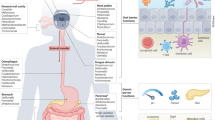
Abstract
Oral vaccines are potential options for preventing global pandemics because of their ability to elicit systemic and mucosal immunity and the high degree of compliance among individuals for oral vaccines. Nevertheless, oral vaccine efficacy is insufficient, and there is heterogeneity in the vaccine response among individuals, which may be attributed to the complex network of interactions in the gastrointestinal tract. An increasing amount of evidence suggests that the composition and function of the gut microbiota are essential factors modulating the efficacy of oral vaccines. Deepening our understanding of the implications and potential mechanisms of the interaction between oral vaccines and the gut microbiota could inspire the design of effective oral delivery methods. Here, the effects of the gut microbiota on the mucosal immune system and oral vaccine efficacy are reviewed. Examples and implications for modulating the gut microbiota are also summarized. We hope that this review inspires a focus on oral vaccines and highlights pivotal crosstalk between the gut microbiota and vaccine effectiveness.
Similar content being viewed by others
Introduction
Vaccination has eradicated or nearly eradicated smallpox and polio, reduced the severity of many diseases, and can prevent the development of cancers, such as cervical cancer1. Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, there has been a growing appreciation of the irreplaceable role of vaccines in protecting against serious diseases2. Vaccination can elicit antigen-specific humoral and cellular immune responses by simulating a response akin to that during the pathogen infection process; therefore, the body’s immune system can establish a strong defense. Conventional vaccines are usually administered intramuscularly or subcutaneously and require professional medical staff, and cause pain at the injection site3. In addition, there are many people with misconceptions and concerns about vaccine safety and the pain caused by injection, which reduces adherence to vaccination, known as vaccine hesitancy4. In recent years, oral vaccines have been studied and are expected to solve these problems.
Oral vaccines do not require injections, thus avoiding the pain and vaccine hesitancy associated with injection5. In addition, the transport and storage conditions for oral vaccines are notably more flexible. For instance, Dukoral (an oral cholera vaccine) can be stored at room temperature (up to 25 °C) for a maximum of two weeks on a single occasion. This characteristic is particularly advantageous for resource-limited countries and regions. These factors suggest that oral vaccination offers both superior compliance and applicability6, thereby facilitating the rapid deployment of oral vaccines worldwide. Furthermore, in the intestinal mucosal system, there are unique lymphoid tissues and abundant lymphocytes. Oral vaccination can provide both systemic immunity and intestinal mucosal immunity realized through the secretion of antigen-specific secretory immunoglobulin A (SIgA), which directly protects against pathogen invasion3. Lymphocytes producing IgA migrate to mucosal sites, such as the lower respiratory tract, nasal cavity, and vagina, increasing protective immunity7,8. Intestinal mucosal vaccination is currently considered the promising way to protect against intestinal pathogens. Despite improvements in the development of oral vaccines, the oral delivery barriers and heterogeneity of the vaccine response among individuals remain key issues. The major challenges for oral vaccine delivery include the following: (1) overcoming gastric degradation, intestinal mucus, and epithelial barriers; (2) delivering intact and active antigens to intestinal mucosal-associated lymphoid tissue; and (3) subsequent antigen uptake by antigen-presenting cells (APCs), antigen presentation, and APC activation9,10.
The heterogeneity of the response of different populations to vaccines is due to a complex network of interactions. The factors that influence the human immune response to vaccines include intrinsic factors (age, genetics, comorbidities, symbiotic microbiota, etc.), extrinsic factors (infection, antibiotic use, invasive microorganisms, etc.), environmental factors, behaviors, and dietary and nutritional habits11. Additionally, the type of vaccine administered, the vaccine dose and adjuvant, and administration route also affect vaccine efficacy12. Recently, the gut microbiota has attracted much attention because of its prominent functions in both the host’s physiological processes and the immune system.
The human gut microbiota contains more than 100 trillion microorganisms, including bacteria, viruses, archaea, fungi, and other complex biological communities13, which perform essential functions in regulating host immunity and nutrient metabolism, maintaining the structural integrity of the intestinal barrier, and defending against pathogens14. The human gut microbiota is dominated by Bacteroidetes and Firmicutes, followed by Actinobacteria, Proteobacteria, and Verrucomicrobia15. Advances in gene sequencing technology and extensive data analysis have revealed that the gut microbiota composition also impacts responses to vaccines16. Scientists have often drawn inspiration from pathogenic microorganisms. There are incredible vaccines that mimic pathogens, ranging from initially attenuated or inactivated microorganism-based vaccines to the microorganism components that provide natural adjuvant activity17,18. Gut microbiota-related molecules may become new oral vaccine adjuvants; for example, flagellin from Salmonella species can be recognized by the pattern recognition receptor (PRR) Toll-like receptor (TLR) 5 on immune cells19. Thus, further understanding the complicated relationships between the gut microbiota and the immune system can provide strong evidence for oral vaccine design.
In this review, we analyzed the impacts of the gut microbiota on the mucosal immune system and oral vaccine efficacy. Examples and implications for modulating the gut microbiota are also summarized to inspire future oral vaccine design. We expect to explore the implications and potential mechanisms of the gut microbiota in depth and provide innovative ideas for oral vaccine development.
Impacts of the gut microbiota on the intestinal mucosal immune system
The intestinal mucosal immune system is a dynamic environment with trillions of microbiota organisms. With the continuous growth and death of microorganisms, the intestinal mucosa periodically interacts with microorganisms and pathogens with different properties. While the gut microbiota has many benefits, its constituents are also recognized as foreign microbes20. Thus, chemical and physical barriers exist that can spatially isolate the gut microbiota and host immune system to prevent an excessive immune response. In the large intestine, there are three physical barriers separating the gut microbiota and epithelial cells: the mucus layer, the glycocalyx on IECs, and the tight and adherent junctions of epithelial cells20. The small intestine produces less mucus than the large intestine; nevertheless, there is a special class of Paneth cells in the small intestine that specializes in the production of chemical barrier molecules, such as antimicrobial peptides and Reg3 family proteins, which are pivotal for the separation of the intestinal microbiota and intestinal epithelial cells (IECs)20. Together, these physical barriers protect intestinal tissues from bacterial pathogens.
From the perspective of delivery of oral vaccines to the mucosal immune system, the pivotal challenges include a mucus barrier characterized by dynamic, continuously renewing gel-like properties and an epithelial barrier composed of IECs21,22,23. The mucus layer covers the surface of the intestinal epithelium21. The mucus layer is a complex gel layer composed of water, inorganic salts, and specific mucus proteins24, with a negatively charged surface and numerous hydrophobic groups. The mucus layer can adsorb and trap foreign pathogens, which are removed with mucus renewal, thus preventing microorganisms from reaching epithelial cells from the intestinal lumen. Most hydrophobic or cationic vaccines are usually captured by the mucus layer and subsequently removed by mucociliary clearance25. Mucus secretion is regulated by the host sensing of the gut microbiota and its metabolites26. The gut microbiota composition critically affects the intestinal mucus, with some bacteria (i.e., Allobaculum mucolyticum) having a greater ability to induce the ability of an impenetrable inner mucus layer, whereas others (i.e., all the bacteria that belong to the Proteobacteria mucolyticum) cannot degrade the mucus layer27. The expression pattern of glycosyltransferases may be the key factor through which the composition of the gut microbiota affects mucus properties28. The last line of defense in the intestinal mucosa is provided by IECs and numerous immune cells residing in the gut-associated lymphoid tissue (GALT). The gut microorganisms themselves can signal IECs via the release of microbial components and metabolites. IECs then modulate the mucosal barrier and transmit signals to lamina propria immune cells to adapt to changes in the intestinal environment. These microbial signals are pivotal for maintaining the integrity, proliferation, and barrier function of IECs.
GALT serves as an important site at which oral vaccines elicit immunity and is indispensable for maintaining gut homeostasis and human health3. The gut microbiota also influences the development of GALTs and the homeostasis of the host immune system29,30. On the basis of differences in lymphocyte composition, genetically determined development, and dependence on environmental stimuli during tissue inflammation and immune response generation, GALTs are divided into Peyer’s patches (PPs), cryptopatches, isolated lymphoid follicles, and mesenteric lymph nodes (MLNs)9. Among them, PPs are abundant in many species and are key targets for oral vaccine design. PPs are not only structurally similar to lymph nodes in that they both have B-cell follicles and T-cell regions, but also have microfold (M) cells for antigen sampling31. M cells internalize intestinal microbial antigens and transport them to dendritic cells (DCs) in GALTs to induce mucosal immune responses32. The specific expression of glycoprotein 2 on both human and mouse M cells initiates mucosal immunity against symbiotic and pathogenic bacteria, such as Escherichia coli and Salmonella32. Thus, oral vaccines could be designed to target glycoprotein-2 or other newly discovered specific receptors on M cells. However, the impacts of the gut microbiota on M-cell development and function remain unclear.
