Abstract
In this study, waste nutrient solution (WNS) was used as a catholyte in a bioelectrochemical cell to directly produce hydrogen peroxide (H2O2), after which the H2O2- containing WNS was integrated with the downstream UV oxidation process to meet quality standards for reuse. The generated current in the bioelectrochemical cell was successfully utilized at the cathode to produce H2O2 in WNS using a two-electron oxygen reduction reaction with different reaction times. The cathodic reaction time with the highest H2O2 production (504 ± 5.2 mg l−1) was 48 h, followed by that obtained from 24 h (368 ± 4.1 mg l−1), 12 h (158.8 ± 2.4 mg l−1), and 6 h (121.1 ± 4.1 mg l−1) reaction times. During H2O2 generation, calcium, magnesium, and phosphate in the WNS were recovered in the form of precipitates under alkaline conditions. The H2O2-containing WNS was further treated with different UV doses. After UV/H2O2 treatment, excitation-emission matrix and molecular weight distribution analyses demonstrated that aromatic compounds were reduced. Moreover, the gene expressions of sul1 (up to 95.65%), tetG (up to 93.88%), and aadA (up to 95.32%) were clearly downregulated compared with those of a control sample. Finally, a high disinfection efficiency was achieved with higher UV doses, resulting in successful seed germination. Thus, our results indicate that the developed method can be a promising process for reusing WNS in hydroponic systems.
Similar content being viewed by others
Introduction
Water is an essential resource with various uses, such as household, industrial, and agricultural use, and both economic and ecological repercussions. Among the fields that use water, agriculture is considered a major freshwater consumer, using about 70% of its freshwater consumption for irrigation1. As water scarcity for irrigation can severely limit socio-economic development, it is necessary to devise alternative water resources for sustainable agriculture and the effective management of water resources2. In addition, to improve global nutrient security and mitigate nutrient pollution in water bodies, nutrients need to be recovered from organic residuals, including crops, food residue, and animal and human manure, for reuse in agricultural production3,4.
In hydroponics, plants are grown in nutrient solutions in the absence of soil. This cultivation method can deliver high-quality plants at accelerated rates of production per unit area5. Hydroponics effectively controls the environmental factors required for continuous plant growth including artificial light, partial pressure of carbon dioxide, nutrient concentrations, and ambient temperature. Liquid culture media containing the necessary nutrients are often supplied to hydroponically raised plants6,7. However, open hydroponic systems (OHS) generate waste nutrient solutions (WNS). WNS are not reused and may be discharged into nearby receiving waters such as lakes and rivers. As WNS contain substantial amounts of nitrogen and phosphorous, their indiscriminate disposal contributes to eutrophication in aquatic ecosystems. OHS must be converted into closed hydroponic systems (CHS) to mitigate WNS generation8,9. In CHS, plant nutrient solutions are recirculated and reused rather than being discharged as effluent. CHS can minimize environmental loading by reducing WNS volumes and output and decrease both freshwater and nutrient solution consumption10.
CHS has high investment and operating costs as it must also integrate a nutrient solution sterilization process9. The nutrient solution needs to be disinfected before reuse to mitigate the risks of plant pathogen infections and the spread of antibiotic resistance genes (ARGs). To achieve this, various methods such as ozonation, UV radiation, filtration, and chemical addition have been attempted, but there are short-term complications associated with the transition from OHS to CHS11. Hydrogen peroxide (H2O2) is often used in hydroponic systems to provide oxygen and reduce microbial populations. Recent studies have also indicated that the advanced oxidation process involving combined UV and H2O2 treatment (UV/H2O2) exhibits enhanced efficacy in decomposing organic acids known to impede germination and plant growth, such as benzoic, phthalic, and succinic acids, within WNS9,12,13. However, the inflow from nearby farms and livestock facilities and the direct use of antibiotics in hydroponic systems, which increase ARG levels, highlight the necessity to investigate ARG levels in hydroponic systems, as they could be associated with severe environmental risks14. Therefore, it is necessary to investigate the potential effect of UV/H2O2 on ARG reduction in WNS for reuse.
Furthermore, H2O2 addition has been revealed to be an obstacle to the cost-effective treatment of WNS8. Bioelectrochemical systems (BES) can be an alternative approach for H2O2 production, with H2O2 being generated in a sustainable way while treating wastewater15,16. In BES, the anodes designed to extract energy from wastewater contain electroactive bacteria that oxidize organic matter and generate electrons that are then transferred from the anodes to the cathodes. In the presence of carbonaceous catalysts at the cathodes, H2O2 is generated in alkaline, neutral, or acidic solution based on the two-electron oxygen reduction reaction (2e-ORR), using electrons from the anode17,18.
In this study, we directly added WNS as a catholyte in a bioelectrochemical cell (BEC) to generate H2O2. The WNS containing the on-site generated H2O2 was then integrated with the downstream UV oxidation process to extend the lifespan of WNS in a CHS. We systematically investigated the feasibility of WNS as a catholyte in a continuous-flow BEC for H2O2 generation as well as the effect of UV/H2O2 treatment on WNS in terms of various aspects, such as organic carbon removal, disinfection, and ARG expression levels.
