Abstract
Whether declined level <10 mIU/ml of antibody to hepatitis B surface antigen (anti-HBs) is still immune to hepatitis B virus (HBV) is controversial. We longitudinally investigated hepatitis B markers in 395 vaccinated children of HBV-infected mothers at a 5.4-years interval. At baseline, they were at average age of 3.2 ± 1.8 years (0.6–12), and 106 (26.8%) children had anti-HBs <10 mIU/ml and 289 (73.2%) others had anti-HBs ≥10 mIU/ml. Of them, 84 (21.3%) were boosted with hepatitis B vaccine and 311 (78.7%) were not boosted. When they were at the age of 8.6 ± 1.9 years (6–18), 166 (42.1%) had anti-HBs <10 mIU/ml and 229 (57.9%) had anti-HBs ≥10 mIU/ml, and none was infected with HBV, including 62 unboosted children with anti-HBs <10 mIU/ml at baseline. Of 311 unboosted participants, 48 (15.4%) had increased anti-HBs levels in the absence of antibody to hepatitis B core antigen, suggesting natural booster immunization. Considering the close contact of children to their HBV-infected mothers, our study showed that successfully vaccinated children are still immune to HBV, even after the anti-HBs levels dropped to <10 mIU/ml. Therefore, anti-HBs levels <10 mIU/ml should not be an indication for booster hepatitis B vaccination.
Similar content being viewed by others
Chronic infection of hepatitis B virus (HBV), positive hepatitis B surface antigen (HBsAg) in circulation for more than 6 months, is a serious global public health issue because of its long-term severe sequelae such as liver cirrhosis and caner and other complications. Mother-to-child transmission (MTCT) of HBV is the main cause of chronic infection, because 70–90% of the infections occurred during infancy will become persistant1,2. Currently, it is recommended that all newborn infants should receive a series of 3-dose hepatitis B vaccine, with the first dose injected within 12 or 24 h after birth, and those born to mothers with positive HBsAg should administer hepatitis B immunoglobulin (HBIG) within 12 h after birth. In addition, peripartum antiviral prophylaxis is recommended in pregnant women with high viral loads (HBV DNA > 2 × 105 IU/ml) or positive hepatitis B e antigen (HBeAg) to further reduce MTCT of HBV3,4,5. Strict adherence with these measures may almost completely prevent MTCT of HBV, as MTCT may reduce to 0–0.03% in infants of HBV-infected mothers6,7,8.
Generally, a level of antibody to HBsAg (anti-HBs) ≥ 10 mIU/ml is considered to be immune to HBV. While the neonatal immunoprophylaxis plays cornerstone roles in preventing MTCT of HBV, whether successfully immunized children in whom levels of anti-HBs declined to <10 mIU/ml are still immune to HBV is controversial. Many studies showed that neonatal immunoprophylaxis against hepatitis B may elicit long-term immunity to HBV, based on the persistence of anti-HBs level ≥10 mIU/ml or swift anamnestic responses as surrogates of the immunity more than 20–35 years9,10,11,12,13,14,15,16,17. These investigations indicate that booster vaccination against hepatitis B in children is not necessary. However, other studies showed that successfully immunized children with positive anti-HBs and negative HBsAg during infancy, who were born to HBV-infected mothers, may encounter breakthrough HBV infection after a few years when their anti-HBs levels waned to <10 mIU/ml18,19, indicating the requirement of booster hepatitis B vaccination in children of HBV-infected mothers. In addition, some scholars proposed that vaccinees with waned anti-HBs levels need booster vaccination because anti-HBs level <10 mIU/ml is considered to be non-protective20. Therefore, whether vaccinated children with anti-HBs declined to <10 mIU/ml require booster hepatitis B vaccination is still a concern. In the present study, we aimed to observe whether anti-HBs level <10 mIU/ml is immune to HBV by longitudinally investigating the hepatitis B serological markers and changes of anti-HBs levels in a cohort of children of HBV-infected mothers.