DCs are professional APCs that initiate and modulate immune responses33. In GALTs, DCs can directly capture antigens, migrate to MLNs, and activate B and T cells34. In homeostasis, conventional DCs require microbial-derived signals to maintain their basal state so that they can quickly respond and initiate adaptive immunity when confronted with pathogens35. In addition, several species of bacteria reportedly affect the immunomodulatory function of DCs. For example, Listeria monocytogenes can activate DCs by binding to PRRs on their surface, and protein components derived from bacteria can also stimulate DC maturation36. Further mechanistic studies have revealed that short-chain fatty acids (SCFAs), gut microbiota metabolites, promote actin polymerization by activating signaling via the Src family kinase/phosphatidylinositol-3 kinase/Rho family GTPase pathway, thereby stimulating dendrite elongation in DCs and increasing antigen uptake and presentation by DCs37. Studies have increasingly confirmed that the gut microbiota can also enhance anticancer immunotherapy by triggering DC activation and maturation and activating an IL-12-dependent Th1 cell immune response38.
The gut microbiota has been confirmed to be beneficial for the development of B and T cells and the production of sIgA. In germ-free animals, the intestinal mucosal immune system shows PP hypoplasia and a reduced number of plasma cells producing sIgA and T cells39. Research has shown that Escherichia coli and Bifidobacterium can facilitate B-cell maturation40. As the most abundant mucosal antibody type, SIgA can protect the mucosa from infection by pathogenic microorganisms and regulate the gut microbiota to promote health41. SIgA can bind to gut microbiota, pathogenic microorganisms, and their products. In general, 20–50% of the gut microbiota is encased in IgA, which is mediated either by the antigen-binding domains or by the glycan moieties of IgA30. SIgA regulates the gut microbiota, including changes in the levels of individual microbial species induced by antibodies that bind directly to microorganisms, and influences complex microbiota ecosystems and interactions between SIgA and the gut microbiota42. Nakajima et al. reported that IgA alters the expression of the mucus-associated functional factor system and further enhances symbiosis with Firmicutes43. IgA also promotes the phagocytosis of pathogens by M cells, DCs, and macrophages, resulting in an efficient local immune response to pathogens30,44. Moreover, the gut microbiota is necessary for normal IgM-to-IgA class-switching recombination.
T follicular helper (Tfh) cells are a specific subset of CD4+ T cells that modulate plasma B-cell differentiation in lymphoid tissues. The development of Tfh cells is deficient in germ-free mice and can be restored via TLR2 ligand-mediated MyD88 signaling45. A lack of MyD88 signaling in T cells reduces high-affinity IgA binding to the microbiota, leading to microbial dysbiosis. Changes in the gut microbiota directly impact the establishment of the Th1/Th2 balance, which in turn leads to reduced secretion of protective cytokines and promotes the occurrence of allergy and autoimmune diseases46. Interestingly, Th17 cells are a subset of CD4+ cells that are independent of Th1 and Th2 cells, which both help the host resist fungal infections, tuberculosis, and other pathogens, and play essential roles in a variety of autoimmune diseases. Th17 cells are most abundant at steady state in GALT, particularly in the small intestinal lamina propria. The composition of the intestinal microbiota impacts the Th17/regulatory T cell (Treg) balance in the lamina propria, thereby affecting intestinal immunity and tolerance47. The adhesion of specific members of the gut microbiome to IEC is essential for inducing Th17 cell differentiation48. A recent study revealed that the gut microbiota can stimulate the gut epithelium to produce Th17 cells by promoting endoplasmic reticulum stress49. The impact of the gut microbiota on the intestinal mucosal immune system is presented in Fig. 1.
Chemical and physical barriers exist that can spatially isolate the gut microbiota and host immune cells to prevent excessive immune responses. Chemical barrier molecules include antimicrobial peptides and Reg3 family proteins. The physical barriers include the mucus layer, the glycocalyx on IECs, and the tight and adhesion junctions of the epithelium. The gut microbiota plays a pivotal role in affecting the intestinal mucus and epithelial barriers. The gut microbiota and their metabolites impact DC maturation, antigen uptake and presentation. The gut microbiota was confirmed to be beneficial for the development of B and T cells and the production of sIgA. Changes in the gut microbiota directly influence the Th1/Th2 and Th17/Treg balance. SIgA, secretory immunoglobulin A; MHC, major histocompatibility complex; TCR, T-cell receptor; DC, dendritic cell; Treg, regulatory T cell. (Created with Adobe illustrator).
Effects of the gut microbiota composition on vaccine efficacy
The composition of the gut microbiota can significantly affect the development and regulation of vaccine-induced immunity. The gut microbiota composition is also closely correlated with age and is consistent with the maturation and function of immune cells and the immunogenicity of vaccines50. In early life, gut microbiota diversity is low, and the immune system is not mature; thus, vaccine immunogenicity is low. Adults have increased gut microbiota diversity and a mature immune system, and vaccines have increased immunogenicity. The diversity of the gut microbiota and vaccine immunogenicity decreases with immunosenescence. Different bacteria have different characteristics and preferences, so their impacts on the immune efficiency of different vaccines differ. On the basis of the results of animal experiments and clinical research, the effects of the gut microbiota on vaccine responses are shown in Table 1.
These studies provide evidence that the gut microbiota composition affects vaccination potency. Species variations and the gut microbiota composition are thus good targets for oral vaccine design22. Specific vaccines may require specific microorganisms to be present in order to enhance their immune effects. Additionally, optimization of vaccine effectiveness by targeting the gut microbiota also needs to be stratified according to different populations. Studies have confirmed that the administration of Bifidobacterium and Lactobacillus in infants enhances humoral immunity51. A systematic review of 26 studies on the use of Bifidobacterium and Lactobacillus and their effects on oral and parental vaccine-induced immunity concluded that the use of these probiotics in infants offers an intervention to improve vaccine efficacy and the duration of protection52. The characteristics of gut dysbiosis in older adults include the presence of many proinflammatory microbes, such as Bacteroides and Enterobacteriaceae, and low production of SCFAs53,54. However, long-lived centenarians generally have a more anti-inflammatory gut microbiota composed of constituents such as Akkermansia or Bifidobacterium. Thus, according to the immune characteristics of different populations, it will be more reasonable and effective to design personalized vaccines to adjust the composition of their gut microbiota.
The mechanisms of the gut microbiota regulating oral vaccination potency
In addition to bacterial components recognized by gut-associated immune cells, bacterial metabolites derived from the gut microbiota also have beneficial modulatory effects on immune responses55. The gut microbial metabolites have also been shown to modulate the microbial community structure and function56. The discovery of host‒microbe interactions and their links to diseases offers opportunities to investigate new therapeutics by regulating the microbiota or their metabolites. Currently, the mechanism by which the microbiota regulates immunity to vaccination still needs to be fully elucidated. Further research into the mechanisms and the interactions between different mechanisms is challenging. The main mechanisms by which the gut microbiota regulates vaccination potency are shown in Fig. 2.
A Acting directly with the vaccine. Many vaccines consist of essential carbohydrates, such as polysaccharide vaccines or targeting ligands, which are polysaccharide structures, so they are easily degraded when exposed to the intestinal environment. B Carrying vaccine-like epitopes induces cross-immunity. The gut microbiota-encoded epitopes increase the ability of B cells or T cells to cross-present antigens and change vaccine-induced immune responses. C Providing immune adjuvants. The gut microbiota provides numerous immune adjuvants that improve vaccine potency. LPS, polysaccharide A, flagellin, and peptidoglycan can activate TLR4, TLR2, TLR5 and NOD2 signaling to modulate immunity. D Immunomodulatory microbiota-derived metabolites. Gut microbial metabolites, such as SCFAs and bile acid, can enhance B-cell metabolism and plasma cell function. SCFAs mediate the inhibition of DC and macrophage maturation, impacting the ability to capture antigens and reducing the production of proinflammatory cytokines. SCFAs can also directly affect the ability of naïve T cells to differentiate into Th1 and Th17 cells. BCR, B-cell receptor; TCR, T-cell receptor; LPS, lipopolysaccharide; TLR, toll-like receptor; APC, antigen-presenting cell; NOD, nucleotide-binding oligomerization domain-containing protein; SCFA, short-chain fatty acid; GPR, G protein-coupled receptor; SIgA, secretory immunoglobulin A. (Created with Adobe illustrator).