Results and discussion
Current generation and H2O2 production in continuous-flow BES
Continuous, stable currents were generated from the duplicate BEC using WNS as the catholyte and heat and acid-treated carbon catalysts on the air-cathode (Fig. 1a). The average current for each reaction time of the cathode chamber was not noticeably different; it was approximately 0.93 A m−2 over the course of the entire continuous operation, with simultaneous, stable H2O2 production. Additionally, while the anode potential was set to −0.3 V, the average cathode potential was maintained at an average value of −235 mV (vs. Ag/AgCl) (Fig. 1b). COD removal ranged from 65.4% to 72.5%, and the final catholyte conductivities decreased from 10.98 mS cm−1 to 8.14–9.08 mS cm−1, probably due to a loss of ions to precipitations (Table 1).
The generated current was successfully utilized at the cathode to produce H2O2 in WNS through 2e-ORR (Fig. 2). The cathodic reaction time with the highest H2O2 production (504 ± 5.2 mg l−1) was 48 h, followed by that of 24 h (368 ± 4.1 mg l−1), 12 h (158.8 ± 2.4 mg l−1), and 6 h (121.1 ± 4.1 mg l−1). H2O2 production was stable over the course of the entire continuous operation. While it seems that H2O2 is concentrated with a longer reaction time, longer reaction times adversely affected H2O2 production rates (Table 1). Cathodic pH was increased up to pH 12 due to OH− accumulation resulting from O2 reduction to H2O2. Even though higher pH conditions are known to mitigate H2O2 losses, the H2O2 conversion efficiency was eventually reduced with longer cathodic reaction times19. Longer reaction times for the cathode have been reported to result in H2O2 decomposition, which became increasingly important in the cathode chamber after 1 day of cathode reaction time20. H2O2 conversion efficiency (58.5%) was the highest at an HRT of 6 h and was maintained at about 34% for HRTs of 12 h and 24 h. The lowest conversion efficiency (27.9%) was obtained at an HRT of 48 h, where the H2O2 concentration was the highest (504 ± 5.2 mg l−1) (Table 1).
Generally, bioelectrochemical H2O2 production at the cathode is performed using a synthetic catholyte made with sodium chloride, phosphate buffer solution, sodium sulfate, or similar substances15,21,22,23. As H2O2 is not highly concentrated in the synthetic catholyte of BES, and its highest concentration reported in a BES is less than 300 ppm, it is strategically feasible to directly use wastewater in the cathode chamber for H2O2 production. That way, H2O2-containing wastewater is directly produced on site and simultaneously used in advanced oxidation processes without the addition of chemicals21,24.
The H2O2 production rates vary widely in bioelectrochemical H2O2 production systems. In this study, the highest H2O2 production rate was obtained with the shortest reaction time (0.79 ± 0.02 04 g l−1 day−1 at 6 h), followed by that obtained at 12 h (0.54 ± 0.04 g l−1 day−1), 24 h (0.51 ± 0.11 04 g l−1 day−1) and 48 h (0.29 ± 0.02 04 g l−1 day−1), which may be attributed to the variation in conversion efficiency with different reaction times (Table 1).
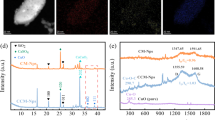
In the cathode chamber, some precipitates were observed in the WNS after H2O2 production (Fig. 3). In general, the ions in the precipitated samples did not significantly differ among the different reaction times. Because various organics and ions exist in WNS, the precipitates mainly appeared to be agglomerates of certain nanospheres. The cathode condition is favorable for the formation of insoluble ionic solid products when there are certain ions present, such as calcium and magnesium ions25. Table 2 shows the ionic composition of the WNS, while the results of the EDS analysis are shown in Supplementary Fig. 1. Among various ions in WNS, calcium, magnesium, and phosphate can precipitate under alkaline conditions. The EDS results showed that the precipitates were composed of calcium, magnesium, and phosphorous. Proportions of up to 11% and 14% of calcium and magnesium, respectively were recovered as solid precipitates (Table 2). The concentration of phosphate ions in the effluent of the catholyte showed a reduction of up to 25%, which may be attributed to precipitation and the transfer from the cathode to the anode chamber through the anion exchange membrane (AEM). The precipitation of calcium and magnesium ions facilitated resource recovery. However, a long-term operation of the reactor may lead to the formation of inorganic scaling on the surfaces of the cathode and the ion exchange membrane. Such scaling could potentially elevate future maintenance costs. Therefore, strategies to control inorganic fouling need to be developed for future implementation. Some nitrites and nitrates were removed in the cathode chamber, but sufficient N and P sources still exist in WNS for reuse in plant cultivation.
