Abstract
Cobalt-based catalysts were synthesized using the molten metal salt method and applied for the degradation of reactive dyeing wastewater. The results demonstrated a degradation of 97.1% for the C.I. Reactive Red 195 solution under the following conditions: 1.0 g/L of Co@MXene, 3 g/L of peroxymonosulfate (PMS), treated at 25 oC for 36 min with initial pH of 7. After adding 20 g/L of NaCl, the dye degradation rate increased to 5.57 times compared to the original rate 0.0894 min−1, but the difference in final degradation was not significant. The enhanced degradation was attributed to the combined action of hydroxyl radicals (•OH), sulfate radicals (SO4•−), and singlet oxygen (1O2). Notably, the Co@MXene catalyst maintained a high dye degradation percentage of 93.5% even after being recycled ten times. The treated dye residue can be recycled for dyeing cotton fabrics with reactive dyes. This study achieves rapid treatment of dye wastewater with wide applicability and provides valuable insights into dye wastewater treatment and environmental remediation.
Similar content being viewed by others
Introduction
With the rapid development of the printing and dyeing industry, a significant volume of hardly degradable organic dyes is being discharged into water bodies, posing a serious threat to human health and natural ecosystems1. Reactive dyes are one of the commonly used dyes in textile dyeing. However, reactive dyes have complex structures and are difficult to degrade, traditional wastewater treatment methods are often unable to completely remove the pollutants2,3. Therefore, it is particularly urgent and necessary to develop an efficient, economical, and environmentally friendly method for the treatment of reactive dye.
Advanced oxidation is an effective method for treating organic pollutants by catalyzing the generation of strong reactive oxygen species (ROS). In recent years, advanced oxidation processes based on sulfate radicals have garnered significant attention in the study of organic pollutant degradation4,5. Peroxymonosulfate (PMS) can be activated to produce sulfate radicals (SO4•−) by breaking the O–O bond through various pathways, including heat treatment, alkali activation, electrochemical activation, UV radiation, photochemistry, acoustic chemistry, and the activation of transition metals (e.g., cobalt, iron, silver, and copper) and carbonaceous materials6,7,8,9,10,11,12. Among these methods, transition metal cobalt activation of PMS is an efficient method to generate SO4•−. Studies have shown that the catalytic performance of the Co(II)/PMS system is superior to that of the conventional Fenton reaction at neutral pH, requiring fewer reagents13. However, the direct use of Co(II) as a homogeneous catalyst typically faces challenges such as low recyclability, metal leakage, increased coloration, and sludge disposal14. To improve the catalytic activity, stability, and recyclability, other carriers loaded with Co are often used to prepare multiphase catalysts.
Two-dimensional transition metal carbides/nitrides (MXene) are unique layered nanomaterials first synthesized by Naguib et al. through the selective etching of the MAX phase15. The surface of MXene features numerous electronegative functional groups (–O, –OH, –F, etc.), which facilitate the adsorption of metal cations, favorable for further reduction and anchoring to the surface. In addition, MXene is widely prepared by etching the MAX phase, which inevitably etches away the metal atoms within the MXene lattice, resulting in the formation of defect sites. The defect sites of unsaturated coordination have certain reducing properties, which also favor the reduction and anchoring of cations16. As a result, composite catalysts formed by loading transition metal ions and metal oxides onto MXene can synergistically activate PMS. Moreover, the low toxicity of MXene has been demonstrated in biomedical studies17, proving the feasibility of application in environmental remediation.
C.I. Reactive Red 195 (RR195) was a monochlorotriazine-vinyl sulfone type reactive dye, higher polarity and reactivity, widely used in textile dyeing and printing. In this study, Co@MXene composite catalysts were prepared using a metal salt melting method. RR195 was selected as the model compound to evaluate the effectiveness of the catalyst in treating reactive dye wastewater. The degradation of the dye solution served as the evaluation index to explore the influence of various parameters, including catalyst concentration, peroxymonosulfate (PMS) concentration, initial pH, temperature, and the concentration of inorganic salts. The kinetics and mechanism of the degradation process were also investigated, and dyeing of wastewater after recycling treatment. This study provides ideas and solutions for the treatment of organic dye wastewater.