Acting directly with the vaccine
The gut microbiota may modify drugs in the host, such as through deglycosylation, demethylation, dehydroxylation, hydrolysis, redox reactions, and other reactions57,58. However, while no studies have shown that the gut microbiota can directly modify vaccine components, it is assumed that the gut microbiota directly affects oral vaccines. Moreover, because oral vaccines are absorbed through the small intestine, their efficacy may also be influenced by microorganisms in the small intestine. The adult distal gut microbial community is mainly composed of Firmicutes and Bacteroidetes59, which all encode abundant glycoside hydrolase genes. Some vaccines that contain carbohydrates, such as polysaccharide vaccines, or targeting ligands that are polysaccharide structures, may degrade easily when exposed to the intestinal environment. Therefore, oral nanovaccine formulations containing glycosidic bonds are easily degraded when exposed to the intestinal environment. This class of vaccines can be modified by glycosylation or carrier inclusion to resist glycosidic bond hydrolysis.
Carrying vaccine epitopes induces cross-immunity
Research has shown that the gut microbiota induces cross-protection by carrying vaccine epitopes, which affects vaccine efficacy and leads to differences in vaccine immune responses in different populations. Su et al. identified CD4 memory T cells specific to HIV-1, cytomegalovirus, and herpes simplex virus with a rich memory phenotype for viral antigens in people who were not infected with these viruses60. The specificity of the T-cell receptor (TCR) in newborns is almost entirely naïve, making them more susceptible to infection. The underlying mechanism may be the cross-reaction of TCRs to epitopes encoded by the gut microbiota. Healthy individuals have many resident CD4 T cells that are responsive to the gut microbiota61. Bioinformatic predictions have revealed extensive TCR epitope library sharing among the human proteome, gut microbiome, and pathogen proteome, thus potentially sharing immunogenic antigens50. A growing body of data suggests that these cross-reactive T cells or B cells can regulate immunity against pathogens by modulating the immunogenicity of antigen epitopes62. In addition, the gut microbiota is thought to induce the production of specific T cells that cross-react with tumor-associated antigens. Intestinal Escherichia coli (E. coli) O86:B7 and Plasmodium spore surfaces express α-gal, which can induce human or mouse production of α-gal-specific IgM antibodies for malaria protection63. Fluckiger et al. discovered major histocompatibility complex (MHC) class I-binding epitopes in the genome of the bacteriophage Enterococcus hirae. Mice harboring this phage showed antigen-specific CD8+ T-cell immunity after immunotherapy64. However, little is known about whether gut microbiota-encoded epitopes activate the ability of B cells and T cells to cross-present antigens and change vaccine-induced immunity, but the underlying mechanisms by which the gut microbiota impacts vaccine-induced immunity warrant further investigation. Bondareva et al. found that several oral commensal bacteria, such as Streptococcus salivarius, can induce salivary anti-SARS-CoV-2 Spike IgG antibodies in mice via a molecular mimicry mechanism, thereby facilitating the clearance of SARS-CoV-265. Furthermore, oral supplementation with Streptococcus salivarius was shown to enhance salivary anti-spike antibody levels in vaccinated individuals. This study suggests that identifying these beneficial commensal microorganisms and leveraging their shared epitopes via molecular mimicry may improve the protective immunity conferred by vaccines.
Providing immune adjuvants
Adjuvants are key components of vaccines that can increase vaccine potency via various mechanisms, such as recruiting and triggering innate immune cell activation, activating PRRs expressed on immune cells, and enhancing antigen presentation66,67. The addition of appropriate adjuvants reduces the required dosage of antigens, decreases the required number of vaccinations, and expands protective immunity68, so adjuvant addition is an important research direction to improve vaccine efficiency. The ideal adjuvants should promote innate and adaptive immunity and induce a durable memory immune response69,70. Cholera toxins produced by Vibrio cholerae and heat-unstable enterotoxins produced by enterotoxigenic E. coli are effective mucosal adjuvants but pose safety issues71. Therefore, there is still an urgent need for powerful mucosal adjuvants that can facilitate oral vaccine efficacy. One study summarized the strengths and weaknesses of common adjuvants, emphasizing promising options derived from the interaction between nutrition and the gut microbiota15.
The abundant gut microbiota and its components are sources of natural immune adjuvants that improve vaccine effectiveness. Adjuvants derived from microorganisms include lipopolysaccharides (LPSs), polysaccharide A, flagellin, and peptidoglycan, which are recognized by PRRs on many immune cells15. LPS is a unique component of the outer wall of gram-negative bacteria and is a complex molecule composed of lipids and polysaccharides. LPS can act as an adjuvant in vaccination by activating TLR472. TLR4 signaling leads to the production of proinflammatory cytokines and type I IFNs and the upregulation of costimulatory molecules, promoting the Th1 response and antibody production. However, different bacterial groups produce different types of LPS with varying immunogenicity, which may further complicate the situation73. In addition, polysaccharide A is a capsular polysaccharide produced by Bacteroides fragilis that is sensed by TLR2 on DCs and inhibits Th1 and Th17 cell activity to modulate inflammatory responses74. Flagellin is the main component of bacterial flagella and a TLR5 ligand, which generates an immune response via the MyD88-dependent pathway signaling. Fu et al. used length-tunable flagella as model protein nanofibers and reported that shorter nanofibers increased IgA levels in mucosal secretions75. The antibody response of TLR5-/- mice to PCV13 was indeed significantly impaired76. Nucleotide-binding oligomerization domain-containing protein 2 (NOD2) sensing of microbiota-derived peptidoglycans can modulate host immunity and maintain homeostasis, which may be required for the development of intranasal immunity to cholera toxin77. Akkermansia muciniphila is a significant gut microbe that plays a crucial role in regulating cancer immunotherapy and homeostatic immunity in humans78,79. Recent studies have demonstrated that the immunomodulatory function of Akkermansia muciniphila is attributed to a diacyl phosphatidylethanolamine with two branched chains (a15:0-i15:0 PE). This compound can activate a non-canonical TLR2-TLR1 heterodimer pathway, leading to the release of inflammatory factors80. This study may inspire the development of novel TLR agonist-like adjuvants aimed at enhancing the immune efficacy of oral vaccines. Thus, other materials or delivery systems that activate signaling via these PRR pathways may also have similar adjuvant effects.
The expression pattern of TLRs varies according to different intestinal sites. Price et al. reported that TLR expression in the colon was greater than that in the small intestine, whereas TLR5 expression was limited to Paneth cells81. The expression and subcellular localization of various TLRs are regulated so that the epithelium is stimulated by appropriate ligands. TLR expression is also age-dependent. In newborns, TLR expression is downregulated, whereas in older adults, TLR expression becomes differentially regulated82. Therefore, on the basis of differences in TLR expression, selecting appropriate TLR-based adjuvants or combinations is the key to improving the immune efficacy of oral vaccines.
Generating immunomodulatory microbial metabolites
The diverse gut microbiota can generate many diverse metabolites that have the potential to regulate immunity. The most studied metabolites are SCFAs and secondary bile acids (BAs). SCFAs include acetate, butyrate, and propionate, which are metabolic end products of bacterial fermentation in the colon50. Bacteroidetes and Firmicutes are the most abundant phyla in the intestine; Bacteroidetes mainly produce acetate and propionate, whereas Firmicutes mainly generate butyrate83. Inspired by “molecular exchange” between intestinal microbiota and host cells, butyrate has been conjugated to polyethylene glycol nanoparticles to promote transepithelial transport and intestinal absorption in the ileum during oral delivery84. Improved transepithelial transport and intestinal absorption were achieved by specific interactions between butyrate and the monocarboxylate transporter on cell membranes. SCFAs have been confirmed to signal through the G protein-coupled receptors (GPRs) GPR41, GPR43 and GPR109A, which are expressed on epithelial cells, neutrophils, macrophages, DCs, B cells and T cells85. The variable expression of these GPRs in different tissues and their different affinities for individual SCFAs are the basis for the versatile functions of SCFAs. SCFAs can induce neutrophil chemotaxis by regulating the production of inflammatory cytokines and chemokines86. Butyric acid increases the expression of tight junction proteins to ensure barrier integrity. In addition, SCFAs reverse increased paracellular permeability and morphological disruption of tight junction proteins by inhibiting inflammatory signaling85. SCFAs have been reported to mediate the inhibition of DC and macrophage maturation through modulating the activity of histone deacetylases, impacting the ability to capture antigens and reducing the production of proinflammatory cytokines86, leading to the generation of tolerogenic T cells. These effects are detrimental to the activation of APCs and the induction of immune responses to oral vaccines in the mucosal immune system. However, SCFAs can directly affect the ability of naïve T cells to differentiate into Th1 and Th17 cells85,87. SCFAs can promote effector T cell or Treg cell differentiation, depending on the immune environment. However, the specific influencing factors need to be further elucidated to guide the design of immune-boosting vaccines or tolerogenic vaccines. SCFAs can also improve oxidative phosphorylation, glycolysis, and fatty acid synthesis in B cells, increasing the expression of genes related to plasma cell differentiation and class switching88. One study analyzed the relationship between the metabolic function of the human gut microbiota and immunity to an inactivated SARS-CoV-2 vaccine89. Vaccination leads to changes in the microbiota composition and enrichment of functional pathways. SCFA levels were significantly greater in a high-antibody-titer group than in a low-antibody-titer group, and the levels of some SCFAs were positively associated with the antibody response. Yang et al. reported that feeding mice SCFAs can potentiate the production of antigen-specific IgA and IgG antibodies against cholera toxin90. SCFAs do not directly mediate the adjuvant activity of cholera toxin but promote plasma cell differentiation by inducing DCs to produce essential signaling molecules. Given the immunoregulatory effects of SCFAs, they may be helpful vaccine components for optimizing the immunogenicity of oral nanovaccines.
