Abstract
The Pfizer COVID-19 vaccines have been associated with an increased risk of myocarditis. However, COVID-19 infection is also associated with complications. A Bayesian network (BN), informed by Australian and international data, was created to determine individual risks and benefits of the Pfizer COVID-19 vaccine in the paediatric. The risk of myocarditis between vaccine-associated, COVID-19 and background rates was compared, as well as secondary outcomes such as hospitalization, and MIS-C. At a population level, hospitalizations, intensive care admissions and MIS-C cases prevented at differing transmission rates and vaccine coverage were analyzed. The model estimated that teenage males were 4.47 times more likely to develop myocarditis from COVID-19 compared to the vaccine. Furthermore, the risk of hospitalizations and MIS-C were more likely in the unvaccinated cohort for all ages. The population level benefits of COVID-19 Pfizer vaccine at mitigating myocarditis are more nuanced, contingent on age, transmission rates and vaccination coverage.
Similar content being viewed by others
Introduction
The SARS-CoV-2 virus first emerged in December 2019, with the World Health Organisation declaring a pandemic on the 11th of March 20201. By April 2024, there have been almost 800 million reported cases worldwide with more than 7 million cumulative deaths2. Research suggests that the paediatric population contributed 1–10% of these cases1. Whilst evidence has emerged that children experience milder illness3, with most cases requiring only supportive care, serious outcomes do occur in this population4. Among the 4.4 million COVID-19 deaths reports in the MPIDR COVerAGE, a database which includes cumulative counts of confirmed COVID-19 cases and death, 0.4% of deaths occurred in children and adolescents, and 53% of these were in adolescents aged 10–19 years and 47% in children aged 0–9 years5.
Different complications from SARS-CoV-2 infection have emerged in the paediatric population compared to the adult population, such as multisystem inflammatory syndrome (MIS-C) a potentially serious illness requiring high rates of intensive care admissions and death6.
The BNT162b2 mRNA COVID-19 Vaccine (Pfizer-BioNTech) first received emergency authorisation from the US FDA in December 2020 for those aged over 16 years, and subsequently, in May 2021 for 12–15-year-olds and in October 2021 for 5–11-year-olds7. Vaccination coverage data in Australia shows that on the 15th March 2023, 38.5% of 5–11 year-olds and 73.0% of 12–15 year-olds had received two doses of a COVID-19 vaccine8. Vaccine hesitancy is prevalent, with a review of 582 adults reporting that 61% were acceptant, 30% hesitant and 9% resistant in March 20229.
Encouraging parents to accept new vaccines for novel pathogens is challenging. Parents and care givers need to weigh up the risks and benefits of vaccinations, and there are often concerns regarding the safety of new vaccines10. Communication requires transparency to understand the differing risk profiles in the paediatric cohort11. Furthermore, information needs to be targeted to the intended audience11, with risk communicated in a sensitive, unbiased manner comparing the risk of harm from vaccine compared to benefit to aid shared decision making12.
The Australian Technical Advisory Group on Immunisation (ATAGI) has provided an informed summary of the risk of myocarditis post Pfizer COVID-19 vaccine13. Furthermore, a full analysis of the risk versus benefits of vaccination in 12–19-year-olds using Bayesian networks (BN), with comparisons of risk of COVID-19-associated myocarditis and background rates of myocarditis has been published by Sinclair et al.14. BN models can amalgamate data from numerous Australian and international sources, as well as incorporating official government statistics and peer reviewed published journals14. The results from BN models have been used to develop an online risk benefit calculator to inform, clinicians and individuals on their risk of developing certain complications from COVID-19 compared to the vaccination. However, to date these analyses have not focused on COVID-19 vaccination in children.
The aim of the study was to assess the individual risk vs benefits of the Pfizer COVID-19 vaccine in children aged 5–17 years old as well at a population level.
Our primary objective is to assess the individual risk of myocarditis from SARS-CoV-2 infection vs Pfizer COVID-19 vaccine associated myocarditis vs background myocarditis over a 3-month period.
Our first secondary objective is to assess total number of cases of myocarditis, at a population level, secondary to SARS-CoV-2 infection vs Pfizer COVID-19 vaccine associated myocarditis vs background myocarditis over a 3-month period, modelled with different vaccination coverage and transmission scenarios.
Our next secondary objective is to investigate total number of hospitalisations and other severe outcomes prevented, at a population level, over a 3-month period with different vaccination coverage and transmission scenarios.