Results
Morphology and elemental analysis of Co@MXene
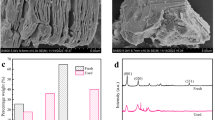
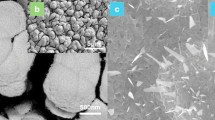
Co@MXene catalysts were synthesized on Ti2AlN MAX phase carriers using molten CoCl2. The morphological structure and composition of the prepared metal catalysts are shown in Fig. 1. The pristine Ti2AlN MAX had an intact bulk structure (Fig. 1a). Upon reaction with molten CoCl2 salt, a large number of cracks appeared in the bulk ceramics (Fig. 1b), and transformation of blocky structures into two-dimensional structures with sparse multilayers. This is attributed to the etching of the Al layer of Ti2AlN by the molten CoCl2 salt18. During this process, Co2+ ions are reduced to Co atoms and then aggregate into Co particles, which is supported by the EDS images (Fig. 1d) and the XRD images (Fig. 1e). Additionally, a few Ti atoms were etched away by the molten metal ions, creating Ti vacancy defects, where Co atoms were subsequently anchored19. Following the loading of CoCl2, the particles covered the surface and interlayers of MXene. The Al layer of Ti2AlN MAX was etched in HF or HCl/LiF solution15,20. As shown in Fig. 1e, the XRD results indicate that the diffraction peaks at 13.04°, 34.62°, 35.28°, 39.72°, 40.08°, 43.9°, 53.68°, 57.5°, 59.4°, 62.04°, 72.3°, 76.4°, and 76.4° correspond to Ti2AlN21. Additionally, characteristic Co diffraction peaks at 27.4°(112)、32.82°(210)、36.76°(104)、41.22°(302)、62.26°(323)、65.16°(413) confirm the successful complexation of Co on MXene. A comparison of the FTIR characterization results of Co@MXene before and after calcination reveals prominent O-H characteristic absorption peaks at 3422 cm−1 and N–H at 1639 cm−1. After high-temperature treatment, most of the intensities of the Co@MXene characteristic peaks were attenuated (Fig. 1f). This functional group information indicates that most of the organic matter was removed. Combined with XRD data analysis, Co was not removed but instead was effectively immobilized on the MXene surface.
Degradation of RR195 in different processes
As mentioned above, Co@MXene possesses a porous structure that enables it to adsorb dye from the dye solution while also acting as a catalyst to activate PMS for dye degradation. Based on this, reactive dyes were treated with 3 g/L of PMS, 1.0 g/L of Co@MXene, and a mixture of both were treated at pH = 7 for 0 ~ 36 min at 25 oC. The dye degradation profiles are shown in Fig. 2.
As shown in Fig. 2, the dye degradation percentage were only 1.64% and 24.5% for 36 min of treatment at 25 oC using PMS or Co@MXene alone, indicating the limited ability of PMS alone to degrade or Co@MXene to adsorb RR195. When PMS was added to Co@MXene process, the degradation percentage of RR195 increased rapidly with the treatment time, and the degradation percentage was as high as 97.1% at 36 min, which indicated that the synergistic effect of PMS/Co@MXene could be utilized to degrade RR195 effectively. These results further confirmed the ability of the prepared Co@MXene catalysts to activate PMS and the better degradation of the PMS/Co@MXene system compared to other systems.
Factors affecting the degradation of RR195
The concentration of the Co@MXene catalyst directly influences the PMS activation capacity and dye degradation. To investigate the effect of Co@MXene concentration on the degradation of RR195, 0.06 g/L of RR195 dye solution was degraded using 0.1–2.0 g/L of Co@MXene activated with 3 g/L of PMS at 25 oC under the initial pH=7 condition. The variation in the degradation of RR195 over time is shown in Fig. 3a.
As shown in Fig. 3a, the dye degradation percentage increased with treatment time for the same catalyst. During the early stage of treatment (0–5 min), the difference in dye degradation was not significant. When the treatment time exceeded 5 min, the dye degradation percentage increased with the Co@MXene concentration. Notably, the degradation of RR195 by PMS using 2.0 g/L of Co@MXene did not show a significant difference in the dye degradation percentage during the later stages of degradation (18–36 min) compared to using 1.0 g/L of Co@MXene. This means that the concentration of the Co@MXene catalyst should not be excessively high when the concentrations of the dye and PMS are determined. In terms of the dye degradation rate, it increased from 0.033 min−1 to 0.0894 min−1 when the Co@MXene concentration was increased from 0.1 g/L to 1.0 g/L. Continuing to increase the catalyst concentration to 2.0 g/L, the dye degradation rate remained at 0.0894 min−1. These results indicated that the catalyst concentration significantly affects dye degradation percentage. When the catalyst concentration is low, PMS activation is insufficient. Conversely, excess Co@MXene rapidly activates PMS in a short time, generating a large number of free radicals. On one hand, the free radicals do not have sufficient time to fully react with the dye molecules. On the other hand, the free radicals can react with each other, losing their ability to degrade the dye22. Therefore, the optimal Co@MXene concentration in this experiment was determined to be 1.0 g/L.