BA is a kind of cholanic acid derivative produced by the catabolism of cholesterol in the body. The microorganisms that participate in BA metabolism include Bacteroides, Clostridium, Lactobacillus, Bifidobacterium, and Listeria91. Microorganisms mainly regulate BAs metabolism through signaling via the farnesoid X receptor and G protein-coupled BA receptor 1, and the resulting BA metabolism affects the abundance of RORγ+ Treg cells and thus affects the IgA content produced in response to vaccine activity on immune cells92,93. Antibiotic treatment can significantly decrease secondary BA levels; decreased secondary BA levels are associated with enhanced inflammation upon influenza vaccine immunization in humans94. Azuar et al. extracted antigenic peptides from group A Streptococcus, bound them to cholic acid via solid-phase peptide synthesis to obtain lipopeptides, and then allowed induced to undergo self-assembly into rod-like nanoparticles95. After intranasal immunization of mice with these nanoparticles, the lipopeptide induced epitope-specific antibody secretion, when administered alone and in a liposomal formulation. The humoral immune response induced by this cholic acid-based conjugate was significantly more robust than that induced by the cholera toxin adjuvant.
Strategies to enhance oral vaccine efficacy through their interaction with gut microbiota
Probiotic-based oral vaccines
Maintaining gut microbiota homeostasis is highly important for disease prevention and treatment. The main approaches used to regulate the composition of the gut microbiota include oral probiotics, prebiotics, symbiotics, and fecal bacterial transplantation96, which can promote the remodeling of microbiota homeostasis97. However, owing to its invasive characteristics and unclear composition of the bacteria, fecal bacteria transplantation has challenges such as poor safety and low compliance of patients98. Oral Lactobacillus plantarum was reported to promote the activation, proliferation, and differentiation of B cells and T cells to enhance the effects of SARS-CoV-2 vaccination99. Further studies are needed to explore more appropriate strains and doses of probiotic therapy. Although oral probiotics are easy to use and have good safety, the study results suggest that their bioavailability is low and that their effectiveness for disease prevention and treatment is limited.
Oral vaccines based on probiotics can induce mucosal immunity and prevent host intestinal infections100. Adding a protective “coat” to probiotics is a common means to protect their function. Cao et al. proposed a convenient single bacterial encapsulation technology that specifically utilizes biological interface supramolecular self-assembly to form an additional layer of phospholipid molecular protection on the surface of probiotics101. The preparation method is simple and efficient, and does not affect the activity of bacteria. This versatile approach is suitable for coating with multiple cell membranes including those derived from erythrocytes, platelets, macrophages, neutrophils, and cancer cells. Moreover, the phospholipid molecule was approved by the U.S. Food and Drug Administration (FDA), which is conducive to subsequent clinical trials and translation. β-glucan can activate signaling via the dectin-1 receptor to promote uptake by M cells. Therefore, Liu’s research team promoted uptake by intestinal M cells by coating probiotics with a functional layer rich in β-glucan102. In this way, the probiotics are protected from gastrointestinal environmental exposure, and oral availability is improved. Such formulations can deliver bacteria to PPs to induce mucosal immunity. Enhanced mucosal immunity helps regulate the microbiota composition. Pathogens such as Salmonella and Escherichia-Shigella were almost completely inhibited, while symbiotic bacteria were retained to avoid infection.
In addition, the use of antibiotics is crucial for regulating gut microecology and influencing vaccine responses. A randomized controlled clinical trial investigating the effects of vancomycin on immunity to rotavirus vaccines in healthy adults revealed that vancomycin did not alter the titer of anti-rotavirus IgA in subjects but enhanced the immune response to rotavirus vaccines seven days after immunization; moreover, vancomycin use can increase the excretion of rotavirus in the stool and rapidly change the diversity of the intestinal flora103. However, Lynn et al. reported that antibiotic-induced intestinal dysbiosis during early life resulted in a reduced antibody response to five different types of vaccines: Bacillus Calmette–Guérin vaccine, Bexsero meningococcal serogroup B vaccine, NeisVac-C meningococcal serogroup C vaccine, Prevenar 13-valent pneumococcal conjugate vaccine, and the INFANRIX Hexa combination vaccine104. Another study showed that antibiotic use-induced microbiota depletion decreases antibody responses in subjects with low preexisting immunity94. Treatment with antibiotics resulted in increased inflammation and a reduction in secondary BA levels, which may explain the impaired immunity. There are conflicting views about how antibiotic use affects vaccine effectiveness, and more research is needed to clarify this issue.
Modulation of gut microbiota-based oral vaccines
Inulin, an ingredient recognized as safe by the FDA, exerts an adjuvant effect by activating TLR4 signaling on APCs105. Inulin was found to target the symbiotic microbiota that is prevalent in the colon. Han et al. prepared an oral inulin gel with a “colon-retentive” property and increased the abundance of SCFA producers, such as Akkermansia, Lactobacillus, and Roseburia, and their metabolites, which can benefit immunity to immune checkpoint blocker therapy106. This led to an amplified memory CD8 + T-cell response and antitumor activity. Notably, oral delivery of PLGA-loaded antimicrobial peptide microspheres was found to enhance the abundance of beneficial bacteria, such as Phocaeicola vulgatus, in the intestinal tract while simultaneously reducing the prevalence of pathogenic bacteria107. For various biomaterial-based nanovaccines, we can screen vaccine candidates that modulate the gut microbiota to design vaccines that target specific microbiota constituents.
The Bacillus Calmette–Guérin (BCG) vaccine is another possible tool to alter the composition of the gut microbiota. The BCG vaccine, which exerts nonspecific immunomodulatory effects, is widely used worldwide108. After BCG vaccination, the abundance of microbial genomes is related to variability in cytokine production and affects metabolite levels in circulation109. Jeyanathan et al. reported that the BCG vaccine induced the production of lung-resident memory macrophages and enhanced immune responses through signaling via the gut‒lung axis110. The specific mechanisms by which parenteral vaccination causes alterations in the gut microbiota are poorly understood. Vaccine-induced immune cells and inflammatory mediators may migrate via the bloodstream from the immunization site to the gut, where they interact with the microbiota111. Interestingly, recent studies comparing the immune efficacy of oral and intradermal BCG have demonstrated that both routes induce a systemic Th1 response capable of producing IFN-γ. Notably, the oral BCG vaccine also elicits a more robust mucosal response112. We hypothesize that oral BCG may possess the potential to modulate gut microbiota in a more direct manner.
Another revelation is how complement components shape the developing gut microbiota of newborns. Newborns lack effective adaptive immunity against most diseases, which can be supplemented with bioactive ingredients through breastfeeding. In addition, breast milk can affect the composition of the infant’s gut microbiota, protecting newborns from intestinal infections. Studies have shown that complement in breast milk can directly lyse specific gram-positive symbiotic bacteria in a C1-activated, antibody-independent, and membrane attack complex-dependent manner113. The activation cascade of the complement system is one of the key mechanisms by which the immune system fights against pathogenic infections. Enhancing a vaccine’s complement activation capacity may also be a strategy to improve the efficacy of oral vaccines. Recently, traditional Chinese medicine (TCM) has attracted much attention because of its strong therapeutic effects and few side effects. An increasing number of studies have shown that TCM can regulate the composition and metabolism of the gut microbiota114. In particular, the polysaccharides in Coptis chinensis Franch can be taken up by PPs in the gut. Therefore, vaccines based on polysaccharides from TCM can activate intestinal immune responses and regulate the gut microbiota115. Future studies could identify more components that simultaneously regulate the gut microbiota and mucosal immune responses for the design of oral vaccine systems. However, the complex interaction between TCMs and the gut microbiota remains unclearly defined, as TCMs have diverse species, various components and multiple targets.