Our final secondary objective is to determine total number of MIS-C cases prevented, at a population level, over a 3-month period with different vaccination coverage and transmission scenarios
Results
Model structure and validation
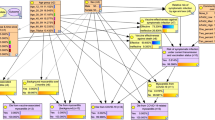
The BN model (see Fig. 1) includes four input nodes, which are shown in orange. Input nodes have no parent nodes and are used for defining the scenarios for the risk-benefit analysis. These include Vaccine (n1), Age (n2), Sex (n3), and Community Transmission over two months (n4).
There are six intermediate nodes, shown in yellow: Vaccine Effectiveness against hospitalisation (n5), Vaccine Effectiveness against severe outcomes (n6), Vaccine Effectiveness against MSI (n7), Vaccine Effectiveness against symptomatic infection (n8), Risk of Symptomatic infection under current transmission (n17), and Vaccination Status (n9).
There are 7 outcome nodes, shown in purple. These are Vaccine Induced Myocarditis, Background myocarditis over 3 months; Hospitalisation from COVID-19, Severe Outcome from COVID-19- ICU or Death, Myocarditis from COVID-19 over a 3-month period, MIS-C from COVID-19, Severe Outcome from MIS-C. See supplementary Table 13 for a summary of the quantitative relationships between each node.
Independent manual calculation of scenarios confirmed that the mathematical assumptions made were correctly implemented in the model (Supplementary Table S14). Model estimates from the sensitivity analysis indicated that the BN was behaving as expected for these scenarios.
Individual risk-benefit analysis for Myocarditis
For the primary objective, our model estimated the risk of COVID-19 induced myocarditis stratified by age and sex, with a direct comparison with the risk of vaccine-induced myocarditis (Fig. 2). For comparison, the background myocarditis risk over 3 months is represented. The risk of myocarditis is higher from COVID-19 compared to Pfizer COVID-19 vaccine associated myocarditis and background rate across all age and sex cohorts. Additionally, males had a higher risk of myocarditis from COVID-19, and Pfizer COVID-19 vaccine compared to females.
Furthermore, teenagers from 12 to 17 years old had a higher risk of both COVID-19 infection induced myocarditis and Pfizer COVID-19 vaccine associated myocarditis compared to the five- to eleven-year-olds. Moreover, the model shows that males aged 5–11 years were 88 times more likely to develop myocarditis after contracting symptomatic COVID-19 than from the second Pfizer COVID-19 vaccine dose (176 per million vs 2 per million). Furthermore, in the 12–15- and 16–17-year-old males, the incidence of myocarditis was 4.47 (95%CI 3.7–5.4) times higher from COVID-19 compared to vaccination (590 per million vs 132 per million).
These results were demonstrated in pictorial format on the CoRICAL tool (https://corical.immunisationcoalition.org.au/children)15, an online COVID-19 risk calculator, which helps members of the public and clinicians make decisions on getting the Pfizer COVID-19 vaccine.
Individual risk-benefit analysis for Hospitalisation, intensive care admission and MIS-C
For our secondary objective, the risk of hospitalisation and severe outcome was stratified by age and vaccination status. Our model shows that in the 5–11-year age group, unvaccinated children were 3.13 times more likely to be hospitalised from COVID-19 (8600 per million vs 2752 per million) and 4.76 times more likely to have a severe outcome, either intensive care admission or death (80 per million vs 16.8 per million). In the 12–17 age group, unvaccinated children were 1.75 times more likely to be hospitalised (7300 per million vs 4161 per million) and 4.76 times more likely to have a severe outcome (80 per million vs 16.8 per million). For both 5–11 and 12–17 year age groups, the model shows that children were 4.55 times more likely to get MIS-C if unvaccinated.
Population level analysis for Myocarditis
Figure 3 shows the risk of background myocarditis vs Pfizer COVID-19 Vaccine associated myocarditis vs SARS-CoV2 associated myocarditis stratified by age and sex across differing vaccine coverage and transmission scenarios.
Cases of myocarditis per million stratified by age and sex attributable to background rates, Pfizer COVID-19 vaccine associated myocarditis, SARS-CoV2 associated at transmission scenarios of 0%, 1%, 2%, 5% and 10% over a 3-month period with vaccine coverage of a 51.4%, b 70% and c 90% of the paediatric population.
