the 2−∆∆CT method and each group contained three replicates. The primer sequences were provided in Supplementary Table 3.
Analysis of pathological sections
Mice spleen, brain, liver with 4% paraformaldehyde fixed (n = 3, each group), paraffin embedding, cut into thin slices of 5 μm, fixed on the slide, by Hematoxylin - Eosin (HE) staining, microscope observation. After image captured, Slide Viewer software was used to analyse each viscera pathological changes.
Lymphocyte proliferation assay
Fourteen days after the last immunization, three mice were anesthetized with isoflurane, and rapid cervical dislocation to ensure humane euthanasia. Mice were autopsied and the spleens were isolated, sterile removed spleen on 70 μm sterile cell mesh internal grinding gently with a syringe, and add the RPMI 1640 medium (Gibico, USA), after filtration tissue residue, made the spleen cell suspension. According to the spleen lymphocyte separation kit (Solarbio, China), separation of lymphocytes. Used trypan blue staining for lymphocytes count, adjust the cell density of 5 × 105 / mL. The above cell suspension inoculation in 96 well plates, with 200 μL/ well; 10 μg/well of purified MEAS was added, cultured for 48 h at 37°C in 5% CO2. And CCK8 reagent (Beyotime, China) was added at 20 μL/ well, the cells were cultured for 4 h at 37°C in 5% CO2. Then, the absorbance value was detected at 450 nm, and the stimulation index (SI) of spleen cells was calculated.
IL-4 and IFN-γ secretion analysis
The lymphocytes administered in 12 wells plate, with 20 μg/well MEAS stimulated spleen lymphocytes, cultured for 48 h at 37°C in 5% CO2, and collected the culture supernatant. The detection of cytokines secretion in the supernatant through Mouse IL-4 and IFN-γ ELISA kit (Proteintech, USA). The operation was performed according to the instructions, and the cytokine content in the supernatants of stimulated lymphocytes were calculated after drawing the standard curve.
Flow cytometry
After the third immunization collected spleen lymphocytes (n = 3), adjusted the cell density to 5 × 106 / tube. Fluorescent-labeled antibodies: APC-CD3 (0.5 μg /106 cells), PE-CD4 (0.25 μg / 106 cells), and FITC-CD8a (1 μg /106 cells) (BioLegend, UK) were added and incubated at room temperature in the dark for 30 min. Then cells were washed with PBS three times and resuspended in 500 μL PBS. CD3+CD4+ and CD3+CD8+ T cells were analyzed by flow cytometer (BD, USA). Data analysis was performed using FlowJo (v10.8.1).
Analysis of opsonization
SS2, SS5, SS9, SS31, SS chz representative strains ZY05719, HN105, GZ0565, WUSS147 and CZ130302 were cultured to logarithmic phase in THB respectively. The bacterial solution was washed three times in PBS and diluted to 107CFU/mL. 100 µL of MEASs immunization serum as well as negative serum were mixed with the same amount of bacterial solution and incubated at 37 °C for 30 min. After incubation, 800 µL of porcine peripheral blood were added and incubated at 37 °C. Samples were taken every 1 h, 2 h, 4 h, and 6 h, and the number of viable bacteria in the blood were calculated by counting on THB agar plates diluted in sterile PBS gradient.
Other all three strains will be diluted to 108 CFU/mL, 100 µL of MEASs immunization serum as well as negative serum were mixed with the same amount of bacterial solution and incubated at 37 °C for 30 min. After centrifugation, the cells were resuspended in 100 µL DMEM medium and seeded in RAW264.7 with MOI = 100, incubated at 37°C in 5% CO2 for 1.5 h. After discarding the supernatant, the cells were washed three times with sterile PBS and then replaced with DMEM medium containing 5 µg/mL penicillin and 100 µg/mL gentamicin to kill the extracellular bacteria. The incubation was continued for 1.5 h. Discarded the supernatant and washed 7 times by PBS, taken the final washing liquid to THB agar plates to detect extracellular bacteria amount. Then added 500 µL ddH2O to destroy the cells, the number of intracellular bacteria was recorded as the amount of bacteria at 0 h, and the phagocytosis rate was calculated. After 1, 2, 3 h of incubation, the number of bacteria was counted, and the survival rate of intracellular bacteria was calculated according to the number of bacteria at 0 h as a reference to evaluate the opsonization effect of serum on macrophages.