Engineered microbial system-based oral vaccines
The live microbiota is considered a promising therapeutic platform. Some bacteria can interact with IECs and actively target specific cells or penetrate challenging biological barriers116. In addition, the microbiota can activate host adaptive immunity, induce IgA secretion, and facilitate microbiota colonization in the intestinal mucosa117. Recently, with the development of synthetic biology, genetically engineered microbiota-based delivery systems have been able to maintain bacterial motility while further empowering bacteria to sense their environment, target specific cells, and “intelligently” deliver cargo118. Moreover, the surface of microbiota organisms has a negative charge, and various substances, such as peptidoglycan, teichoic acid, lipids, and proteins, facilitate surface modification116. These structures can be modified through diverse physical and chemical strategies. The surface components of bacteria are rich in thiol, amino, hydroxyl, and carboxyl groups119, which can be modified, such as in functional targeting ligands and even nanoparticles. The advantages of engineered microbial systems and their products are functionalization by various modifications, retention of the surface morphology, and the use of antigens and adjuvants. As a result, the microbiota can be genetically engineered or surface modified to sense and respond to environmental signals, which is a safer and more efficient approach for oral vaccines. However, maintaining the viability of sufficient numbers of bacteria, the modification efficiency of functional groups, the protection of functional groups, the expression efficiency of target antigens, the neutralization of toxicity, and the adjuvant efficiency are all essential factors to be considered. In addition, the safety and ethical considerations surrounding engineered microbial systems, particularly those involving genetically modified organisms, must not be overlooked. Before advancing to clinical trials, it is imperative to conduct a comprehensive safety assessment to ensure that there are no unacceptable risks posed to patients or the environment120. Safety risks include the stability of genetic modifications, potential gene transfer and virulence, as well as unknown long-term consequences118. Ethical considerations include ensuring informed consent and autonomy121. Given the complexity and novelty of engineered microbial systems, it is important that patients are adequately informed about the scientific principles underlying their treatment, potential outcomes, and associated uncertainties. Furthermore, we should respect patients’ autonomous decisions throughout this process. Meanwhile, operations should be conducted in strict adherence to relevant standards and guidelines to ensure their compliance.
Genetic modification of the gut microbiota to express specific antigens to induce antigen-specific immunity is the most common approach. The specific engineering strategy depends heavily on the unique properties of the microbiota. E. coli is the preferred vector for the production of recombinant proteins because of its fast propagation and high efficiency of protein expression. It can affect mucosal inflammation by regulating the levels of inflammatory cytokines and has been used in clinical trials to treat inflammatory bowel disease (IBD) and regulate dysbiosis and other intestinal diseases. Sarnelli et al. engineered the E. coli Nissle 1917 strain with a SARS-CoV-2 spike protein-encoding plasmid, which elicited systemic and mucosal immunity after oral vaccination122. The surface expression of viral epitopes on E. coli can extend the half-life of these epitopes when administered to immunized animals, while simultaneously acting as adjuvants to enhance the overall immune response. Salmonella has also been reported to bind selectively to M cells, thereby demonstrating its potential for use as an oral vector to carry antigens123. Probiotics, such as Lactobacillus, Bifidobacterium, and Enterococcus, can be administered to treat diseases such as diarrhea, irritable bowel syndrome, and IBD124. The use of probiotic vectors, especially lactic acid bacteria, as promising food-grade vaccine delivery vectors has the advantages of oral delivery and mucosal immunoregulation125. Mohamadzadeh et al. reported the development of a recombinant probiotic vaccine in which Lactobacillus acidophilus was used to deliver a protective antigen from Bacillus anthracis126. In this study, the levels of antigen-specific antibodies and IgA-expressing cells were comparable to those with the aluminum-adjuvanted vaccine, demonstrating that Lactobacillus is an effective bacterial adjuvant.
In addition to whole microorganism-based biological therapies, the properties of bacterial spores confer their potential as drug delivery vehicles127. Spores, which are metabolically dormant bacteria that can resist extreme environmental stress, have also aroused great interest for oral administration. An oral spore-based nanoparticle generator modified with deoxycholic acid was created, which crosses multiple biological barriers and penetrates epithelial cells to increase basolateral drug release128. The most common use of spores is to express or surface display antigens. Bacillus subtilis spores are among the most studied spore surface display systems. They are “generally recognized as safe” probiotics approved by the FDA and can colonize the intestinal microenvironment128. The use of Bacillus subtilis spores as vaccine delivery vectors for multiple antigens has resulted in good immune effects129,130. In particular, an oral vaccine against anthrax was developed with Sterne spores encapsulated in alginate and coated with a poly-L-lysine and a vitelline protein B shell for protection from the gastrointestinal environment131. Furthermore, bacterial ghosts consist of empty bacterial cell envelopes expressing the PhiX174 lysis gene E. These ghosts preserve pathogen-associated molecular patterns (PAMPs)132, which can be used for constructing oral vaccine delivery carriers against Helicobacter pylori and hand-foot-and-mouth disease virus133,134, eliciting antigen-specific humoral and mucosal immune responses.
Outer membrane vesicles (OMVs) are natural nanoparticles derived from gram-negative bacteria that stimulate the host immune system through abundant PAMPs135. OMV-based vaccines against serogroup B Neisseria meningitidis are licensed and provide cross-species protection against Neisseria gonorrheae136. Gut bacteria can also secrete immunoregulatory OMVs. More importantly, OMVs can efficiently cross the intestinal epithelial barrier and interact with immune cells137, especially DCs, which play a role in antigen presentation, to exert immunoregulatory effects. In addition, some bacterial extracellular vesicles also contain SCFAs, which may work together with PAMPs to regulate the immune response138. Yue et al. genetically engineered E. coli and established an oral tumor vaccine based on OMVs derived from these genetically engineered bacteria139. After oral administration, OMVs carrying tumor antigens can overcome the intestinal epithelial barrier and be internalized by DCs in the lamina propria of the intestine, which then present the tumor antigen. Vaccine-activated tumor antigen-specific immunity inhibits tumor growth and plays a long-term protective role against the recurrence and metastasis of tumors. Regulation of the gut microbiota composition and engineering of microbial system-based vaccines are shown in Fig. 3. The efficacy, mechanisms, and limitations of the above oral vaccine platforms are shown in Table 2.
A Design of β-glucan-encapsulated probiotics to induce mucosal immunity by modulating the gut microbiota. B An orally administered inulin gel increased the abundance of SCFA producers, such as Akkermansia, Lactobacillus, Roseburia, and their metabolites, which can benefit T-cell immunity. C Spore-based oral autonomous nanoparticles were fabricated to penetrate multiple barriers in the gastrointestinal tract. D Genetically engineered E. coli-derived OMVs that can cross the intestinal epithelial barrier and be internalized by APCs in the intestine to promote tumor antigen presentation can be used to construct oral tumor vaccines. Panel A was reproduced under the terms of the Creative Commons CC-BY 4.0 license102; Copyright 2021, The Authors, published by the American Association for the Advancement of Science. Panel B was reproduced with permission106; Copyright 2021, Springer Nature. Panel C was reproduced with permission128; Copyright 2019, John Wiley and Sons. Panel D was reproduced with permission139; Copyright 2022, Springer Nature.
Conclusions and perspectives
Oral vaccines do not require injection and offer advantages in terms of convenience and the induction of mucosal immunity. Despite the progress on oral vaccine formulation design and delivery technologies over the last decade, various challenges remain to be addressed for clinical translation140. First, the gastrointestinal tract is a complicated physiological system comprising diverse molecular landscapes and variations in pH and ionic strength141. The physiological environments and functions of different intestinal segments differ8. Regulating the effective delivery of oral vaccines in the gut requires further consideration. We can customize and integrate various strategies into one delivery system to enable co-delivery of antigens and adjuvants, targeted delivery of vaccines to intestinal APCs, improved antigen uptake and presentation, and stimulation of APCs. However, the complexity involved in manufacturing such oral delivery systems (often comprising multiple functional modules) and the variability observed between production batches may hinder their industrial application142.
Finally, the heterogeneity of gut microbiota within the population is indeed a significant influencing factor, complicating the regulation of immune responses through gut microbiota in current practice. There is an increasing need for comprehensive studies to clarify how the modulation of gut microbiota by various factors impacts vaccine efficacy in the population. The differences in the gut microbiota between individuals, which are influenced by many factors, such as age, environmental exposure, health status, genetics, geography, and diet9,143. In addition, exercise, antibiotic use, and surgical intervention can also change the composition of the gut microbiota144,145. In a mouse model, nutritional supplements composed of spirulina, amaranth, flaxseed, and micronutrients augmented the secretion of antigen-specific IgA, which was microbiota-dependent51. Future vaccine design should take into account how the microbiota modulates vaccination efficacy in diverse backgrounds, which will also guide more targeted adjuvant discovery.