UV treatment with H2O2-containing WNS
The H2O2-containing WNS was treated with different UV doses. Figure 4 shows the rates of reduction in DOC, UV254, and SUVA throughout the UV/H2O2 treatment. DOC was not completely mineralized as the DOC concentration (8.8–9.3 mg l−1) was maintained throughout the UV/H2O2 treatment. However, UVC irradiation with H2O2 decreased the UV254 and SUVA (Fig. 4), indicating that the degradation of UV-absorbing moieties was much greater than the removal of DOC. The UV254 absorbances at UV0 (0.357 cm−1) and UV10 (0.352 cm−1) were comparable to that at the control condition (0.359 cm−1), but they continuously decreased from 0.345 cm−1 to 0.275 cm−1 according to the UV dose. UV254 provides an estimation of the concentrations of organic matter, specifically those that contain aromatic rings in their molecular structures. Accordingly, the initial SUVA for WNS was ≥4, indicating that the organic material had a relatively high molecular weight. As the UV dosage increased, however, the SUVA approached 3.
The observed decreasing UV254 and SUVA values are also in accordance with the fluorescence spectra, as shown by the three specific peaks observed in Fig. 5. Organic matter can be identified by the positions of fluorescence maxima identified in previous studies26,27. There was a low-intensity peak A (Ex/Em = 275/345) in the UV0 sample similar to peaks previously associated with protein-like substances that became immediately invisible once the UV dose was increased. The intensities of peaks B (Ex/Em = 250/420) and C (Ex/Em = 295/410) decreased by 26% and 36% with increased UV intensities, respectively (Fig. 5 and Supplementary Table 1). Peaks B and C mainly resembled those of humic and fulvic-like substances, which are natural organic matter. Specifically, peak C could represent benzoic acid, a secondary metabolite released through root exudates in hydroponic systems27,28.
Figure 6 and Supplementary Table 3 show the average molecular weight and molecular weight distributions for all UV/H2O2 treatments. Typically, the molecular weight of DOC in the treated WNS was less than 10 kDa (Supplementary Table 3). The proportion of high-molecular-weight substances (between 1 kDa and 10 kDa) gradually decreased from 54.3% to 47.3% with the increased UV dosage, and the percentage of low-molecular-weight substances (<0.5 kDa) also decreased. However, the percentage of DOC with middle-molecular weight (0.5–1 kDa) increased from 35.1% to 43.8%. This means that DOC with low-molecular weight were completely demineralized and DOC with high-molecular weight were converted into middle-molecular weight DOC. Figure 6 shows that the peaks gradually decreased at approximately 1.5–1.7 kDa, while the peaks increased at approximately 0.8–0.9 kDa. Thus, the UV/H2O2 treatment converted some of the polymers to middle-molecular-weight forms (≤1 kDa). The excitation-emission matrix (EEM) and molecular weight distribution analyses demonstrated that the changes in the properties of the soluble material could be confirmed based on the UV/H2O2 throughput.
Effects of microbial disinfection and ARG reduction
The disinfection efficiency was investigated by comparing the number of bacteria before and after UV/H2O2 treatment. Under UV0 (i.e., sole H2O2 addition), only 7.81% of disinfection efficiency (11,800 CFU ml−1) was achieved, implying a necessity of combining the treatment with UV irradiation (Fig. 7). Contrastingly, in the presence of low UV doses, UV/H2O2 application showed excellent disinfection efficiency. Under UV10, 96.41% of disinfection efficiency was achieved, which was further enhanced with increasing UV doses. Particularly, under UV1000 and UV2000, all bacteria were found to be eliminated (Fig. 7). Given that one of the most significant concerns regarding the reuse of WNS in CHS is the potential spread of root and plant pathogens, WNS should be completely disinfected before recirculation29. In that regard, the feasibility of the suggested UV/H2O2 process using bioelectrochemically synthesized H2O2 is clearly demonstrated by the results of this study.
Antibiotics, which are emerging environmental pollutants that can induce resistance genes, have been extensively applied in agricultural farms and livestock sheds, from where they can potentially leach into groundwater. This could negatively affect hydroponic facilities using groundwater as their main water source. In addition, direct application of antibiotics in hydroponics could also lead to the development of antibiotic-resistant bacteria. Thus, ARGs in WNS also need to be monitored and reduced before the reuse of WNS along with a complete microbial disinfection owing to their potential threat to health.
Among the target ARGs, sul1, tetG, and aadA were detected in WNS, while blaTEM was not found (Fig. 8). The detection of each ARG in WNS seems to be strongly related to the use extent and purpose of relevant antibiotics. Sulfonamides and tetracyclines, frequently detected in aquatic environments30,31,32,33, are the most commonly used antibiotics, applied in livestock facilities and aquaculture, among others. In addition, streptomycin is an antibiotic widely used in agriculture to prevent bacterial diseases30, whereas beta-lactam antibiotics are extensively used for medical purposes.
UV/H2O2 treatment clearly reduced the gene expressions of sul1 (up to 95.65%), tetG (up to 93.88%), and aadA (up to 95.32%) compared with those of the control. The reduction trend was similar to that observed in a previous study, where UV irradiation with 0.01 mol l−1 H2O2 at pH 3.5 highly decreased the frequency of ARG transfers. This was attributed to the cells being damaged by UV light and OH radicals generated by the reaction of UV and H2O234. Nevertheless, in the present study, the relative gene expression levels of the foregoing genes widely varied with UV doses (Fig. 8). Interestingly, the relative gene expression levels were higher under UV10 than those under UV0. This may be explained by the low UV intensity possibly inducing bacteria from a dormant state to an active state.