Data statistics
All data were analyzed by t-test or one-way ANOVA in GraphPad Prism 6.0 software (San Diego, CA, USA) to evaluate the difference between groups. Data were expressed as mean ± standard deviation (mean ± SD), and differences were indicated when p-values < 0.05 (ns P ≥ 0.05, *P < 0.05, **P < 0.01, ***P < 0.001).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Tan, C., Zhang, A., Chen, H. & Zhou, R. Recent proceedings on prevalence and pathogenesis of Streptococcus suis. Curr. issues Mol. Biol. 32, 473–520 (2019).
Segura, M. Streptococcus suis research: progress and challenges. Pathogens 9, 707 (2020).
Bonifait, L., Veillette, M., Létourneau, V., Grenier, D. & Duchaine, C. Detection of Streptococcus suis in bioaerosols of swine confinement buildings. Appl. Environ. Microbiol. 80, 3296–3304 (2014).
Dutkiewicz, J. et al. Streptococcus suis: a re-emerging pathogen associated with occupational exposure to pigs or pork products. Part II - pathogenesis. Ann. Agric. Environ. Med. AAEM 25, 186–203 (2018).
Dong, X. et al. The global emergence of a novel Streptococcus suis clade associated with human infections. EMBO Mol. Med. 13, e13810 (2021).
Feng, Y., Zhang, H., Ma, Y. & Gao, G. F. Uncovering newly emerging variants of Streptococcus suis, an important zoonotic agent. Trends Microbiol. 18, 124–131 (2010).
Segura, M. et al. Update on Streptococcus suis research and prevention in the era of antimicrobial restriction: 4th international workshop on S. suis. Pathogens (Basel, Switzerland) 9, 374 (2020).
Bojarska, A. et al. Diversity of serotypes and new cps loci variants among Streptococcus suis isolates from pigs in Poland and Belarus. Vet. Microbiol. 240, 108534 (2020).
Zhu, J. et al. Streptococcus suis serotype 4: a population with the potential pathogenicity in humans and pigs. Emerg. microbes Infect. 13, 2352435 (2024).
Goyette-Desjardins, G., Auger, J. P., Xu, J., Segura, M. & Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. microbes Infect. 3, e45 (2014).
Kang, W. et al. Investigation of genomic and pathogenicity characteristics of Streptococcus suis ST1 human strains from Guangxi Zhuang Autonomous region (GX) between 2005 and 2020 in China. Emerg. Microbes Infect. 13, 2339946 (2024).
Maneerat, K. et al. Virulence genes and genetic diversity of Streptococcus suis serotype 2 isolates from Thailand. Transbound. Emerg. Dis. 60, 69–79 (2013).
Atanassov, C., Bonifait, L., Perivier, M., Gottschalk, M. & Grenier, D. Candidate proteomic biomarkers for three genogroups of the swine pathogen Streptococcus suis serotype 2. BMC Microbiol. 15, 84 (2015).
Segura, M. Streptococcus suis vaccines: candidate antigens and progress. Expert Rev. Vaccines 14, 1587–1608 (2015).
Fan, Q., Wang, H., Wang, Y., Yi, L. & Wang, Y. Evaluation of the protective efficacy of three novel identified membrane associated proteins of Streptococcus suis serotype 2. Micro. Pathog. 193, 106759 (2024).
Yi, L. etal. Evaluation of immune effect of Streptococcus suis biofilm-associated protein PDH. Vet. Microbiol 263, (2021).
Wang, H., Fan, Q., Wang, Y., Yi, L. & Wang, Y. Rethinking the control of Streptococcus suis infection: Biofilm formation. Vet. Microbiol. 290, 110005 (2024).
Wang, J. et al. Identification of a novel linear B cell epitope on the sao protein of Streptococcus suis serotype 2. Front. Immunol. 11, 1492 (2020).