The differences in gut microbiota between primate and rodent models may have contributed to the observed discrepancies in findings. While human and mouse gut microbiota share approximately 90% overlap at the phylum and genus levels, there are significant variations in microbial composition and abundance146. For instance, humans exhibit a markedly higher Firmicutes/Bacteroidetes ratio compared to mice. Furthermore, both humans and mice harbor specific bacterial genera147. Another important consideration is the differing conditions under which the microbiome is established. In humans, host genetics and environmental factors influence the establishment and stability of the gut microbiome148. The genetic background of rodents with different receptors strongly influences the colonization of the human gut microbiota and determines the biological applicability of different animal models148. Conversely, rodent gut microbiota can be modulated under laboratory conditions to obtain germ-free animal models or specific microbial colonization animal models. Consequently, both human and rodent models possess unique advantages as well as limitations that must be carefully considered when translating findings from rodents to humans. In the future, additional interdisciplinary studies are needed to investigate various aspects of the relationship between the intestinal flora and oral vaccines to optimize vaccine design.
While oral vaccines offer numerous advantages, they are accompanied by an evolutionary tolerance response from the host. For the development of effective oral vaccines, it is essential to elucidate how these inherent tolerance systems function and to propose strategies for overcoming them. The oral tolerance mechanism primarily involves: the uptake process of antigens from the intestinal lumen, which is a critical step in establishing oral tolerance; antigen presentation by APCs to CD4+ T cells, leading to their differentiation into FOXP3+ Tregs; and RORγt+ FOXP3+ Tregs play a pivotal role in mediating the tolerance to the microbiota149. Whether immune enhancement or tolerance occurs via the oral route hinges on T-cell activation or anergy. We posit that overcoming the tolerogenic effects of oral vaccines relies on optimizing dosages and types of antigens as well as adjuvants to prevent excessive stimulation of the immune response while modulating Treg cell functionality. Furthermore, the composition and diversity of gut microbiota significantly influence both the establishment and maintenance of immune tolerance. Gonzalez-Visiedo et al. have demonstrated manipulation of microbiomes to enhance oral tolerance in food allergies150. This indicates that targeted modulation of specific gut microbiota may also help surmount immune tolerance barriers, thereby enhancing the immunogenic efficacy of oral vaccines. This intriguing avenue warrants further investigation and clarification.
As highly adapted tissue-specific adjuvants, gut microbiota constituents modulate immune function and influence vaccine efficacy39. The existing studies on the effect of the gut microbiota on vaccine efficacy are mostly cross-sectional studies that relate the microbiota to the vaccine response only at specific time points. Since the composition of the gut microbiota varies with environmental exposure, longitudinal studies are needed to assess its impact on the vaccine response and the specific molecular mechanisms involved. In addition, further studies on the mechanisms by which vaccination may cause changes in the gut microbiota are lacking. The question of how vaccines alter the composition of the gut microbiota and how these changes affect vaccine efficacy is of critical importance for optimizing vaccine design, especially in low-immunogenicity settings. Bacterial species that promote the response to a vaccine can be administered in the form of probiotics prior to vaccination in individuals at risk of low vaccine immunogenicity. This requires studying the composition of the gut microbiota and testing translational methods in vitro and in vivo. Focusing on health-promoting microbiota and exploiting their immunomodulatory properties or targeting harmful bacteria to optimize the composition of the gut microbiota may further improve the efficacy of oral vaccines.
This review primarily focuses on the general roles of gut microbiota in modulating the mucosal immune system and influencing vaccination efficacy. It also discusses strategies to enhance the effectiveness of oral vaccines through their interactions with gut microbiota. However, a limitation of this review is its lack of recent real-world case studies. Moving forward, we will place greater emphasis on examining the impact of gut microbiota on specific oral vaccines (e.g., rotavirus or cholera vaccines) to uncover additional patterns and associations. In future oral vaccine research, more advanced technologies, such as reverse vaccinology, artificial intelligence, big data screening, and other interdisciplinary approaches, can also be combined. Stratification on the basis of individual gut microbiota characteristics, metabolism, and host genetic factors may be critical. Notably, the gut microbiota composition is also strongly related to the risk of other immune- and non-immune-mediated diseases. As the population increasingly ages worldwide, consideration of the gut microbiota will become essential for regulating aging-related processes151,152. Therefore, vaccination that optimizes the composition of the microbiota can be used to study more diseases and potentiate modern fields of research.
Data availability
No datasets were generated or analysed during the current study.
References
Chen, F. et al. Nanobiomaterial-based vaccination immunotherapy of cancer. Biomaterials 270, 120709 (2021).
He, C., Qin, M. & Sun, X. Highly pathogenic coronaviruses: thrusting vaccine development in the spotlight. Acta Pharm. Sin. B. 10, 1175–1191 (2020).
Kwong, K. W. et al. Oral vaccines: a better future of immunization. Vaccines 11, 1232 (2023).
McIntosh, E. D., Janda, J., Ehrich, J. H., Pettoello-Mantovani, M. & Somekh, E. Vaccine hesitancy and refusal. J. Pediatr. 175, 248–249.e1 (2016).
Abeer, M. M. et al. Silica nanoparticles: a promising platform for enhanced oral delivery of macromolecules. J. Control Rel. 326, 544–555 (2020).
Miao, Y. B. et al. Engineering nano-and microparticles as oral delivery vehicles to promote intestinal lymphatic drug transport. Adv. Mater. 33, e2104139 (2021).
Davitt, C. J. H. & Lavelle, E. C. Delivery strategies to enhance oral vaccination against enteric infections. Adv. Drug Deliv. Rev. 91, 52–69 (2015).
Zhang, Y., Li, M., Du, G., Chen, X. & Sun, X. Advanced oral vaccine delivery strategies for improving the immunity. Adv. Drug Deliv. Rev. 177, 113928 (2021).
Lee, Y., Kamada, N. & Moon, J. J. Oral nanomedicine for modulating immunity, intestinal barrier functions, and gut microbiome. Adv. Drug Deliv. Rev. 179, 114021 (2021).
Frizzell, H. & Woodrow, K. A. Biomaterial approaches for understanding and overcoming immunological barriers to effective oral vaccinations. Adv. Funct. Mater. 30, 1907170 (2020).
Ponziani, F. R. et al. Factors influencing microbiota in modulating vaccine immune response: a long way to go. Vaccines 11, 1609 (2023).
Zimmermann, P. & Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 32, e00084–18 (2019).
Jordan, A., Carding, S. R. & Hall, L. J. The early-life gut microbiome and vaccine efficacy. Lancet Microbe 3, e787–e794 (2022).
Gentile, C. L. & Weir, T. L. The gut microbiota at the intersection of diet and human health. Science 362, 776–780 (2018).
Yakabe, K., Uchiyama, J., Akiyama, M. & Kim, Y. G. Understanding host immunity and the gut microbiota inspires the new development of vaccines and adjuvants. Pharmaceutics 13, 163 (2021).
Roy, S. & Trinchieri, G. Microbiota: a key orchestrator of cancer therapy. Nat. Rev. Cancer 17, 271–285 (2017).
Wu, S., Xia, Y., Hu, Y. & Ma, G. Bio-mimic particles for the enhanced vaccinations: lessons learnt from the natural traits and pathogenic invasion. Adv. Drug Deliv. Rev. 176, 113871 (2021).
Wu, J. & Ma, G. Imitation of nature: bionic design in the study of particle adjuvants. J. Control Rel. 303, 101–108 (2019).
Luchner, M., Reinke, S. & Milicic, A. TLR agonists as vaccine adjuvants targeting cancer and infectious diseases. Pharmaceutics 13, 142 (2021).
Kayama, H., Okumura, R. & Takeda, K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu Rev. Immunol. 38, 23–48 (2020).
Chen, Y. et al. Reinforcement of the intestinal mucosal barrier via mucus-penetrating PEGylated bacteria. Nat. Biomed. Eng. 8, 823–841 (2024).
Chen, C., Beloqui, A. & Xu, Y. Oral nanomedicine biointeractions in the gastrointestinal tract in health and disease. Adv. Drug Deliv. Rev. 203, 115117 (2023).
Deng, B. et al. Oral nanomedicine: challenges and opportunities. Adv. Mater. 36, e2306081 (2024).
Bansil, R. & Turner, B. S. The biology of mucus: composition, synthesis and organization. Adv. Drug Deliv. Rev. 124, 3–15 (2018).
Cone, R. A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 61, 75–85 (2009).
Marques, R. & Boneca, I. G. Expression and functional importance of innate immune receptors by intestinal epithelial cells. Cell Mol. Life Sci. 68, 3661–3673 (2011).
Johansson Malin, E. V. et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 18, 582–592 (2015).
Paone, P. & Cani, P. D. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut 69, 2232–2243 (2020).
Schluter, J. et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 588, 303–307 (2020).
Kim, M. & Kim, C. H. Regulation of humoral immunity by gut microbial products. Gut Microbes 8, 392–399 (2017).
Bemark, M., Pitcher, M. J., Dionisi, C. & Spencer, J. Gut-associated lymphoid tissue: a microbiota-driven hub of B cell immunity. Trends Immunol. 45, 211–223 (2024).