Results
Demographic characteristics of participants
A total of 395 children/adolescents born to HBV-infected (positive HBsAg) mothers in Nanjing Drum Tower Hospital and Zhenjiang Fourth People’s Hospital between January 2004 and March 2012, with 219 (55.4%) male and 176 (44.6%) female, were included in this study (Fig. 1). None of their mothers received anti-HBV therapy during pregnancy. All participants received neonatal immunoprophylaxis after birth. None of them was positive HBsAg and only one (0.2%) was positive for antibody to hepatitis B core antigen (anti-HBc), tested from November 2011 to November 2012, when they were at the average age of 3.2 ± 1.8 years (0.6–12 years) (hereafter named the first follow-up)21,22. These participants were followed up for hepatitis B serological markers from January to September 2017 (hereafter named the second follow-up) when they were at an average age of 8.6 ± 1.9 years (6–18 years) (Fig. 1). Thus, the average interval between the first and second follow-up was 5.4 years.
Children born to mothers infected with hepatitis B virus (HBV) received hepatitis B immunoglobulin (HBIG) and hepatitis B vaccine after birth. They were followed up for hepatitis B surface antigen (HBsAg), antibody to HBsAg (anti-HBs), and antibody to hepatitis B core antigen (anti-HBc) twice, from November 2011 to November 2012 and from January to September 2017, respectively.
Changes of anti-HBs levels in boosted and unboosted children
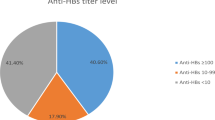
Of the 395 participants, 106 (26.8%) had anti-HBs <10 mIU/ml and 289 (73.2%) had anti-HBs ≥10 mIU/ml in the first follow-up. Although booster immunization is not recommended in general children and adolescents23, considering that children in the present study were at risk of exposure to HBV because they had close contact with their HBV-infected mothers, we advised booster immunization with one vaccine dose for children with anti-HBs <10 mIU/ml in the first follow-up, as studies showed that one dose booster can efficiently elicit robust anti-HBs response24. Whether booster vaccination or not and the number of booster vaccination dose were determined by the parents and physicians in the designed hospital responsible for vaccinations. Finally, 84 (21.3%) of the 395 participants had been boosted with various doses (10 μg/dose) of hepatitis B vaccine, 53 with three doses at 0, 1, and 6 months schedule, 7 with two doses at 0 and 1 month schedule, and 24 with one dose, and 311 (78.7%) participants were not boosted (Table 1).
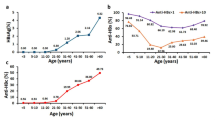
The 84 boosted participants included 44 (52.4%) children with anti-HBs <10 mIU/ml and 40 (47.6%) children with anti-HBs ≥10 mIU/ml in the first follow-up, with a median anti-HBs level 9.06 mIU/ml (0.34–>1000) (Table 1). We did not measure anti-HBs response 1–2 months after the final booster dose, but just tested anti-HBs in the second follow-up. As anticipated, the positive rate (91.7%) of anti-HBs and the median anti-HBs levels (138.95 mIU/ml) in the boosted participants were both significantly increased in the second follow-up (Table 1). On the other hand, 311 unboosted participants comprised of 62 (19.9%) children with anti-HBs <10 mIU/ml and 249 (80.1%) children with anti-HBs ≥10 mIU/ml in the first follow-up, with a median anti-HBs level 53.34 mIU/ml (0.34–>1000). In the second follow-up, the positive rate (48.9%) of anti-HBs and the median anti-HBs level (9.32 mIU/ml) in these unboosted participants were both significantly decreased (Table 1). In addition, the boosted participants had significantly higher rate of anti-HBs ≥10 mIU/ml (91.7% vs 48.9%, χ2 = 49.707, P < 0.001) and higher median anti-HBs titer than unboosted children (138.95 vs 9.32 mIU/ml, Z = 79.606, P < 0.001) (Table 1). Overall, of the 395 participants in the second follow-up, 229 (57.9%) had anti-HBs ≥10 mIU/ml and 166 (42.1%) had anti-HBs <10 mIU/ml.
Changes of anti-HBs levels in unboosted children during 5.4-year interval
Although the rate of anti-HBs ≥10 mIU/ml and the overall median anti-HBs level in 311 unboosted individuals were decreased in the second follow-up (Table 1), 48 (15.4%) of them had elevated anti-HBs levels, compared to those in the first follow-up. We further analyzed the frequency of elevated anti-HBs levels in the second follow-up based on the anti-HBs levels in the first follow-up. Table 2 shows that the proportion (48.4%) of elevated anti-HBs levels in individuals with anti-HBs <10 mIU/ml was significantly higher than that with anti-HBs 10–<100 (10.2%), 100–<1000 (5.2%), and ≥1000 mIU/ml (0%) respectively, and the lower anti-HBs levels in the first follow-up, the more proportion of elevated anti-HBs levels in the second follow-up (χ2 = 66.548, P < 0.0001).