Li, W., Wan, Y., Tao, Z., Chen, H. & Zhou, R. A novel fibronectin-binding protein of Streptococcus suis serotype 2 contributes to epithelial cell invasion and in vivo dissemination. Vet. Microbiol. 162, 186–194 (2013).
Ji, Y., Sun, K., Yang, Y. & Wu, Z. Dihydroartemisinin ameliorates innate inflammatory response induced by Streptococcussuis-derived muramidase-released protein via inactivation of TLR4-dependent NF-κB signaling. J. Pharm. Anal. 13, 1183–1194 (2023).
Garibaldi, M. et al. Immunoprotective activities of a Streptococcus suis pilus subunit in murine models of infection. Vaccine 28, 3609–3616 (2010).
Li, Q. et al. Identification of novel laminin- and fibronectin-binding proteins by far-western blot: capturing the adhesins of Streptococcus suis type 2. Front. Cell. Infect. Microbiol. 5, 82 (2015).
Li, Q. et al. Live attenuated salmonella enterica serovar choleraesuis vector delivering a conserved surface protein enolase induces high and broad protection against Streptococcus suis serotypes 2, 7, and 9 in mice. Vaccine 38, 6904–6913 (2020).
Zhang, S. et al. Discovery of oligosaccharide antigens for semi-synthetic glycoconjugate vaccine leads against Streptococcus suis serotypes 2, 3, 9 and 14*. Angew. Chem. (Int. ed. Engl.) 60, 14679–14692 (2021).
Wisselink, H. J., Vecht, U., Stockhofe-Zurwieden, N. & Smith, H. E. Protection of pigs against challenge with virulent Streptococcus suis serotype 2 strains by a muramidase-released protein and extracellular factor vaccine. Vet. Rec. 148, 473–477 (2001).
Li, Y. A. et al. Salmonella enterica serovar Choleraesuis vector delivering a dual-antigen expression cassette provides mouse cross-protection against Streptococcus suis serotypes 2, 7, 9, and 1/2. Vet. Res. 53, 46 (2022).
Tahir Ul Qamar, M. et al. Reverse vaccinology assisted designing of multiepitope-based subunit vaccine against SARS-CoV-2. Infect. Dis. poverty 9, 132 (2020).
Goodswen, S. J., Kennedy, P. J. & Ellis, J. T. A guide to current methodology and usage of reverse vaccinology towards in silico vaccine discovery. FEMS Microbiol. Rev. 47, fuad004 (2023).
Albutti, A. An integrated multi-pronged reverse vaccinology and biophysical approaches for identification of potential vaccine candidates against Nipah virus. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 31, 101826 (2023).
Tang, X. D. et al. In vitro and ex vivo evaluation of a multi-epitope heparinase vaccine for various malignancies. Cancer Sci. 105, 9–17 (2014).
Cong, H. et al. Comparative efficacy of a multi-epitope DNA vaccine via intranasal, peroral, and intramuscular delivery against lethal toxoplasma gondii infection in mice. Parasit. Vectors 7, 145 (2014).
Zhu, S. et al. Hepatitis B virus surface antigen as delivery vector can enhance Chlamydia trachomatis MOMP multi-epitope immune response in mice. Appl. Microbiol. Biotechnol. 98, 4107–4117 (2014).
Liao, J. et al. A rational designed multi-epitope vaccine elicited robust protective efficacy against Klebsiella pneumoniae lung infection. Biomed. Pharmacother. Biomed. Pharmacotherapie 174, 116611 (2024).
Tahir Ul Qamar, M. et al. Designing multi-epitope vaccine against Staphylococcus aureus by employing subtractive proteomics, reverse vaccinology and immuno-informatics approaches. Comput. Biol. Med. 132, 104389 (2021).
Kalita, A., Kalita, M. & Torres, A. G. Exploiting the power of OMICS approaches to produce E. coli O157 vaccines. Gut Microbes 5, 770–774 (2014).
Zhang, Y. et al. Development and evaluation of a multi-epitope subunit vaccine against group B Streptococcus infection. Emerg. Microbes Infect. 11, 2371–2382 (2022).