Across all vaccine coverage and transmission scenarios, cases of myocarditis attributable to vaccine were higher in males aged 12–17 years compared to myocarditis attributable to COVID-19. For example, with a 10% transmission scenario with 90% vaccine coverage, there would be 2.5 times more cases of myocarditis attributable to vaccine compared to SARS-CoV2 infection in the 12–17 age group (122.4 vs 48.3) over a 3-month period.
Conversely, in all vaccine coverage and transmission scenarios, cases of myocarditis attributable to SARS-CoV2 infection were higher than from the Pfizer COVID-19 vaccine in the 5–11 female group, with zero cases of Pfizer COVID-19 vaccine associated myocarditis in this age group. For males aged 5–11, the only scenario where more cases of myocarditis were attributable to vaccine compared to infection, were with 90% vaccine coverage and 1% transmission over 3 months (1.8 cases per million vs 1.49 cases per million). Lastly, in females aged 12–17 with a 70% or 90% vaccine coverage rate, cases of myocarditis attributable to infection were higher only with a transmission rate of 10%. With a vaccination coverage of 51.4%, cases attributable to vaccine are higher in 1 and 2% transmission scenarios, however lower with 5% and 10% transmission.
Population level analysis for Hospital, intensive care admissions and MSI cases
Over a 3-month period, if 10% of the population was infected, 1849 children per million aged between 5 and 11 years and 1757 per million between 12 and 17 would be admitted to hospital in an unvaccinated population (Fig. 4a, b).
Number of hospitalisations and hospitalisations prevented over a 3-month period stratified by different scenarios of transmission and vaccination coverage; a the 5–11 year and b 12–17-year age groups. Number of intensive care admissions, and intensive care admissions prevented over a 3-month period stratified by different scenarios of transmission and vaccination coverage; c the 5–11 year and d 12–17-year age groups. Number of MIS-C cases per million and prevented cases stratified by different vaccination and transmission scenarios; e 5–11 year and f 12–17-year age group.
If 51.4% of the population was vaccinated, 697 hospitalisations per million in children aged between 5–11 and 537 hospitalisations per million in those aged between 12 and 17 would be prevented (Fig. 4a, b). Furthermore, the vaccine would prevent 7 admissions per million to intensive care for those aged of 5–11 years and 8 per million for those aged 12–17 years (Fig. 4c, d), compared to 1 case per million of Pfizer COVID-19 Vaccine associated myocarditis in males aged between 5–11 and 84 cases per million of Pfizer associated myocarditis for those aged between 12 and 17 (Fig. 3).
However, if vaccination coverage was 90% with 10% transmission of SARS-CoV2 infection over a 3-month period, 1220 hospitalisations per million would be prevented in the 5–11 age cohort and 943 hospitalisations per million in the 12–17 age group. With a subsequent 13 per million and 15 admissions per million to the ICU prevented in the 5–11 and 12–17 age cohorts, respectively (Fig. 4a, b). In addition, 14 cases per million and 10 cases per million of MIS-C would be prevented in the 5–11 and 12–17 age cohorts (Fig. 4e, f), compared to 2 cases per million of Pfizer COVID-19 vaccine associated myocarditis in a 5–11 aged male and 148 cases per million in the 12–17 age group (Fig. 3).
Discussion
A Bayesian Network was developed for risk-benefit and scenario analysis regarding Pfizer COVID-19 vaccination in the paediatric population during the Omicron wave. The model showed an increased risk of myocarditis in male teenagers from background myocarditis, vaccine-associated myocarditis, and COVID-19. This correlates with a systematic review of 46 studies between October 2020 and January 2022, showed the highest incidence in myocarditis in males aged 12–17 (50–139 cases per million) compared to lowest incidence in girls and boys aged 5–11 (<20 cases per million)16. However, the model also highlights the potential benefits of Pfizer COVID-19 vaccine, especially for boys aged 5–11 years. A reduced risk of hospitalisations, severe outcomes, and multisystem inflammatory syndrome (MIS-C) in vaccinated individuals across all age and sex groups compared to the unvaccinated was also identified.
The model demonstrates the elevated risk of myocarditis among male teenagers across all three outcomes: background myocarditis, vaccine-associated myocarditis, and myocarditis from COVID-19. Conversely, the model offers reassurance regarding the benefits of Pfizer COVID-19 vaccination, particularly for males aged 5–11 years. The findings also clearly demonstrate the reduced risk in hospitalisations, severe outcomes and multisystem inflammatory syndrome across all age and sex cohorts in the vaccinated compared to unvaccinated population.
The model can be used both at an individual level as well as for population health planning.