HBsAg and anti-HBc in the second follow-up
None of the 311 participants had the history of being diagnosed with hepatitis B during the 5.4-year period between the first and second follow-up. One child with anti-HBc positive in the first follow-up was still anti-HBc positive in the second follow-up and all 310 others were still anti-HBc negative in the second follow-up. All 311 participants was negative for HBsAg in the second follow-up.
Discussion
In the current study, 395 children born to HBsAg positive mothers who had not been infected with HBV were followed up after an average of 5.4 years. None of them developed acute hepatitis B or chronic HBV infection, although 26.8% and 42.1% of the participants already had anti-HBs <10 mIU/ml before 5.4 years and at this follow-up respectively. Since all participants had closely contacted with HBV-infected mothers, this study demonstrated that successfully immunized children are still immune to HBV even after anti-HBs levels declined below 10 mIU/ml. The results indicate that booster immunization is not necessary in vaccinees with anti-HBs levels declined to lower than 10 mIU/ml.
Based on the documented literature, real breakthrough infection of wild-type HBV in vaccinated individuals in who anti-HBs was efficiently elicited and then declined to undetectable level was extremely rare25,26. Our present study also did not find novel HBV infection in successfully vaccinated offspring of HBV-infected mothers during a period of 5.4 years, even in the children with anti-HBs level declined to lower than 10 mIU/ml. By contrast, several reports from China showed that successfully vaccinated individuals had frequent HBsAg seroconversion18,27. However, whether the positivity of HBsAg in vaccinated individuals in these reports was caused by carry-over from early life or by the novel infection after successful vaccination was unknown, and there were several other concerns on the reliability of HBV infection28,29. In the present study, blood collection by peripheral venipuncture and measurement of hepatitis B serological markers in both follow-ups were all completed by our own investigators, rather than by using remained blood samples from the clinical laboratory or reported results of hepatitis B markers from different hospitals or clinics. Thus, the identity of each participant was ascertained and the quality of tests for hepatitis B markers was guaranteed. In addition, we used the most popular reagents (Abbott) to test hepatitis B markers. Thus, the results obtained in the present study should be accurate and reliable.
Previous studies on the long-term protective efficacy of hepatitis B vaccination were conducted in general participants14,24, or in offspring of HBV-infected mothers without the detailed anti-HBs levels in the vaccinees9,30. No HBV infection in vaccinated general participants may be attributed to the protection by hepatitis B vaccination, yet an alternative explanation may be that the vaccinees were not exposed to HBV. In the present investigation, because all participants were born to HBsAg positive mothers, they had evidently close contacts with HBV. The results that 48 (15.4%) of all 311 unboosted children, particularly nearly half (48.4%) of children with anti-HBs <10 mIU/ml, had increased anti-HBs levels during 5.4 years (Table 2) demonstrated the HBV exposure. Thus, the current study provides novel evidence that vaccinees in whom anti-HBs levels declined below 10 mIU/ml remain to be immunity to HBV. This is meaningful to rationalize booster hepatitis B vaccination. For successfully vaccinated individuals, anti-HBs level <10 mIU/ml should not be an indication for booster vaccination, even in offspring of HBV-infected mothers.
Notably, 15.4% of the unboosted children had higher anti-HBs levels in the second follow-up after 5.4 years than in the first follow-up (Table 2). Unquestionably, these children had exposed to HBsAg, indicating the exposure to HBV. However, none of them was anti-HBc positive. Such a phenomenon was previously observed worldwide with different frequencies31,32,33,34. Theoretically, anti-HBc should be positive after natural HBV infection, since hepatitis B core antigen is the most robust antigenic component of HBV. However, none of the subjects seroconverted to anti-HBc positive during the 5.4-year period in the current study. The absence of anti-HBc may be associated with the occurrence of “abortive HBV infection” in the vaccinees, a transient HBV infection that is quickly terminated by brisk anamnestic specific immune responses because of the preexisting immunity induced by the vaccination, during which hepatitis B core antigen was not sufficiently produced to elicit immune response.