Afshari, E., Cohan, R. A., Sotoodehnejadnematalahi, F. & Mousavi, S. F. In-silico design and evaluation of an epitope-based serotype-independent promising vaccine candidate for highly cross-reactive regions of pneumococcal surface protein A. J. Transl. Med. 21, 13 (2023).
Zhang, Y. et al. Development of a universal multi-Eeitope vaccine candidate against Streptococcus suis infections using immunoinformatics approaches. Vet. Sci. 10, 383 (2023).
Liang, S. et al. Combined immunoinformatics to design and evaluate a multi-epitope vaccine candidate against Streptococcus suis infection. Vaccines 12, 137 (2024).
Wu, Z. et al. The Streptococcus suis transcriptional landscape reveals adaptation mechanisms in pig blood and cerebrospinal fluid. RNA 20, 882–898 (2014).
Yu, Y. et al. Infection and adaption-based proteomic changes of Streptococcus suis serotype 2 in a pig model. J. Proteom. 180, 41–52 (2018).
Segura, M., Fittipaldi, N., Calzas, C. & Gottschalk, M. Critical Streptococcus suis virulence factors: are they all really critical? Trends Microbiol. 25, 585–599 (2017).
Ferrante, A., Beard, L. J. & Feldman, R. G. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatr. Infect. Dis. J. 9, S16–S24 (1990).
Vidarsson, G., Dekkers, G. & Rispens, T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 5, 520 (2014).
Ni, C. et al. Fpr2 exacerbates Streptococcus suis-induced streptococcal toxic shock-like syndrome via attenuation of neutrophil recruitment. Front. Immunol. 14, 1094331 (2023).
Xia, T. et al. Screening optimal DC-targeting peptide to enhance the immune efficacy of recombinant Lactobacillus expressing RHDV VP60. Virulence 15, 2368080 (2024).
Golani-Armon, A., Golan, M., Shamay, Y., Raviv, L. & David, A. DC3-decorated polyplexes for targeted gene delivery into dendritic cells. Bioconjug. Chem. 26, 213–224 (2015).
Xia, T. et al. Human dendritic cell targeting peptide can be targeted to porcine dendritic cells to improve antigen capture efficiency to stimulate stronger immune response. Front. Immunol. 13, 950597 (2022).
Takeuchi, D. et al. Impact of a food safety campaign on Streptococcus suis infection in humans in Thailand. Am. J. Tropical Med. Hyg. 96, 1370–1377 (2017).
Kerdsin, A. et al. Genotypic diversity of Streptococcus suis strains isolated from humans in Thailand. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 37, 917–925 (2018).
Gottschalk, M., Xu, J., Calzas, C. & Segura, M. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 5, 371–391 (2010).
Scherrer, S. et al. Genetic diversity and antimicrobial susceptibility of Streptococcus suis from diseased Swiss pigs collected between 2019 - 2022. Vet. Microbiol. 293, 110084 (2024).
Liu, Z. et al. The characteristics of population structure and antimicrobial resistance of Streptococcus suis serotype 8, a non-negligible pathotype. Transbound. Emerg. Dis. 69, e2495–e2505 (2022).
Goyette-Desjardins, G. et al. Structure determination of Streptococcus suis serotypes 7 and 8 capsular polysaccharides and assignment of functions of the cps locus genes involved in their biosynthesis. Carbohydr. Res. 473, 36–45 (2019).
Pan, Z. et al. Novel variant serotype of streptococcus suis isolated from piglets with meningitis. Appl. Environ. Microbiol. 81, 976–985 (2015).
Coullon, H. et al. Peptidoglycan analysis reveals that synergistic deacetylase activity in vegetative Clostridium difficile impacts the host response. J. Biol. Chem. 295, 16785–16796 (2020).
Vollmer, W. & Tomasz, A. Peptidoglycan N-acetylglucosamine deacetylase, a putative virulence factor in Streptococcus pneumoniae. Infect. Immun. 70, 7176–7178 (2002).
Vuong, C. et al. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 279, 54881–54886 (2004).
Dangel, M. L. et al. The 5’-nucleotidase S5nA is dispensable for evasion of phagocytosis and biofilm formation in Streptococcus pyogenes. PloS one 14, e0211074 (2019).