IntI1, a gene related to an enzyme involved in gene insertion during HGT, also showed a similar trend to that of target ARG expression (Fig. 7). This gene is an indicator of the presence of ARGs in natural and man-made environments and is an effective platform to propagate ARGs via HGT35,36,37. Pearson’s correlation coefficient was analyzed to confirm if the propagation of resistance genes was due to HGT. As a result, positive correlations were confirmed in sul1 (0.996, p < 0.01), tetG (0.998, p < 0.01), and aadA (0.996, p < 0.01). These results indicate that intI1 in WNS could serve as an important carrier of genes for resistance to sulfonamides, tetracyclines, and aminoglycosides via HGT. In this regard, further studies are needed to identify ARG propagation through intI1 by confirming the distribution and changes of antibiotic-resistant bacteria in WNS under specific treatment conditions.
Some organic substances detected in the WNS before and after UV/H2O2 treatment are believed to be natural organic matters, including humic or fulvic materials, and their decomposition products. Particularly, humic materials, such as aromatic carboxylic, aromatic dicarboxylic, phenolic hydroxyl, and salicylic acid, could have many of the observed functionalities38, which may be dissociated from humic substances during the oxidation process. Unfortunately, the aromatic carboxylic and dicarboxylic structures are the constituents of benzoic acid and phthalic acid, respectively39,40. In particular, benzoic acid is known as a typical secondary metabolite and a strong inhibitor of seed germination and plant growth even at low concentrations39,41,42. Moreover, benzoic acid-driven phytotoxicity was not alleviated despite the increased oxidant concentration in O3/H2O229. As byproducts of natural organic matter could retard or inhibit seed germination and plant growth, a phytotoxicity assessment was carried out for lettuce seed germination. Regardless of the UV dose, in this study, no significant effects on seed germination were observed in any treatment (Supplementary Fig. 2). Conversely, microbial disinfection and ARG reduction efficiency were substantially improved. These findings clearly show the feasibility of the suggested UV/H2O2 process combined with BES for WNS reuse in CHS.
WNS can be reused in applications where microbiological and chemical water quality demands are less stringent, as in agriculture. In this study, a BEC was successfully used to supply H2O2 to WNS by on-site generation using a current generated from the anode treating wastewater. The highest H2O2 production rate (0.79 ± 0.02 g l−1 day−1 at 6 h) was obtained with the shortest reaction time of WNS in the cathode chamber, whereas the highest H2O2 concentration (504 ± 5.2 mg l−1) was achieved with the longest reaction time (48 h). Certain amounts of minerals and nutrients were recovered by precipitation in the BEC with sufficient amounts of nutrients remaining in the WNS for reuse. Further treatment of the H2O2-containing WNS by UV achieved microbial disinfection, reduced ARG levels, and aromatic compound removal. Seed germination was successfully carried out by reusing the treated WNS. This strategy could be significantly contributed to the circular economy by recovering and recycling nutrients as well as by alleviating water scarcity. Further investigation is required not only economic feasibility but also to validate the effectiveness of the proposed process across various types of hydroponic nutrient solutions, thus fully demonstrating its efficacy.
Methods
Reactor and electrode preparation
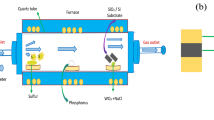
A two-chambered reactor was constructed to evaluate the H2O2 production in continuous operation mode during synthetic wastewater treatment at the anode. Each chamber was 10 cm × 10 cm × 1.5 cm and it was divided by a cation exchange membrane (Neosepta AMX, ASTOM Corporation, Japan). The anode chamber had a working volume of 150 ml and contained graphite felt anodes 5 mm in thickness (GF-20-5F; Nippon Carbon, Japan) connected to a titanium (Ti) wire current collector. First, the anode was inoculated in a microbial fuel cell at 1,000 Ω external resistance to develop a biofilm of exoelectrogens on the anode surface; activated sludge from a wastewater treatment plant (Jeju, Korea) was used as a seed sludge. In the microbial fuel cell, the cathode was made of a carbon cloth (30% wet-proofed; NARA Cell Tech, Japan), with a projected surface area of 100 cm2. The layer facing the liquid phase was coated with a platinum (Pt) catalyst (0.5 mg Pt cm−2). The other side, facing the gas phase, had a gas diffusion layer prepared with a mixture of polytetrafluoroethylene (PTFE) suspension (60 wt%; Sigma-Aldrich, USA) and carbon black powder (Vulcan XC-72; Cabot Corp., USA), as previously reported15. The cathode chamber had a working volume of 150 ml. After inoculation of the anode in the microbial fuel cell with Pt catalysts on the cathode, the cathode was shifted to a new cathode with a gas diffusion layer loaded with modified carbon black (CB; Alfa Aesar, USA). It was treated in a furnace at 600 °C (AJ-MB7; Ajeon Heating Industrial Co. Ltd., Korea), after which it was subjected to acid treatment in 20% (v/v) HNO3 for 2-e ORR. During the heat treatment of CB, the furnace temperature was increased from room temperature to 600 °C over a period of 120 min, after which the CB was annealed at 600 °C for 30 min and cooled in the furnace to room temperature. The heat-treated CB and the 20% (v/v) HNO3 were combined at a 1:4 volumetric ratio to fabricate the acid-treated CB. The mixture was stirred at 80 °C for 6 h and washed more than 10 times with deionized water. The acid-treated CB was then vacuum-dried in an oven at 50 °C for 12 h. The catalyst slurry (60 mg of the treated CB and 0.75 ml of 8% [w/v] PTFE binder) was uniformly coated onto the liquid side of the cathode at a loading rate of 0.5 mg cm−2 and dried for 24 h before the subsequent experiments15.