There were several several limitations in this study. First, the size of children with anti-HBs below 10 mIU/ml was relative small. Second, whether booster hepatitis B vaccination in children was determined by the parents, but not by the investigators. Third, the anti-HBs response after the primary series of hepatitis B vaccination was unknown in a considerable proportion of children, which could not completely exclude the potentiality that anti-HBs was produced by the natural resolved HBV infection. However, only one child showed anti-HBc positive, and all others were anti-HBc negative in both surveys, indicating the successful vaccination. Fourth, we did not measure the anti-HBs levels 1–2 months after the final booster vaccination dose and every year in the subjects, and the dynamic changes of anti-HBs levels during the 5.4-year period were not precisely observed. Fifth, we did not measure the cellular immune response in the children, as cellular immunity to HBV in vaccinees may also provide long-term protection35. On the other hand, the strength of our study is that we not only measured the declining anti-HBs levels, but also collected the clinical outcomes of the children during the following period, and found that none of the children with closely contacts with HBV had evidence of HBV infection (absence of clinical hepatitis and negative for both HBsAg and anti-HBc) even after anti-HBs levels declined below 10 mIU/ml.
In summary, this study directly demonstrates the sustained protection against clinical hepatitis B and chronic HBV infection over a 5.4-year follow-up in children with close contacts with HBV, even after the anti-HBs levels dropped to below 10 mIU/ml. Therefore, anti-HBs levels <10 mIU/ml should not be an indication for booster hepatitis B vaccination.
Patients and methods
Study subjects
From November 2011 to November 2012, we tested hepatitis B serological markers in 502 children born to HBV-infected (positive HBsAg) mothers between January 2004 and March 2012 (the first follow-up)21,22. Of them, 423 (84.3%) received HBIG within 24 h after birth and 79 (15.7%) others did not administer HBIG. The reason for not applying HBIG in neonates was that the parents were not able to afford the financial cost of HBIG then, because China did not provide charge-free HBIG to infants of HBV-infected mothers until 2011. All children completed a full 3-dose serial hepatitis B vaccination, 478 (95.2%) timely vaccinated with birth vaccine dose within 24 h after birth and 24 (4.8%) received delayed (3–45 days after birth) the first vaccine dose because of the preterm birth and/or other medical conditions. Overall, four (0.8%) children were defined with chronic HBV infection based on the presence of HBsAg, and one (0.2%) was defined with resolved HBV infection based on the positive antibody to hepatitis B core antigen (anti-HBc) and negative HBsAg21,22. In the present study, we planned to follow up 498 children/adolescents with negative for HBsAg in the first follow-up for hepatitis B serological markers from January to September 2017 (named the second follow-up, Fig. 1).
The parents of all children were contacted by phone calls. Children who participated in the study were interviewed together with their parents or guardians by investigators at the follow-up. Information on the history of viral hepatitis and booster hepatitis B vaccine was collected by a questionnaire. Booster vaccination was verified based on the children’s vaccination card. Peripheral blood was collected from each participant and stored at −30 °C. The peripheral blood samples collected during the first follow-up were all archived at −30 °C.
This study was approved by the Institutional Review Board of the Ethics Committee of each hospital (Nanjing Drum Tower Hospital, XK200709E; Zhenjiang Fourth People’s Hospital, 2011016). Written informed consent was obtained from the parents/guardians of each child. This study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
Laboratory tests
Serum samples were tested for HBsAg, anti-HBs, and anti-HBc with commercial ELISA kits for HBsAg, anti-HBs, and anti-HBc with microparticle enzyme immunoassay (Architect HBsAg, Abbott, North Chicago, USA) at Nanjing Drum Tower Hospital. Based on the instructions, a measurement of HBsAg ≥0.05 IU/ml was defined with positive HBsAg, anti-HBs ≥10 mIU/ml was defined positive anti-HBs, and antibody to HBV core antigen (anti-HBc) ≥ 1.0 S/CO was defined positive anti-HBc. To avoid the variation in the quantification of anti-HBs levels between the follow-up November 2011 to November 2012 and January to September 2017, we measured anti-HBs levels in the paired serum samples collected in both follow-ups in parallel at same time.
Statistical analysis
All analyses in this study were conducted using R software, version 4.10. Quantitative data that did not follow a normal distribution were described using medians (range from minimum to maximum). Comparisons over time within the same population were performed using the paired Wilcoxon signed-rank test, and comparisons with groups were performed using the Wilcoxon Mann–Whitney test. Categorical data were represented by frequencies and percentages. Changes in rates over time within the same population were assessed using the McNemar test, while comparisons of rates across multiple independent groups were analyzed using the chi-square test. Differences were considered statistically significant at P < 0.05.