For individual outcomes, the model demonstrates the elevated risk of myocarditis among male teenagers across all three outcomes: background myocarditis, vaccine-associated myocarditis, and myocarditis from COVID-19. This was most prominent in males aged 12–17 years, with a 66-fold increase in the likelihood of vaccine-associated myocarditis compared to their younger counterparts. This is consistent with previous findings from a systematic review of COVID-19 vaccine induced myocarditis in young males which identified six studies reporting an incidence greater than 15 cases per 100,000 individuals among males aged 12–24 years following the second dose of an mRNA-based vaccine17.
Conversely, the model offers reassurance regarding the benefits of Pfizer COVID-19 vaccination, particularly for males aged 5–11 years. Most publicity has been associated with the risk of vaccine associated myocarditis18, however our model shows males and females aged 12–17 years were more likely to get myocarditis from COVID-19 compared to the vaccine. This is a key example of information that can be used for individual decision making and highlights that risks and benefits differ across sub-populations. Research shows that key parental concerns about COVID-19 vaccines for their children are safety and side effects19.
The findings also clearly demonstrate the reduced risk in hospitalisations, severe outcomes and MIS-C across all age and sex cohorts in the vaccinated compared to unvaccinated population. The model reports absolute risk of severe outcomes (ICU and death) being quite low. This likely correlates with the reduced virulence of new Omicron strains8,20. Despite the reduced virulence of Omicron, the risk of hospitalisation or MIS-C was higher in the unvaccinated population across age groups. This finding confirms that vaccination remains one of the most important methods for preventing severe illness and lowering mortality rates associated with COVID-19.
The model was used to evaluate outcomes under different coverage rates which could provide additional insights for public health decision-making. Although at a population-level, the benefits of the Pfizer COVID-19 vaccine in mitigating myocarditis risk appear nuanced, contingent upon factors such as vaccination coverage, transmission rates, sex, and age. Notably, among females aged 5–11, there were consistently more cases of myocarditis attributable to infection compared to vaccination across all scenarios over a three-month period. Conversely, among teenage males, cases of myocarditis attributable to vaccination outweigh those from infection across all transmission and vaccination coverage scenarios. This highlights the importance of risk benefit analysis specific for this age group, with similar studies in adults showing the development of COVID-19-related myocarditis is generally higher than vaccine-associated myocarditis19.
The strengths of the model are (a) its versatility (it can easily be updated to represent new variants, vaccine and outcomes if relevant data is available, b) it is informed by best available evidence and (c) independent of both vaccine manufacturers and the government.
Model estimates and outcomes should be interpreted in the context of data limitations. First, the model does not have provisions for co-morbidities and underlying immunodeficiency because data was not available. This would both vary the risk of hospitalisations, severe outcomes and possibly MIS-C, as well as influence vaccine effectiveness for such outcomes. In these specialised population groups, further individualised discussions between clinician and patient are required. Second, many patients now have previously had a COVID-19 infection, either from ancestral variants, delta variants or previous omicron strains, which would impact the immune response to COVID-19 and alter the additive effects of vaccination. Thirdly, the model does not account for waning efficacy of vaccination for the outcomes of interest, as this data was not currently available. The protective effects of vaccination on hospitalisation and severe outcome may reduce progressively with time from vaccination21. Fourth, this data represent the information available at the time of writing in December 2023. Estimates for the various outcomes will likely change over time, so the model will be updated onto the online tool once available, one of the key benefits to using a BN. For the risk of severe outcomes and MIS-C, authors of the Paediatric Active Enhanced Surveillance group provided Australian data. However, the data were not stratified by vaccination status, therefore the model possibly underestimates the risk of these outcomes in the unvaccinated paediatric population8. Fifth, multiple data sources are used for the BN network, introducing potential for biases due to the heterogeneity and differences in methodology of data collection. This, however, is an issue with any analyses with modelling and not specific to the BN approach. To ensure the most reliable data was chosen, sources were discussed with experts in the field, with rigorous assessment of the study design of collected data sets. Sixth, the model would benefit from external validation, once independent published information with analogous outcomes becomes available.