Reactor set-up and operation
The anode chamber of the BEC was fed with a synthetic wastewater solution consisting of 1.5 g l−1 sodium acetate in phosphate buffer nutrient medium containing 50 mM phosphate-buffered solution (4.57 g l−1 Na2HPO4, 2.45 g l−1 NaH2PO4 ∙ H2O (pH 7.04), 0.31 g l−1 NH4Cl, 0.13 g l−1 KCl, trace vitamins, and minerals). The anode potential was set to −0.3 V vs. Ag/AgCl (RE-1B, BASi® Research Products, USA) with a potentiostat (Ivium Technologies, Netherlands) over the course of the continuous operation, while the generated current was simultaneously monitored. During the continuous operation, the anode chamber was operated at 1-d hydraulic retention time (HRT). The catholyte during continuous H2O2 production was WNS (pH 7.11, conductivity 10.98 mS cm−1) collected from a hydroponic culture facility for paprika (sweet pepper) in Jinju, Korea. The cathode chamber was operated in semi-batch mode and its catholyte was replaced every 6 h, 12 h, 24 h, and 48 h. Both chambers were equipped with Ag/AgCl to monitor the electrode potential, and the reactors were operated in a temperature-controlled room (25 °C).
After producing H2O2 in WNS, the WNS was diluted to achieve an H2O2 concentration of approximately 8 mg l−1. This is because the minimum phytotoxicity thresholds of H2O2 in the nutrient solution have been determined to be within the 8 mg l−1 range8,29. The H2O2-containing WNS was further treated with UVC at 254 nm using a collimated beam apparatus with a 20 W low-pressure monochromatic mercury lamp (KOREX UV Co., Korea); detailed information is described in a previous study22. The UVC treatment was conducted in an open crystalline petri dish at doses of approximately 0 (UV0), 10 (UV10), 50 (UV50), 100 (UV100), 250 (UV250), 500 (UV500), 1000 (UV1000), and 2000 (UV2000) mJ cm−2. The control condition was a WNS without H2O2 and UV irradiation.
Analytical methods and calculation
The H2O2 concentration in each sample was measured with the vanadate method, using a UV/Vis spectrophotometer (DR6000, HACH)43. The calibration solution consisted of 0.06 M NH4VO3 (Samchun Chemicals, Korea), 0.28 M H2SO4 (Daejung Chemicals, Korea), and H2O2 (30% w/w; Sigma-Aldrich, USA). A 5 mM stock solution (0.51 ml of 30% w/w H2O2) and samples of different concentrations were prepared at regular intervals to plot the calibration curve. For the vanadate method, 0.4 ml of each sample concentration was added to 0.4 ml calibration solution, the mixture was diluted to 4 ml with deionized water, and the absorbance of the final product was measured with the UV-Vis spectrophotometer at 450 nm.
The H2O2 conversion efficiency (CE, %) was calculated as shown in Eq. (1):
where I is the ORR current (A), n is the number of transferred electrons (n = 2), V is the electrolyte volume (l), F is Faraday’s constant (F = 96,485 C mol−1), t is the operation time (s), M (H2O2) is the molar mass of H2O2 (34 g mol−1), and c (H2O2) is the concentration of H2O2 generated (g l−1).
The WNS samples were collected at every HRT and immediately filtered using a 0.20 μm syringe filter (PTFE; Advantec Group, Japan) to remove fine suspended particles. The filtrate was used in the anion and cation measurements. The concentrations of the anions NO3−-N, NO2−-N, PO43−, and Cl− and the cations K+, Mg2+, and Ca2+ were determined by ion chromatography (ICS-1600; Thermo Fisher Scientific, USA). Total organic carbon, dissolved organic carbon (DOC), and total nitrogen were determined with a total organic carbon analyzer (Shimadzu Corp., Japan). The specific UV absorbance (SUVA) value was estimated as UV254 divided by the DOC of the WNS sample; UV254 was measured with the UV/Vis spectrophotometer. The pH and conductivity (mS cm−1) were directly measured with a multimeter (SevenMulti; Mettler-Toledo, USA).
Solid samples precipitated from the WNS outlet of the BEC were collected at the end of the experimental period. Each sample was dried at room temperature for approximately 3 days. The dried powder (precipitates) was analyzed with a field emission scanning electron microscope (MIRA3; TESCAN, Czech Republic) and energy dispersive spectrometer (EDS, MIRA; TESCAN, Czech Republic).