Ohno, H. Intestinal M cells. J. Biochem. 159, 151–160 (2016).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–245 (1998).
Lelouard, H. et al. Pathogenic bacteria and dead cells are internalized by a unique subset of Peyer’s patch dendritic cells that express lysozyme. Gastroenterology 138, 173–184 (2010).
Schaupp, L. et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells. Cell 181, 1080–1096 (2020).
Mirzaei, R. et al. Identification of proteins derived from Listeria monocytogenes inducing human dendritic cell maturation. Tumor Biol. 37, 10893–10907 (2016).
Inamoto, T. et al. Short-chain fatty acids stimulate dendrite elongation in dendritic cells by inhibiting histone deacetylase. FEBS J. 290, 5794–5810 (2023).
Lu, Y. et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J. Hematol. Oncol. 15, 47 (2022).
Pabst, O. & Hornef, M. Gut microbiota: a natural adjuvant for vaccination. Immunity 41, 349–351 (2014).
Lundell, A.-C. et al. Infant B cell memory differentiation and early gut bacterial colonization. J. Immunol. 188, 4315–4322 (2012).
Fadlallah, J. et al. Microbial ecology perturbation in human IgA deficiency. Sci. Transl. Med. 10, eaan1217 (2018).
Pabst, O. & Izcue, A. Secretory IgA: controlling the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 19, 149–150 (2022).
Nakajima, A. et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J. Exp. Med 215, 2019–2034 (2018).
Kadaoui, K. A. & Corthésy, B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment. J. Immunol. 179, 7751–7757 (2007).
Kubinak, J. L. et al. MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17, 153–163 (2015).
Wills-Karp, M., Nathan, A., Page, K. & Karp, C. L. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. 3, 104–110 (2010).
Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008).
Atarashi, K. et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015).
Duan, J. et al. Endoplasmic reticulum stress in the intestinal epithelium initiates purine metabolite synthesis and promotes Th17 cell differentiation in the gut. Immunity 56, 1115–1131.e9 (2023).
Lynn, D. J., Benson, S. C., Lynn, M. A. & Pulendran, B. Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. Nat. Rev. Immunol. 22, 33–46 (2022).
Luccia, B. D. et al. Combined prebiotic and microbial intervention improves oral cholera vaccination responses in a mouse model of childhood undernutrition. Cell Host Microbe 27, 899–908.e5 (2020).
Zimmermann, P. & Curtis, N. The influence of probiotics on vaccine responses – A systematic review. Vaccine 36, 207–213 (2018).
Cianci, R. et al. The interplay between immunosenescence and microbiota in the efficacy of vaccines. Vaccines 8, 636 (2020).
Mangiola, F., Nicoletti, A., Gasbarrini, A. & Ponziani, F. R. Gut microbiota and aging. Eur. Rev. Med. Pharm. Sci. 22, 7404–7413 (2018).
Bittinger, K. et al. Bacterial colonization reprograms the neonatal gut metabolome. Nat. Microbiol 5, 838–847 (2020).
Krautkramer, K. A., Fan, J. & Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol 19, 77–94 (2021).
Nothaft, H. & Szymanski, C. M. Protein glycosylation in bacteria: sweeter than ever. Nat. Rev. Microbiol 8, 765–778 (2010).
Lynch, T. & Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 76, 391–396 (2007).
Mahowald, M. A. et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl Acad. Sci. USA 106, 5859–5864 (2009).
Su, L. F., Kidd, B. A., Han, A., Kotzin, J. J. & Davis, M. M. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity 38, 373–383 (2013).
Hegazy, A. N. et al. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology 153, 1320–1337 (2017).
Williams, W. B. et al. HIV-1 VACCINES. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science 349, aab1253 (2015).
Yilmaz, B. et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell 159, 1277–1289 (2014).
Fluckiger, A. et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 (2020).
Bondareva, M. et al. Cross-regulation of antibody responses against the SARS-CoV-2 Spike protein and commensal microbiota via molecular mimicry. Cell Host Microbe 31, 1866–1881.e10 (2023).
Reed, S. G., Orr, M. T. & Fox, C. B. Key roles of adjuvants in modern vaccines. Nat. Med 19, 1597–1608 (2013).
Pulendran, B., Arunachalam, P. S. & O’Hagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 20, 454–475 (2021).
Peletta, A., Lemoine, C., Courant, T., Collin, N. & Borchard, G. Meeting vaccine formulation challenges in an emergency setting: towards the development of accessible vaccines. Pharm. Res. 189, 106699 (2023).
Wu, T. Y. et al. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 6, 263ra160 (2014).
Pereira, B., Xu, X. N. & Akbar, A. N. Targeting inflammation and immunosenescence to improve vaccine responses in the elderly. Front. Immunol. 11, 583019 (2020).
Holmgren, J. & Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 11, S45–S53 (2005).
Georg, P. & Sander, L. E. Innate sensors that regulate vaccine responses. Curr. Opin. Immunol. 59, 31–41 (2019).
Vatanen, T. et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165, 842–853 (2016).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Fu, D. et al. Self-assembled flagella protein nanofibers induce enhanced mucosal immunity. Biomaterials 288, 121733 (2022).
Oh, J. Z. et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 41, 478–492 (2014).
Kim, D. et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat. Med. 22, 524–530 (2016).
Derosa, L. et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 28, 315–324 (2022).
Depommier, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103 (2019).
Bae, M. et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 608, 168–173 (2022).
Price, A. E. et al. A map of toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity 49, 560–575.e6 (2018).
Kollmann, T. R., Levy, O., Montgomery, R. R. & Goriely, S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 37, 771–783 (2012).
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71 (2021).
Wu, L. et al. Bioinspired butyrate-functionalized nanovehicles for targeted oral delivery of biomacromolecular drugs. J. Control Rel. 262, 273–283 (2017).
Mann, E. R., Lam, Y. K. & Uhlig, H. H. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat. Rev. Immunol. 24, 577–595 (2024).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
Kim, C. H., Park, J. & Kim, M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 14, 277–288 (2014).
Kim, M., Qie, Y., Park, J. & Kim, C. H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20, 202–214 (2016).
Tang, B. et al. Correlation of gut microbiota and metabolic functions with the antibody response to the BBIBP-CorV vaccine. Cell Rep. Med. 3, 100752 (2022).
Yang, W. et al. Microbiota metabolite short-chain fatty acids facilitate mucosal adjuvant activity of cholera toxin through GPR43. J. Immunol. 203, 282–292 (2019).
Gérard, P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens 3, 14–24 (2014).
Song, X. et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415 (2020).
Kuipers, F., de Boer, J. F. & Staels, B. Microbiome modulation of the host adaptive immunity through bile acid modification. Cell Metab. 31, 445–447 (2020).
Hagan, T. et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell 178, 1313–1328.e13 (2019).
Azuar, A. et al. Cholic acid-based delivery system for vaccine candidates against group A streptococcus. ACS Med. Chem. Lett. 10, 1253–1259 (2019).
Kaźmierczak-Siedlecka, K. et al. Therapeutic methods of gut microbiota modification in colorectal cancer management-fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes 11, 1518–1530 (2020).
Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R. & Rastall, R. A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 16, 605–616 (2019).
Suez, J., Zmora, N., Segal, E. & Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729 (2019).
Xu, J. et al. Boosting vaccine-elicited respiratory mucosal and systemic COVID-19 immunity in mice with the oral Lactobacillus plantarum. Front. Nutr. 8, 789242 (2021).
Singh, S., Singh, M. & Gaur, S. Probiotics as multifaceted oral vaccines against colon cancer: a review. Front. Immunol. 13, 1002674 (2022).
Cao, Z., Cheng, S., Wang, X., Pang, Y. & Liu, J. Camouflaging bacteria by wrapping with cell membranes. Nat. Commun. 10, 3452 (2019).
Lin, S. et al. Mucosal immunity–mediated modulation of the gut microbiome by oral delivery of probiotics into Peyer’s patches. Sci. Adv. 7, eabf0677 (2021).
Harris, V. C. et al. Effect of antibiotic-mediated microbiome modulation on rotavirus vaccine immunogenicity: a human, randomized-control proof-of-concept trial. Cell Host Microbe 24, 197–207.e4 (2018).
Lynn, M. A. et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe 23, 653–660.e5 (2018).
Kumar, S., Kesharwani, S. S., Kuppast, B., Bakkari, M. A. & Tummala, H. Pathogen-mimicking vaccine delivery system designed with a bioactive polymer (inulin acetate) for robust humoral and cellular immune responses. J. Control Rel. 261, 263–274 (2017).
Han, K. et al. Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel. Nat. Biomed. Eng. 5, 1377–1388 (2021).