This model informed a new online risk calculator which provides a user-friendly interface for discussing the risks and benefits of Pfizer COVID-19 Vaccination in the paediatric population. A separate adult COVID Risk Calculator (CoRiCal) was first developed to inform risk-benefit analysis for the Astra-Zeneca COVID-19 Vaccination in an Australian Population due to concern of Thrombosis and Thrombocytopenia Syndrome22 and later updated to evaluate the risk of myocarditis from the Pfizer COVID-19 Vaccine compared to COVID-1914. However, vaccine associated myocarditis is seen to be a milder illness compared to COVID-19 induced myocarditis18. Users can access both the adult and children’s online tool and input their age and sex to find out their personalised risk calculation, such as the risk of myocarditis from COVID-19 versus Pfizer COVID-19 vaccine. The results from this study informed the new tool for children with outcomes such as hospitalisation and intensive care admission. A tool is required to communicate risks versus benefits in a child-centred way, as well as addressing concerns that parents may have.
The BN model can be used to predict hospitalisation levels and severe outcomes at a population level, providing policy makers with the tools to inform public health action.
In conclusion COVID-19 vaccine uptake is complex, and an individualised approach will be of benefit when it comes to discussions of risks and benefits. This model is a useful tool to inform a patient-centred discussion. This BN mode. This BN model provides a framework for assessing the impact of multiple variables during a pandemic, including changes in transmission, vaccination coverage or variant.
Materials and methods
Bayesian networks
Bayesian networks are graphical models representing relationships between different variables23. They use conditional probabilities to describe how likely it is for one event to occur given the occurrence of other related events. The BN shows associations between variables, with relationships represented as conditional probabilities23. Nodes represent variables, each with multiple potential states (e.g., 5–11;12–15 and 16–17 years old). Associations, such as Fig. 5, between variables are represented by arrows originating from a parent node (independent variable) to a child node (dependent variable). Probability tables, such as Fig. 6, define the probability of a node being in a given state, based on either a defined prior distribution (for nodes with no parent nodes) or the observed states of the parent node (i.e., the existing evidence). Probability tables can be populated using multiple data sources including published articles, government data, expert opinion and grey literature.
BN networks have previously been used in the Australian context for risk benefit analysis of the AstraZeneca COVID-19 vaccine and the Pfizer COVID-19 vaccine in adults14,22. As well as for assessing risk factors for COVID-19 disease progression24,25. BN networks allow for a flexible model structure, fast computation (over manual calculations, that would be prone to error and take months to complete) and the ability to use different information sources to inform different components of the system, that can be updated as and when new data sources emerge.
Model design
The model was adapted from a previous Bayesian network analysis assessing adult risk associated with the COVID-19 Pfizer vaccine. The methods from this model have been described in detail elsewhere14. The current model utilises evidence gathered from multiple sources and designed through collaboration with clinicians and researchers with expertise in paediatrics, infectious disease epidemiology, general practice, virology, and public health (PB, SL, CLL, KRS, JL, TS). Modellers and infectious diseases epidemiologists developed the model (CLL, HJM, TM). Collaborators agreed on variables to be included and their relationships with each other, as well as data sources for each variable. The model was then fine-tuned to include only nodes and relationships with reliable quantitative evidence.
BN structure
The BN structure was developed in several stages. The first stage included using previous BN14,26 designed by the team as a backbone structure. The previous model14, had identified vaccine associated myocarditis as a concern with the Pfizer COVID-19 vaccine and deemed an important variable in this model. The second stage required the agreement by all authors and experts to modify this list of relevant variables for the paediatric population dependent on the concerns with SARS-CoV-2 and the vaccine. These included defining meaningful outputs in the paediatric population, such as MIS-C, intensive care admissions and hospitalisations. The third stage involved defining the state of each node with the design discussed in detail below. The final stage of the model was critically evaluated by the experts in the field to ensure that authoritative evidence was used.
Conditional probability tables were derived from data extracted from a variety of sources including the Therapeutic Goods Administration (TGA) COVID-19 weekly safety reports17, ATAGI data27 as well as published literature. If available, Australian data were preferred over international sources. Prior distributions for age group and sex were used based on Australian census data28. Furthermore, the Paediatric Active Enhanced Disease Surveillance (PAEDS) network29, a collaboration of clinicians and public health researchers who monitor children hospitalised with infectious diseases to inform immunisation policy, were contacted directly to provide aggregate Australian data on the “Severe outcome from COVID-19” and “Multisystem Inflammatory Syndrome from COVID-19.” Few deaths were reported in children and therefore severe outcome incorporates both intensive care admissions and death. Due to the paucity of data on sub-lineages of Omicron variants, our model is applicable to Omicron variants in general.