The upper limit of test for anti-HBs is 1000 mIU/ml. In the statistical analysis, those with anti-HBs ≥1000 mIU/ml were arbitrarily determined as 1100 mIU/ml. Since the median anti-HBs titer was used in the analysis, the arbitrary determination did not influence the analysis.
Data availability
The datasets generated and analyzed in this study are presented in the manuscript. Original raw data are also available from the corresponding authors upon reasonable request.
References
Tang, J., Zhao, H. & Zhou, Y. H. Screening for viral hepatitis carriage. Best. Pract. Res. Clin. Obstet. Gynaecol. 96, 102523 (2024).
Tang, J., Xiang, K. & Zhou, Y. H. Making elimination of perinatal hepatitis B infection a reality: the Chinese contribution. Matern. Fetal Med. 6, 67–69 (2024).
Zhou, Y. H. et al. CSOG MFM Committee Guideline: management of hepatitis B during pregnancy and prevention of mother-to-child transmission of hepatitis B virus (2020). Matern. Fetal Med. 3, 7–17 (2021).
Kumar, M. et al. Asian Pacific association for the study of liver (APASL) guidelines: hepatitis B virus in pregnancy. Hepatol. Int. 16, 211–253 (2022).
World Health Organization. Guidelines for the diagnosis, care and treatment of persons with chronic hepatitis B infection. https://www.who.int/publications/i/item/9789240090903 (2024).
Zeng, Q. L. et al. Tenofovir alafenamide for pregnant Chinese women with active chronic hepatitis B: A multicenter prospective study. Clin. Gastroenterol. Hepatol. 20, 2826–2837.e9 (2022).
Zeng, Q. L. et al. Expected 8-week prenatal vs 12-week perinatal tenofovir alafenamide prophylaxis to prevent mother-to-child transmission of hepatitis B virus: a multicenter, prospective, open-label, randomized controlled trial. Am. J. Gastroenterol. 120, 1045–1056 (2025).
Yin, X. et al. Real-world implementation of a multilevel interventions program to prevent mother-to-child transmission of HBV in China. Nat. Med. 30, 455–462 (2024).
Poovorawan, Y. et al. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J. Viral Hepat. 18, 369–375 (2011).
Roznovsky, L. et al. Long-term protection against hepatitis B after newborn vaccination: 20-year follow-up. Infection 38, 395–400 (2010).
Wang, Z. Z. et al. Long-term persistence in protection and response to a hepatitis B vaccine booster among adolescents immunized in infancy in the western region of China. Hum. Vaccin. Immunother. 13, 909–915 (2017).
Ma, J. C. et al. Long-term protection at 20-31 years after primary vaccination with plasma-derived hepatitis B vaccine in a Chinese rural community. Hum. Vaccin. Immunother. 16, 16–20 (2020).
Chang, K. C. et al. Universal infant hepatitis B virus (HBV) vaccination for 35 years: moving toward the eradication of HBV. J. Infect. Dis. 225, 431–435 (2022).
Bruce, M. G. et al. Protection and antibody levels 35 years after primary series with hepatitis B vaccine and response to a booster dose. Hepatology 76, 1180–1189 (2022).
Spradling, P. R. et al. Immunity to hepatitis B virus (HBV) infection two decades after implementation of universal infant HBV vaccination: association of detectable residual antibodies and response to a single HBV challenge dose. Clin. Vaccin. Immunol. 20, 559–561 (2013).
Chiara, F. et al. Hepatitis B vaccination at three months of age: a successful strategy?. Vaccine 31, 1696–1700 (2013).
Trevisan, A. et al. Significance of anti-HB levels below 10 IU/L after vaccination against hepatitis B in infancy or adolescence: an update in relation to sex. Hum. Vaccin Immunother. 16, 460–464 (2020).
Zheng, Z. et al. Seroconversion of hepatitis B surface antigen among those with previously successful immune response in Southern China. Hum. Vaccin. Immunother. 17, 845–851 (2021).
Song, Y. et al. A booster hepatitis B vaccine for children with maternal HBsAg positivity before 2 years of age could effectively prevent vaccine breakthrough infections. BMC Infect. Dis. 22, 863 (2022).