Molecular weight analysis by the size exclusion chromatography principle and fluorescence spectra analysis were used to evaluate the concentrations and characteristics of the organic compounds in UV/H2O2-sterilized WNS. The molecular weight analysis was performed using a high-performance liquid size-exclusion chromatograph (LC-20A series; Shimadzu Corp.) fitted with a UV detector. The fluorescence spectra were collected using a spectrofluorophotometer (RF 6000; Shimadzu Corp.) and were based on 1 mg l−1 DOC. Three-dimensional spectra were obtained by repeatedly measuring the emission (Em) spectra within a 280–600 nm range, with excitation (Ex) wavelengths from 220 to 400 nm, spaced at 10-nm intervals in the excitation domain. The spectra were then concatenated into an excitation-emission matrix.
Microbial disinfection assessment
The relative microbial disinfection rates of the various UV/H2O2 irradiation-treated samples were assessed by enumerating the bacteria before and after each treatment44,45,46. Samples exposed to each UV irradiation intensity were extracted and inoculated onto a bacterial count plate (PetrifilmTM aerobic count; 3 M Corp., USA) in a plant growth chamber (GC-300TLH; JeioTech, Daejeon, Korea) at 35 °C for 48 h, after which colonies were directly enumerated.
The colonies were enumerated and reported in log colony forming unit (CFU) ml−1. Each colony number was calculated as follows:
where N is the number of colonies, \(\sum C\) is the sum of all colonies on all plates, n1 and n2 are the numbers of plates calculated from the first and second diluted multiples, respectively, and d is the number of diluted multiples of plates calculated from the first diluted multiples. In principle, the colony number is calculated by selecting a plate with 15–300 colonies.
Reduction of ARGs
After UV/H2O2 treatment, 100 ml of WNS sample were passed through a 45-µm membrane filter (hydrophilic PTFE membrane filter, SciLab Korea Co., Ltd., Korea), and DNA was extracted using the DNeasy PowerSoil Pro Kit (Qiagen, Germany) according to the manufacturer’s manual. DNA concentration was immediately measured with a high-quality monochromator-based UV/VIS spectrophotometer (Thermo Scientific, USA), diluted to a 2 ng µl−1 concentration, and stored at −20 °C until analysis.
Target ARGs that are resistant to sulfonamide (sul1), tetracycline (tetG), streptomycin (aadA), and beta-lactam (blaTEM) antibiotics were selected as the genes to be used in this study. In addition, 16s rRNA and intI1 were respectively selected to estimate the total bacterial abundance and confirm the role of ARGs as genes directly involved in horizontal gene transfer (HGT)47.
16S rRNA, intI1, and target genes were quantified by CFX ConnectTM Real-Time PCR (Bio-rad, USA). The 20 µl of PCR reaction mixtures consisted of 6.4 µl nuclease-free water (Qiagen, Germany), 10 µl SYBR green Realtime PCR Master Mix (Toyobo, Japan), 2 µl template DNA, and 1.6 µl primer. The amplification procedure consisted of an initial denaturation at 95 °C for 30 s and 40 cycles of 30 s at 95 °C (denaturation), 20 s at different temperatures for each primer (Supplementary Table 1) (annealing), and 15 s (most genes) or 30 s (aadA and blaTEM) at 72 °C (extension). To compare reduction efficiencies, relative gene expression levels were calculated using the Ct and 2−ΔΔCt methods48:
In Eqs. (3) and (4), Ct represents the threshold cycle for the fluorescence signal to reach a certain level, while ΔCt is the difference in Ct values between the target and reference genes, and ΔΔCt is the difference in ΔCt between the target and reference samples.
Phytotoxicity assessment
A seed germination test was conducted to investigate the phytotoxicity of WNS after different UV/H2O2 treatments. Lettuce (Lactuca sativa. L) seeds were sterilized using 70% ethanol and 2% sodium hypochlorite solution and washed with deionized water. Forty sterilized seeds were then placed on a Petri dish (90 × 20 mm; SPL 10091, Gyeonggi-do, Korea) layered with filter paper (90 mm; Whatman, Maidstone, UK). After injecting 4 ml of UV/H2O2-treated samples, Petri dishes were sealed and cultured with a 24-h photoperiod at 25 ± 1 °C for 8 days in the plant growth chamber (GC-300TLH; Jeio Tech, Daejeon, Korea). Seed germination was recorded at intervals of 24 h during incubation.
Data availability
The authors confirm the data supporting the findings of the study are found within the main article and the Supporting Information.
References
Saccon, P. Water for agriculture, irrigation management. Appl. Soil Ecol. 123, 793–796 (2018).
Kumar, R. R. & Cho, J. Y. Reuse of hydroponic waste solution. Environ. Sci. Pollut. Res. Int. 21, 9569–9577 (2014).
Rosemarin, A. et al. Circular nutrient solutions for agriculture and wastewater—a review of technologies and practices. Curr. Opin. Environ. Sustain. 45, 78–91 (2020).