We assumed that vaccine-associated myocarditis occurred in the first 3 months post-vaccination. Therefore, for our primary objective of comparing the individual risk of myocarditis from SARS-COV-2 infection with vaccine associated myocarditis from the second dose of Pfizer COVID-19 vaccine, and background myocarditis, we represented the data over a three-month period. The probability of background myocarditis risk was calculated over three months. Data analysis was therefore required to obtain probabilities by converting background myocarditis incidence to probability over three months.
The risk of vaccine-induced myocarditis was sourced from the TGA weekly safety report17. The TGA does not define explicitly the total time period post-vaccination that myocarditis cases occurred. However, it reports that most cases occurred between 3 and 5 days and the majority within 10 days. Singer et al. reported that the majority of myocarditis cases from COVID-19 occur in the first 21 days post infection30. The incidence of background myocarditis was seen to be significantly higher in the 16–17 age cohort compared to the 12–15 age cohort and therefore the 12–17 age group was split accordingly. The authors agreed this was better to highlight the significant finding in epidemiology data compared to averaging out to a 12–17 age group when evaluating the myocarditis data.
For the risk of hospitalisation, no Australian data were available stratified to vaccination status, Therefore, for the 5–11-year-old age group, we used Singaporean data31 collected during the Omicron period and for the 12–17-year-old age group, data were acquired from a study in New York32
See table 1 and supplementary Tables 1–12 for node definitions, with the full description of the data source and assumptions made.
Individual risk-benefit analysis
The BN was used to estimate the individual risk of COVID-19 associated myocarditis stratified by age and sex, with a direct comparison with the risk of Pfizer COVID-19 vaccine induced myocarditis and background myocarditis. For our secondary objective, the risk of hospitalisation, severe outcome and MSI was stratified by age and vaccination status to define individual risk. The BN assumes a patient has contracted symptomatic SARS-CoV-2 infection for the purpose of the risk-benefit analysis.
Population level risk-benefit analysis
We analyzed the risks vs benefits of the Pfizer COVID-19 vaccine at a population level under scenarios of vaccine coverage of 51.4%, 70.0% and 90.0% of the population for two doses. Vaccine coverage of 51.4% was used as that was the vaccine coverage in Australia in February 2023. Vaccine coverage of 70% and 90% were used to predict outcomes under these vaccination coverage levels to help guide public health interventions. We assumed the same vaccine coverage for all age groups.
Transmission scenarios were defined as the percentage of the total paediatric population with SARS-CoV-2 infection over a two-month period. Various scenarios were selected for risk-benefit analysis at a population level. Scenarios of zero, one, two, five and ten percent transmission were selected to mirror the Centres for Disease Control and Prevention (CDC) indicators of community transmission (low [1–2%]; moderate [5%] and high [10%]). For example, if there were 30,000 cases reported per week, this is equivalent to 240,000 cases over 2 months. With a total population in Australia of children aged 5–17 years old of 8 million28, 240,000 cases over 2 months equates to 3% of the population infected over 2 months.
Using the BN model, the cases per million over 2 months of background myocarditis, Pfizer COVID-19 induced myocarditis, SARS-CoV-2 induced myocarditis, hospitalisations, severe outcomes and MIS-C were calculated. Subsequently, the number of hospitalisations, severe outcome and MIS-C cases prevented were estimated under varying community transmission scenarios with vaccine coverage scenarios of 0%, 51.4%, 70.0% and 90.0%).
Sensitivity analysis
The two most likely variables to fluctuate over time were community transmission and vaccine coverage. Therefore, the above population level analysis also acted as a sensitivity analysis to evaluate the requirement to update model assumptions.
Model validation
Collaborators evaluated the model structure, variables and relationships as well as assumptions made to ensure it accurately represented the current best evidence. A statistician (SJB) was provided with the assumptions and the data used to calculate the CPTs. Then using pre-defined scenarios, outcomes were manually calculated. Further opportunity was provided to discuss assumptions with subject experts SW and PB.
Data availability
All data supporting the findings of this study are available within the paper and its supplementary information.
References
Irfan, O. et al. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch. Dis. Child 106, 440–448 (2021).
WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. https://covid19.who.int/.
Hoang, A. et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine 24, 100433 (2020).
Castagnoli, R. et al. Severe acute respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 174, 882–889 (2020).
COVID-19 confirmed cases and deaths - UNICEF DATA. https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-dashboard/.
Feldstein, L. R. et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 383, 334–346 (2020).
Frenck, R. W. et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N. Engl. J. Med. 385, 239–250 (2021).