Jiao, X. et al. Exploring PLGA-OH-CATH30 microspheres for pral therapy of Escherichia coli-induced enteritis. Biomolecules 14, 86 (2024).
Garly, M. L. et al. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine 21, 2782–2790 (2003).
Stražar, M. & Mourits, V. P. Koeken VACM, de Bree LCJ, Moorlag SJCFM, Joosten LAB, van Crevel R, Vlamakis H, Netea MG, Xavier RJ. The influence of the gut microbiome on BCG-induced trained immunity. Genome Biol. 22, 275 (2021).
Jeyanathan, M. et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut–lung axis. Nat. Immunol. 23, 1687–1702 (2022).
Zimmermann, P. The immunological interplay between vaccination and the intestinal microbiota. npj Vaccines 8, 24 (2023).
Hoft, D. F. et al. PO and ID BCG vaccination in humans induce distinct mucosal and systemic immune responses and CD4+ T cell transcriptomal molecular signatures. Mucosal Immunol. 11, 486–495 (2018).
Xu, D. et al. Complement in breast milk modifies offspring gut microbiota to promote infant health. Cell 187, 750–763 (2024).
Feng, W., Ao, H., Peng, C. & Yan, D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharm. Res 142, 176–191 (2019).
Chen, Q. et al. Coptis chinensis Franch polysaccharides provide a dynamically regulation on intestinal microenvironment, based on the intestinal flora and mucosal immunity. J. Ethnopharmacol. 267, 113542 (2021).
Guo, P. et al. Advancement of engineered bacteria for orally delivered therapeutics. Small 19, e2302702 (2023).
Donaldson, G. P. et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 360, 795–800 (2018).
Zhou, S., Gravekamp, C., Bermudes, D. & Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 18, 727–743 (2018).
Lin, S. et al. Surface-modified bacteria: synthesis, functionalization and biomedical applications. Chem. Soc. Rev. 52, 6617–6643 (2023).
Forbes, N. S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 10, 785–794 (2010).
Liu, D., Yu, L., Rong, H., Liu, L. & Yin, J. Engineering microorganisms for cancer immunotherapy. Adv. Health. Mater. 13, e2304649 (2024).
Sarnelli, G. et al. Oral immunization with Escherichia coli Nissle 1917 expressing SARS-CoV-2 spike protein induces mucosal and systemic antibody responses in mice. Biomolecules 13, 569 (2023).
Hathaway, L. J. & Kraehenbuhl, J. P. The role of M cells in mucosal immunity. Cell Mol. Life Sci. 57, 323–332 (2000).
Sola-Oladokun, B., Culligan, E. P. & Sleator, R. D. Engineered probiotics: applications and biological containment. Annu Rev. Food Sci. Technol. 8, 353–370 (2017).
Oh, S. H. et al. Cytoplasmic expression of a model antigen with M Cell-Targeting moiety in lactic acid bacteria and implication of the mechanism as a mucosal vaccine via oral route. Vaccine 39, 4072–4081 (2021).
Mohamadzadeh, M., Duong, T., Sandwick, S. J., Hoover, T. & Klaenhammer, T. R. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc. Natl Acad. Sci. USA 106, 4331–4336 (2009).
Holay, M. et al. Bacteria-inspired nanomedicine. ACS Appl Bio Mater. 4, 3830–3848 (2021).
Song, Q. et al. A probiotic spore-based oral autonomous nanoparticles generator for cancer therapy. Adv. Mater. 31, e1903793 (2019).
Uyen, N. Q., Hong, H. A. & Cutting, S. M. Enhanced immunisation and expression strategies using bacterial spores as heat-stable vaccine delivery vehicles. Vaccine 25, 356–365 (2007).
Lin, P. et al. Progress in research and application development of surface display technology using Bacillus subtilis spores. Appl Microbiol. Biotechnol. 104, 2319–2331 (2020).
Benn Felix, J. et al. Protective antibody response following oral vaccination with microencapsulated bacillus anthracis sterne strain 34F2 spores. npj Vaccines 5, 59 (2020).
Chen, H. et al. Bacterial ghosts-based vaccine and drug delivery systems. Pharmaceutics 13, 1892 (2021).
Chen, J., Li, N. & She, F. Helicobacter pylori outer inflammatory protein DNA vaccine-loaded bacterial ghost enhances immune protective efficacy in C57BL/6 mice. Vaccine 32, 6054–6060 (2014).
Gong, S. et al. Protective immunity elicited by VP1 chimeric antigens of bacterial ghosts against hand-foot-and-mouth disease virus. Vaccines 8, 61 (2020).
Shen, H. et al. Engineered microbial systems for advanced drug delivery. Adv. Drug Deliv. Rev. 187, 114364 (2022).
Leduc, I. et al. The serogroup B meningococcal outer membrane vesicle-based vaccine 4CMenB induces cross-species protection against Neisseria gonorrhoeae. PLoS Pathog. 16, e1008602 (2020).
Bittel, M. et al. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe-host-communication in vivo. J. Extracell. Vesicles 10, e12159 (2021).
Huang, J. et al. Extracellular vesicles as a novel mediator of interkingdom communication. Cytokine Growth Factor Rev. 73, 173–184 (2023).
Yue, Y. et al. Antigen-bearing outer membrane vesicles as tumour vaccines produced in situ by ingested genetically engineered bacteria. Nat. Biomed. Eng. 6, 898–909 (2022).
Lou, J. et al. Advances in oral drug delivery systems: challenges and opportunities. Pharmaceutics 15, 484 (2023).
Aljabbari, A., Kihara, S., Rades, T. & Boyd, B. J. The biomolecular gastrointestinal corona in oral drug delivery. J. Control Rel. 363, 536–549 (2023).
Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013).
Gong Y., Liu Z., Zhou P., Li J., Miao Y.-B. Biomimetic nanocarriers harnessing microbial metabolites usher the path for brain disease therapy. Nano TransMed. 2, 100020 (2023).
Monda, V. et al. Exercise modifies the gut microbiota with positive health effects. Oxid. Med Cell Longev. 2017, 3831972 (2017).
Guyton, K. & Alverdy, J. C. The gut microbiota and gastrointestinal surgery. Nat. Rev. Gastroenterol. Hepatol. 14, 43–54 (2017).
Park, J. C. & Im, S.-H. Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp. Mol. Med. 52, 1383–1396 (2020).
Hou, K. et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 7, 135 (2022).
Wos-Oxley, M. L. et al. Comparative evaluation of establishing a human gut microbial community within rodent models. Gut Microbes 3, 234–249 (2014).
Cerovic, V., Pabst, O. & Mowat, A. M. The renaissance of oral tolerance: merging tradition and new insights. Nat. Rev. Immunol. 25, 42–56 (2025).
Gonzalez-Visiedo, M., Kulis, M. D. & Markusic, D. M. Manipulating the microbiome to enhance oral tolerance in food allergy. Cell Immunol. 382, 104633 (2022).
Ghosh, T. S., Shanahan, F. & O’Toole, P. W. The gut microbiome as a modulator of healthy aging. Nat. Rev. Gastroenterol. Hepatol. 19, 565–584 (2022).
Xu, X. et al. Gut microbiota is associated with aging-related processes of a small mammal species under high-density crowding stress. Adv. Sci. 10, e2205346 (2023).
Swaminathan, G. et al. Vaccine hyporesponse induced by individual antibiotic treatment in mice and non-human primates is diminished upon recovery of the gut microbiome. Vaccines 9, 1340 (2021).
Zhang, Y. et al. Composition of the murine gut microbiome impacts humoral immunity induced by rabies vaccines. Clin. Transl. Med. 10, e161 (2020).
Huda, M. N. et al. Stool microbiota and vaccine responses of infants. Pediatrics 134, e362–e372 (2014).
Harris, V. et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes 9, 93–101 (2018).
Huda, M. N. et al. Bifidobacterium abundance in early infancy and vaccine response at 2 years of age. Pediatrics 143, e20181489 (2019).
Moroishi, Y. et al. A prospective study of the infant gut microbiome in relation to vaccine response. Pediatr. Res. 93, 725–731 (2023).
Ng, S. C. et al. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut 71, 1106–1116 (2022).
Alexander, J. L. et al. The gut microbiota and metabolome are associated with diminished COVID-19 vaccine-induced antibody responses in immunosuppressed inflammatory bowel disease patients. EBioMedicine 88, 104430 (2023).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82304408) and the Sichuan Science and Technology Program (2024NSFSC1738).
Author information
Authors and Affiliations
Contributions
Y.Y.H. wrote the paper and drew the figures. J.Q.L. and Y.W. revised the paper and supervised the work. All the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hou, Y., Li, J. & Wu, Y. Modulation of oral vaccine efficacy by the gut microbiota. npj Vaccines 10, 179 (2025). https://doi.org/10.1038/s41541-025-01240-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-025-01240-8