Sohn, W. et al. Nutrients in a circular economy: role of urine separation and treatment. Desalination 560, 116663 (2023).
Richa, A., Touil, S., Fizir, M. & Martinez, V. Recent advances and perspectives in the treatment of hydroponic wastewater: a review. Rev. Environ. Sci. Biotechnol. 19, 945–966 (2020).
Kannan, M., Elavarasan, G., Balamurugan, A., Dhanusiya, B. & Freedon, D. Hydroponic farming—a state of art for the future agriculture. Mater. Today Proc. 68, 2163–2166 (2020).
Kwon, M. J. et al. Waste nutrient solutions from full-scale open hydroponic cultivation: dynamics of effluent quality and removal of nitrogen and phosphorus using a pilot-scale sequencing batch reactor. J. Environ. Manag. 281, 111893 (2021).
Hosseinzadeh, S. et al. Degradation of root exudates in closed hydroponic systems using UV/H2O2: kinetic investigation, reaction pathways and cost analysis. Sci.Total Environ. 687, 479–487 (2019).
Hosseinzadeh, S., Bonarrigo, G., Verheust, Y., Roccaro, P. & Van Hulle, S. Water reuse in closed hydroponic systems: comparison of GAC adsorption, ion exchange and ozonation processes to treat recycled nutrient solution. Aquac. Eng. 78, 190–195 (2017).
Ajeng, A. A. et al. Resource recovery from hydroponic wastewaters using microalgae-based biorefineries: a circular bioeconomy perspective. J. Biotech. 360, 11–22 (2022).
Ehret, D. L., Alsanius, B., Wohanka, W., Menzies, J. G. & Utkhede, R. Disinfestation of recirculating nutrient solutions in greenhouse horticulture. Agronomie 21, 323–339 (2001).
Lau, V. & Mattson, N. Effects of hydrogen peroxide on organically fertilized hydroponic lettuce (Lactuca sativa l.). Horticulturae 7, 106 (2021).
Hosseinzadeh, S., Verheust, Y., Bonarrigo, G. & Van Hulle, S. Closed hydroponic systems: operational parameters, root exudates occurrence and related water treatment. Rev. Environ. Sci. Biotechnol. 16, 59–79 (2017).
McCorquodale-Bauer, K., Grosshans, R., Zvomuya, F. & Cicek, N. Critical review of phytoremediation for the removal of antibiotics and antibiotic resistance genes in wastewater. Sci. Total Environ. 870, 161876 (2023).
Eom, H. et al. Surface modification of carbon catalysts for efficient production of H2O2 in bioelectrochemical systems. Environ. Sci. Water Res. Technol. 8, 2304–2313 (2022).
Arends, J. B. A., Van Denhouwe, S., Verstraete, W., Boon, N. & Rabaey, K. Enhanced disinfection of wastewater by combining wetland treatment with bioelectrochemical H2O2 production. Bioresour. Technol. 155, 352–358 (2014).
Zhao, Q., An, J., Wang, X. & Li, N. In-situ hydrogen peroxide synthesis with environmental applications in bioelectrochemical systems: a state-of-the-art review. Int. J. Hydrog. Energy 46, 3204–3219 (2021).
Zhang, H. et al. Janus electrode of asymmetric wettability for H2O2 production with highly efficient O2 utilization. ACS Appl. Energy Mater. 3, 705–714 (2020).
Sim, J., Reid, R., Hussain, A., An, J. & Lee, H. S. Hydrogen peroxide production in a pilot-scale microbial electrolysis cell. Biotechnol. Rep. 19, e00276 (2018).
Ki, D., Popat, S. C., Rittmann, B. E. & Torres, C. I. H2O2 production in microbial electrochemical cells fed with primary sludge. Environ. Sci. Technol. 51, 6139–6145 (2017).
Yoon, Y. et al. Combination of H2O2-producing microbial desalination cells and UV/H2O2 advanced oxidation process: water salinity reduction and microbial inactivation. J. Environ. Chem. Eng. 11, 110110 (2023).
Khan, W. et al. A proof of concept study for wastewater reuse using bioelectrochemical processes combined with complementary post-treatment technologies. Environ. Sci. Water Res. Technol. 5, 1489–1498 (2019).
Zou, R. et al. Elimination of recalcitrant micropollutants by medium pressure UV-catalyzed bioelectrochemical advanced oxidation process: influencing factors, transformation pathway and toxicity assessment. Sci. Total Environ. 828, 154543 (2022).
Zhao, Y. et al. On-site H2O2 electro-generation process combined with ultraviolet: a promising approach for odorous compounds purification in drinking water system. Chem. Eng. J. 430, 132829 (2022).
Jwa, E. et al. Energy-efficient seawater softening and power generation using a microbial electrolysis cell-reverse electrodialysis hybrid system. Chem. Eng. J. 391, 123480 (2020).
Yu, H. et al. Relationship between soluble microbial products (SMP) and effluent organic matter (EfOM): characterized by fluorescence excitation emission matrix coupled with parallel factor analysis. Chemosphere 121, 101–109 (2015).