COVID-19 Vaccine Roll-out (2023). Available at COVID-19 vaccine rollout update – 31 March 2023 | Australian Government Department of Health and Aged Care. Available at: https://www.health.gov.au/resources/publications/covid-19-vaccine-rollout-update-31-march-2023?language=en. Accessed 15/3/2023
Kleitman, S. et al. The psychology of COVID-19 booster hesitancy, acceptance and resistance in Australia. Vaccines 11, 907 (2023).
Esposito, S. et al. Knowledge on parental hesitancy toward COVID-19 vaccination of children 5–11 Years Old. Vaccines 11, 587 (2023).
Chou, W.-Y. S., Burgdorf, C. E., Gaysynsky, A. & Hunter, C. M. (2020). COVID-19 Vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. national institutes of health. Retrieved from https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf.
Bonner, C. et al. Current best practice for presenting probabilities in patient decision aids: fundamental principles. Med. Decis. Mak. 41, 821–833 (2021).
Australian Government Department of Health, ATAGI, CSANZ, RACGP, ACRRM, ACEM. COVID-19 vaccination – Guidance on myocarditis and pericarditis after mRNA COVID-19 vaccines. Canberra: Department of Health; 2021 Nov 8. Available from: https://acem.org.au/getmedia/44c79b6b-3482-469a-85fe-bb97ed81d845/covid-19-vaccination-guidance-on-myocarditis-and-pericarditis-after-mrna-covid-19-vaccines.
Sinclair, J. E. et al. A Bayesian network analysis quantifying risks versus benefits of the Pfizer COVID-19 vaccine in Australia. NPJ Vaccines 7, 93 (2022).
Immunisation Coalition. CoRiCal: COVID-19 Risk Calculator [Internet]. Melbourne: Immunisation Coalition; [updated 2023 Feb 27; cited 2023 Mar 23]. Available from: https://corical.immunisationcoalition.org.au/children.
Pillay, J. et al. IncidIncidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditisfollowing COVID-19 vaccination: living evidence syntheses and review. BMJ. 378, e069445 (2022).
Therapeutics Goods Administration. COVID-19 vaccine weekly safety report - 06-01-2022 | Therapeutic Goods Administration (TGA). Australian Government Department of Health https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-06-01-2022#section-1372 (2022).
Knudsen, B. & Prasad, V. COVID-19 vaccine induced myocarditis in young males: a systematic review. Eur. J. Clin. Investig. 00, 13947 (2022).
Kohler, R. E. et al. Parents’ intentions, concerns and information needs about COVID-19 vaccination in new jersey: a qualitative analysis. Vaccines11, 1096 (2023).
Nealon, J. & Cowling, B. J. Comment Omicron severity: milder but not mild. Lancet 399, 412–413 (2022).
Menegale, F. et al. Evaluation of waning of SARS-CoV-2 vaccine-induced immunity: a systematic review and meta-analysis. JAMA Netw. Open 6, E2310650 (2023).
Mayfield, H. J. et al. Designing an evidence-based Bayesian network for estimating the risk versus benefits of AstraZeneca COVID-19 vaccine. Vaccine 40, 3072–3084 (2022).
Probabilistic Reasoning in Intelligent Systems. Probabilistic Reasoning in Intelligent Systems https://doi.org/10.1016/C2009-0-27609-4 (1988).
Mascaro, S. et al. Modeling COVID-19 disease processes by remote elicitation of causal Bayesian networks from medical experts. BMC Med. Res. Methodol. 23, 76 (2023).
Prodhan, G. & Fenton, N. Extending the range of COVID-19 risk factors in a Bayesian network model for personalised risk assessment. Epidemiol. Int. J. 4, 170–171 (2020).
Australian Government Department of Health and Aged Care. COVID-19 vaccine rollout update – 12 April 2024. Canberra: Department of Health and Aged Care; 2024 Apr 12. Available from: https://www.health.gov.au/resources/publications/covid-19-vaccine-rollout-update-12-april-2024.
Australian Technical Advisory Group on Immunisation (ATAGI) | Australian Government Department of Health and Aged Care. https://www.health.gov.au/committees-and-groups/australian-technical-advisory-group-on-immunisation-atagi.
Statistics, A. B. of. Stat Data Explorer (BETA) • Quarterly Population Estimates (ERP), by State/Territory, Sex and Age. https://explore.data.abs.gov.au/vis?tm=quarterly population&pg=0&df[ds]=ABS_ABS_TOPICS&df[id]=ERP_Q&df[ag]=ABS&df[vs]=1.0.0&hc[Frequency]=Quarterly&pd=2019-Q2%2C&dq=1.3…Q&ly[cl]=TIME_PERIOD&ly[rw]=REGION (2022).