Phattraprayoon, N. et al. Duration of hepatitis B vaccine-induced protection among medical students and healthcare workers following primary vaccination in infancy and rate of immunity decline. Vaccines. 10, 267 (2022).
Xu, C. et al. Protective effect of hepatitis B vaccine and hepatitis B immunoglobulin on infants against mother-to-infant transmission of hepatitis B virus. Prog. Obstet. Gynecol. 22, 14–18 (2013). [in Chinese].
Rui, Y. et al. Evaluation for effects of routine administration of hepatitis B vaccine and hepatitis B immunoglobulin on preventing mother-to-infant transmission of HBV-infected mothers with negative HBeAg. J. Practical Obstet. Gynecol. 29, 206–209 (2013). [in Chinese].
European Consensus Group on Hepatitis B Immunity Are booster immunisations needed for lifelong hepatitis B immunity?. Lancet 355, 561–565 (2000).
McMahon, B. J. et al. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J. Infect. Dis. 200, 1390–1396 (2009).
Boot, H. J. et al. Acute hepatitis B in a healthcare worker: a case report of genuine vaccination failure. J. Hepatol. 50, 426–431 (2009).
Zhao, H. & Zhou, Y. H. Revaccination against hepatitis B in late teenagers who received vaccination during infancy: yes or no?. Hum. Vaccin. Immunother. 14, 456–463 (2018).
Wang, Y. et al. Adolescent booster with hepatitis B virus vaccines decreases HBV infection in high-risk adults. Vaccine 35, 1064–1070 (2017).
Zhou, Y. H. Be cautious for exceptional results in evaluating the effect of adolescent booster of hepatitis B vaccine. Int. J. Infect. Dis. 66, 150–152 (2018).
Zeng, Q. L. & Zhou, Y. H. Requirement for further validation on the seroconversion of hepatitis B surface antigen in successful vaccinees. Hum. Vaccin. Immunother. 17, 2773–2774 (2021).
Lin, A. W. & Wong, K. H. Long-term protection of neonatal hepatitis B vaccination in a 30-year cohort in Hong Kong. J. Hepatol. 59, 1363–1364 (2013).
Coursaget, P. et al. Seven-year study of hepatitis B vaccine efficacy in infants from an endemic area (Senegal). Lancet 2, 1143–1145 (1986).
Lai, C. L. et al. Five-year follow-up of a prospective randomized trial of hepatitis B recombinant DNA yeast vaccine vs. plasma-derived vaccine in children: immunogenicity and anamnestic responses. Hepatology 18, 763–767 (1993).
Lee, P. I. et al. Long-term efficacy of recombinant hepatitis B vaccine and risk of natural infection in infants born to mothers with hepatitis B e antigen. J. Pediatr. 126, 716–721 (1995).
Bulkow, L. R. et al. Increases in levels of antibody to hepatitis B surface antigen in an immunized population. Clin. Infect. Dis. 26, 933–937 (1998).
Simons, B. C. et al. A longitudinal hepatitis B vaccine cohort demonstrates long-lasting hepatitis B virus (HBV) cellular immunity despite loss of antibody against HBV surface antigen. J. Infect. Dis. 214, 273–280 (2016).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81672002), the Science and Technology Department of Jiangsu Province (BK20221169), and Jiangsu Province Center for Innovation in Obstetrics and Gynecology (CXZX202229), China. The funders had no role in study design, data collection and analysis, preparation and writing of the manuscript and its submission.
Author information
Authors and Affiliations
Contributions
P.S., C.X., T.L., Y.Z., and Y-H.Z. contributed to the conceptualization and design of this study. C.X., Y.R., and Y.H. followed up the participants and collected the clinical and vaccination data. P.S., and Y.R. tested for hepatitis B serological markers. T.L. performed the statistical analysis. Y.H., Y.Z., and Y-H.Z. supervised subjects follow-up and laboratory tests. P.S., C.X., and T.L. wrote the original manuscript draft. Y.Z. and Y-H.Z. critically revised the manuscript. All authors interpreted the data, edited the manuscript, and approved the submitted version of manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, P., Xu, C., Li, T. et al. Level of antibody to hepatitis B surface antigen declined below 10 mIU/ml is still protective. npj Vaccines 10, 126 (2025). https://doi.org/10.1038/s41541-025-01188-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-025-01188-9