Ferreira, M. P. S., Santos, P. S. M. & Duarte, A. C. Oxidation of small aromatic compounds in rainwater by UV/H2O2: optimization by response surface methodology. Sci. Total Environ. 815, 152857 (2022).
Santos, P. S. M., Cardoso, H. B., Rocha-Santos, T. A. P. & Duarte, A. C. Oxidation of benzoic acid from biomass burning in atmospheric waters. Environ. Poll. 244, 693–704 (2019).
Han, U., Lee, Y. G., Byeon, J., Chon, K. & Cho, S. K. Mitigation of benzoic acid-driven autotoxicity in waste nutrient solution using O3 and O3/H2O2 treatments: seed germination and root growth of Lactuca sativa. L. Environ. Poll. 331, 121930 (2023).
Kümmerer, K. Antibiotics in the aquatic environment—a review—part I. Chemosphere 75, 417–434 (2009).
Kümmerer, K. Antibiotics in the aquatic environment—a review—part II. Chemosphere 75, 435–441 (2009).
Tamminen, M. et al. Tetracycline resistance genes persist at aquaculture farms in the absence of selection pressure. Environ. Sci. Technol. 45, 386–391 (2011).
Thevenon, F., Adatte, T., Wildi, W. & Poté, J. Antibiotic resistant bacteria/genes dissemination in lacustrine sediments highly increased following cultural eutrophication of Lake Geneva (Switzerland). Chemosphere 86, 468–476 (2012).
Zhang, Y. et al. Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes. Sci. Total Environ. 550, 184–191 (2016).
Gillings, M. et al. The evolution of class 1 integrons and the rise of antibiotic resistance. J. Bacteriol. 190, 5095–5100 (2008).
Ma, L., Li, A. D., Yin, X. L. & Zhang, T. The prevalence of integrons as the carrier of antibiotic resistance genes in natural and man-made environments. Environ. Sci. Tech. 51, 5721–5728 (2017).
Zhang, Y. et al. Deciphering of antibiotic resistance genes (ARGs) and potential abiotic indicators for the emergence of ARGs in an interconnected lake-river-reservoir system. J. Hazard. Mater. 410, 124552 (2021).
Elkins, K. M. & Nelson, D. J. Spectroscopic approaches to the study of the interaction of aluminum with humic substances. Coord. Chem. Rev. 228, 205–225 (2002).
Lee, J. G., Lee, B. Y. & Lee, H. J. Accumulation of phytotoxic organic acids in reused nutrient solution during hydroponic cultivation of lettuce (Lactuca sativa L.). Sci. Hortic. 110, 119–128 (2006).
Pramanik, M. H. R., Nagai, M., Asao, T. & Matsui, Y. Effects of temperature and photoperiod on phytotoxic root exudates of cucumber (Cucumis sativus) in hydroponic culture. J. Chem. Ecol. 26, 1953–1967 (2000).
Asaduzzaman, M. & Asao, T. Autotoxicity in beans and their allelochemicals. Sci. Hortic. 134, 26–31 (2012).
Asao, T. et al. Autotoxicity in some ornamentals with the means to overcome it. HortScience 42, 1346–1350 (2007).
Nogueira, R. F. P., Oliveira, M. C. & Paterlini, W. C. Simple and fast spectrophotometric determination of H2O2 in photo-Fenton reactions using metavanadate. Talanta 66, 86–91 (2005).
Farhat, N., Kim, L. H. & Vrouwenvelder, J. S. Online characterization of bacterial processes in drinking water systems. npj Clean Water 3, 16 (2020).
Haque, S. et al. Effects of adding household water filters to Rwanda’s Community-Based Environmental Health Promotion Programme: a cluster-randomized controlled trial in Rwamagana district. npj Clean Water 5, 42 (2022).
Wang, H. B. et al. Biofouling characteristics of reverse osmosis membranes by disinfection-residual-bacteria post seven water disinfection techniques. npj Clean Water 6, 24 (2023).
Pallares-Vega, R. et al. Determinants of presence and removal of antibiotic resistance genes during WWTP treatment: a cross-sectional study. Water Res. 161, 319–328 (2019).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25, 402–408 (2001).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (Nos. 2020R1C1C1009444 and 2022M3I3A1081850).
Author information
Authors and Affiliations
Contributions
J.Y.N. and S.K.C. conceived the idea of the study and designed the experiments. J.Y.N., H.E. and U.H. performed the experiments, analyzed the data and wrote the first draft of the article. S.Y.H. and H.K. contributed part of the experiments and supported data analysis. J.Y.N., S.K.C. and S.K. supervised the research activities and contributed to writing and proof-reading the manuscript. J.Y.N. and E.J. contributed to funding acquisition. J.Y.N., H.E. and U.H. contributed equally as co-first authors and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nam, JY., Eom, H., Han, U. et al. Unravelling the UV/H2O2 process using bioelectrochemically synthesized H2O2 to reuse waste nutrient solution. npj Clean Water 7, 66 (2024). https://doi.org/10.1038/s41545-024-00363-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41545-024-00363-0