Zheng, L. et al. Streptococcal 5’-nucleotidase A (S5nA), a novel streptococcus pyogenes virulence factor that facilitates immune evasion. J. Biol. Chem. 290, 31126–31137 (2015).
Firon, A. et al. Extracellular nucleotide catabolism by the group B Streptococcus ectonucleotidase NudP increases bacterial survival in blood. J. Biol. Chem. 289, 5479–5489 (2014).
Liu, P. et al. Streptococcus suis adenosine synthase functions as an effector in evasion of PMN-mediated innate immunit. J. Infect. Dis. 210, 35–45 (2014).
Wu, Z., Zhang, W. & Lu, C. Comparative proteome analysis of secreted proteins of Streptococcus suis serotype 9 isolates from diseased and healthy pigs. Micro. Pathog. 45, 159–166 (2008).
Glazunova, O. O., Raoult, D. & Roux, V. Partial sequence comparison of the rpoB, sodA, groEL and gyrB genes within the genus Streptococcus. Int. J. Syst. Evolut. Microbiol. 59, 2317–2322 (2009).
Tang, Y., Zhang, X., Wu, W., Lu, Z. & Fang, W. Inactivation of the sodA gene of Streptococcus suis type 2 encoding superoxide dismutase leads to reduced virulence to mice. Vet. Microbiol. 158, 360–366 (2012).
Bonifait, L. et al. The cell envelope subtilisin-like proteinase is a virulence determinant for Streptococcus suis. BMC Microbiol. 10, 42 (2010).
Bonifait, L., Vaillancourt, K., Gottschalk, M., Frenette, M. & Grenier, D. Purification and characterization of the subtilisin-like protease of Streptococcus suis that contributes to its virulence. Vet. Microbiol. 148, 333–340 (2011).
Weiße, C. et al. Immunogenicity and protective efficacy of a Streptococcus suis vaccine composed of six conserved immunogens. Vet. Res. 52, 112 (2021).
Ogunniyi, A. D. et al. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 23, 731–738 (2009).
Shao, Z. et al. HtpS, a novel immunogenic cell surface-exposed protein of Streptococcus suis, confers protection in mice. FEMS Microbiol. Lett. 314, 174–182 (2011).
Mehne, F. M. et al. Cyclic di-AMP homeostasis in bacillus subtilis: both lack and high level accumulation of the nucleotide are detrimental for cell growth. J. Biol. Chem. 288, 2004–2017 (2013).
Gundlach, J. et al. An essential poison: synthesis and degradation of cyclic Di-AMP in Bacillus subtilis. J. Bacteriol. 197, 3265–3274 (2015).
Backert, S., Bernegger, S., Skórko-Glonek, J. & Wessler, S. Extracellular HtrA serine proteases: an emerging new strategy in bacterial pathogenesis. Cell. Microbiol. 20, e12845 (2018).
Chitlaru, T. et al. A novel live attenuated anthrax spore vaccine based on an acapsular Bacillus anthracis Sterne strain with mutations in the htrA, lef and cya genes. Vaccine 35, 6030–6040 (2017).
Bartolini, E. et al. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J. Extracell Vesicles 2, 20181 (2013).
Stevens, T. L. et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334, 255–258 (1988).
Li, M. et al. The type II histidine triad protein HtpsC is a novel adhesion with the involvement of Streptococcus suis virulence. Virulence 6, 631–641 (2015).
Liu, L. et al. Identification and experimental verification of protective antigens against Streptococcus suis serotype 2 based on genome sequence analysis. Curr. Microbiol. 58, 11–17 (2009).
Jo, E. K. Interplay between host and pathogen: immune defense and beyond. Exp. Mol. Med. 51, 1–3 (2019).
Chabot-Roy, G., Willson, P., Segura, M., Lacouture, S. & Gottschalk, M. Phagocytosis and killing of Streptococcus suis by porcine neutrophils. Micro. Pathog. 41, 21–32 (2006).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Page, A. J. et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31, 3691–3693 (2015).