PAEDS. COVID-19 in Australia. NCIRS https://paeds.org.au/ (2022).
Singer MPH, M. E., Taub, I. B., Kaelber, D. C., Singer, M. E. & Associate Professor, M. Risk of Myocarditis from COVID-19 infection in people under age 20: a population-based analysis. https://doi.org/10.1101/2021.07.23.21260998.
Tan, S. et al. Effectiveness of BNT162b2 vaccine against omicron in children 5 to 11 Years. SSRN Electron. J. 525–532 https://doi.org/10.2139/ssrn.4052133 (2022).
Dorabawila, V. et al. Effectiveness of the BNT162b2 vaccine among children 5–11 and 12–17 years in New York after the Emergence of the Omicron Variant [Preprint]. medRxiv. https://doi.org/10.1101/2022.02.25.22271454.
Fleming-Dutra, K. E. et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infectionin children and adolescents during Omicron predominance. JAMA. 327, 2210–2219 (2022).
New South Wales Government. NSW Respiratory Surveillance Report – Epidemiological week 30, ending 30 July 2022. Sydney: NSW Health; 2022 Jul 30. Available from: https://www.health.nsw.gov.au/Infectious/covid-19/Documents/weekly-covid-overview-20220730.pdf.
Australian Bureau of Statistics. National, state and territory population [Internet]. Canberra: ABS; 2021 December [cited 2022 August 22]. Available from: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release.
Nasreen, S. et al. Background incidence rates of adverse events of special interest related to COVID-19 vaccines in Ontario, Canada, 2015 to 2020, to inform COVID-19 vaccine safety surveillance. Vaccine. 40, 3305–3312 (2022).
Health, A. G. D. of. COVID-19 vaccine safety report - 25-08-2022 | Therapeutic Goods Administration (TGA). https://www.tga.gov.au/news/covid-19-vaccine-safety-reports/covid-19-vaccine-safety-report-25-08-2022.
Block, J. P. et al. Cardiac Complications After SARS-CoV-2 Infection and mRNA COVID-19 Vaccination — PCORnet, United States, January 2021–January 2022. MMWR Morb. Mortal. Wkly Rep. 71, 517–523 (2022).
Price, A. M. et al. BNT162b2 protection against the omicron variant in children and adolescents. N. Engl. J. Med. 386, 1899–1909 (2022).
Zambrano, L. D. et al. BNT162b2 mRNA vaccination against COVID-19 is associated with a decreased likelihood of multisystem inflammatory syndrome in children aged 5–18 Years—United States, July 2021 – April 2022. Clin. Infect. Dis. https://doi.org/10.1093/CID/CIAC637 (2022).
Acknowledgements
Our BN model was built using GeNIe Modeller (Bayes Fusion 2019), available for free for teaching and academic use at https:www.bayesfusion.com. This research did not receive any specific funding or grant from funding agencies in the not-for-profit, commercial or public sectors. The CoRiCal project was supported by the immunisation coalition through educational grants received from pharmaceutical companies that manufacture vaccines. However, the CoRiCAL project did not receive any direct funding from Pfizer, Astra Zeneca or any other pharmaceutical company that produces COVID-19 vaccines. All co-authors in this paper provided in-kind contribution of their expertise and time to develop the interactive tool and the modelling and research that underpins it. C.L.L. and K.R.S. were supported by the Australian National Health and Medical Research Council (NHMRC) investigator grants (1193826 and 2007919).
Author information
Authors and Affiliations
Contributions
Conception and design: T.S., J.L., K.R.S., C.L., A.D. Acquisition of data: T.S., O.W., S.J.B., J.S., P.J.B., R.P. Analysis and Interpretation: T.S., S.J.B., T.M., A.D., S.W. Drafting the article: T.S., J.L., C.L., H.M., A.D. Revising article for intellectual content: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the paper. Author K.R.S. serves as an editor of this journal, with A.D. an associate editor of NPJ Digital Public Health, both had no role in the peer-review or decision to publish this manuscript. Both K.R.S. and A.D. declares no financial competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shukla, T., Mayfield, H.J., Litt, J.C.B. et al. A Bayesian network analysis of the Pfizer COVID-19 vaccine in the paediatric population. npj Vaccines 10, 174 (2025). https://doi.org/10.1038/s41541-025-01237-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-025-01237-3