Doytchinova, I. A. & Flower, D. R. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics 8, 4 (2007).
Saha, S. & Raghava, G. P. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 65, 40–48 (2006).
Saha, S. & Raghava, G. P. Prediction methods for B-cell epitopes. Methods Mol. Biol. 409, 387–394 (2007).
Karosiene, E., Lundegaard, C., Lund, O. & Nielsen, M. NetMHCcons: a consensus method for the major histocompatibility complex class I predictions. Immunogenetics 64, 177–186 (2012).
Gutiérrez, A. H. et al. In vivo validation of predicted and conserved T cell epitopes in a Swine influenza model. PloS one 11, e0159237 (2016).
Ishida, S. et al. Development of an appropriate PCR system for the reclassification of Streptococcus suis. J. Microbiol. Methods 107, 66–70 (2014).
Wilkins, M. R. et al. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 112, 531–552 (1999).
Curiel, T. J. et al. Peptides identified through phage display direct immunogenic antigen to dendritic cells. J. Immunol. 172, 7425–7431 (2004).
Sharma, N., Naorem, L. D., Jain, S. & Raghava, G. P. S. ToxinPred2: an improved method for predicting toxicity of proteins. Briefing Bioinform. 23, bbac174 (2022).
Buchan, D. W. A. & Jones, D. T. The PSIPRED protein analysis workbench: 20 years on. Nucleic Acids Res. 47, W402–w407 (2019).
Jones, D. T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292, 195–202 (1999).
Zhou, X. et al. I-TASSER-MTD: a deep-learning-based platform for multi-domain protein structure and function prediction. Nat. Protoc. 17, 2326–2353 (2022).
Yang, J. & Zhang, Y. I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 43, W174–181 (2015).
Ko, J., Park, H., Heo, L. & Seok, C. GalaxyWEB server for protein structure prediction and refinement. Nucleic Acids Res. 40, W294–297 (2012).
Vajda, S. et al. New additions to the ClusPro server motivated by CAPRI. Proteins 85, 435–444 (2017).
Kozakov, D. et al. The ClusPro web server for protein-protein docking. Nat. Protoc. 12, 255–278 (2017).
Rego, N. & Koes, D. 3Dmol.js: molecular visualization with WebGL. Bioinformatics 31, 1322–1324 (2015).
Wallace, A. C., Laskowski, R. A. & Thornton, J. M. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 8, 127–134 (1995).
Rapin, N., Lund, O., Bernaschi, M. & Castiglione, F. Computational immunology meets bioinformatics: the use of prediction tools for molecular binding in the simulation of the immune system. PLoS ONE 5, e9862 (2010).
Acknowledgements
This research was supported by the National Key Research and Development Program of China (2022YFD1800904) and the National Natural Science Foundation of China (31972650).
Author information
Authors and Affiliations
Contributions
J.M. and H.Y. supervised the project and evaluated the results. J. L., W.P., X.P. performed most of the experiments and interpreted the data. J.L. and W.P. performed parts of protein purification and immunization. J.L. performed parts of mice challenged and observed. Z.Z. performed with the collation of supplementary material and text checking. C.L. and Z.Z. performed parts of the immunoblotting. J.L. analyzed the data. P.L. Q.B. and S.L. provided some technical help. Y.Y. provided some materials. J.M. is the lead contact of corresponding authors. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Jiale Ma, Jianan Liu. Wanxia Pu, Zongfu Wu and Huochun Yao are inventors on a patent applied for by the Nanjing Agricultural University, China. Patent application CN202410182539.7 pertains to all aspects of the multiple-epitope antigen. It is still pending and has not been issued. All the authors declare no competing interests. We have obtained Publication and Licensing Rights from BioRender.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, J., Zhang, Z., Pu, W. et al. A multi-epitope subunit vaccine providing broad cross-protection against diverse serotypes of Streptococcus suis. npj Vaccines 9, 216 (2024). https://doi.org/10.1038/s41541-024-01015-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-01015-7
This article is cited by
-
Adjuvant screening of an inactivated Pasteurella multocida vaccine in mice and evaluation of its immunogenicity in sheep
Veterinary Research Communications (2025)