After PMS is activated by Co@MXene, the peroxy bonds break to form free radicals such as SO4•−, •OH, and 1O2, which are essential for the degradation of dye molecules. To elucidate the relationship between PMS concentration and dye degradation, experiments were conducted at an initial pH of 7, with 1.0 g/L of Co@MXene, using 1–5 g/L of PMS at 25 oC to degrade a 0.06 g/L of RR195 dye solution over 0–36 min. The dye degradation curves are shown in Fig. 3b. It can be seen that the dye degradation percentage corresponding to 5 g/L of PMS was relatively low during the early stages of degradation. The PMS concentration had a significant effect on the dye degradation percentage when the treatment time was between 5 and 18 min. At 12 min, the dye degradation percentage for PMS concentrations of 1, 2, 3, 4, and 5 g/L was 68.8%, 64.1%, 67.1%, 82.1%, and 77.2%, respectively. The dye degradation percentage showed an increasing and then decreasing trend with increasing PMS concentration. With further extension of the treatment time, the PMS concentration had little effect on the extent of the dye, especially on the final degradation percentage. Apparently, the rate of dye degradation was closely related to the concentration of PMS. Overall, the 4 g/L of PMS dye degradation rate was faster, and either increasing or decreasing PMS slowed down the dye degradation percentage, but had little effect on the final degradation percentage of the dye. It may be due to the depletion from free radical interactions, which led to a decrease in the dye degradation rate22. Considering the dye degradation percentage and degradation rate, the PMS concentration was determined to be 3 g/L in the experiment.
The pH of the solution is an important factor in the non-homogeneous catalytic degradation of organic pollutants, which affects the PMS activation, the catalyst surface charge, and the types of free radicals in the system23. To elucidate the effect of initial pH on dye degradation, the pH of the initial dye solution was adjusted to 3, 7, and 10. Subsequently, 1.0 g/L of Co@MXene and 3 g/L of PMS were added to the dye solution and treated at 25 oC for 0–36 min. The resulting dye degradation is depicted in Fig. 3c. When the initial pH was set at 3 or 7, dye degradation percentage increased gradually over time, little difference in trends in degradation rates. However, when the pH was raised to 10, dye degradation exhibited three distinct stages: 0–8 min, 8–26 min, and beyond 26 min. In the initial phase, dye degradation percentage surged rapidly, reaching 89.48%, which is 36.28% higher than the degradation percentage observed at pH 3 within the same timeframe. This phase can be characterized as the rapid growth period. When the treatment time exceeded 8 min and continued to extend, the dye degradation percentage incrementally increased to 97.85%, reflecting an 8.37% rise. Beyond 26 min of treatment, the dye degradation percentage plateaued, entering a stable equilibrium phase. The degradation rates corresponding to pH values of 3, 7, and 10 were 0.1068, 0.0894, and 0.1172 min−1, respectively. These results indicate that dye degradation was fastest under alkaline conditions, slowest under neutral conditions, and intermediate under acidic conditions. This can be attributed to the alkaline reaction conditions potentially enhancing the decomposition of PMS by Co@MXene to produce singlet oxygen (1O2)24. Although the initial pH influences the dye degradation rate, the final degradation percentage levels exceeded 97.1% in all cases, demonstrating the efficacy and wide applicability of the catalytic system.
Temperature plays a crucial role in the effectiveness of wastewater treatment reactions. To determine the relationship between temperature and dye degradation, the dye solution was treated at 25–60 oC for 0–36 min, using 3 g/L and 1.0 g/L concentrations of PMS and Co@MXene, respectively, with an initial pH of 7. The dye degradation results are presented in Fig. 3d. At 25 oC, dye degradation percentage increased gradually, reaching a maximum value of 97.12% at 36 min. However, upon elevating the treatment temperature, dye degradation percentage accelerated significantly. For instance, at 5 min, the dye degradation percentage was 39.51%, 66.57%, and 92.08% for temperatures of 25 oC, 40 oC, and 60 oC, respectively. The calculated activation energy for the degradation of RR195 by Co@MXene-activated PMS was relatively low at 40.31 kJ/mol, indicating that the dye is more readily degraded within this system. From the thermodynamic perspective, as the temperature increases, the likelihood of collisions between PMS molecules and the cobalt monoatomic catalyst also increases, or the O–O bond in PMS breaks more readily. In other words, higher temperatures enhance the HSO5− breaking reaction, generating more SO4•−, which accelerates the degradation of RR19525.
As a dyeing promoter for reactive dyes, NaCl is an unavoidable chemical in dyeing wastewater. To elucidate the relationship between inorganic salts and the degradation efficiency of dyeing wastewater, varying concentrations of NaCl (0–25 g/L) were added to the original degradation system (PMS = 3 g/L, Co@MXene = 1.0 g/L, pH = 7). The degradation performance of the dyes is presented in Fig. 3e.
Figure 3e illustrates that the addition of NaCl accelerated the rate of dye degradation without affecting the final degradation outcome. The degradation percentage was 97.12% for 36 min of dye treatment without NaCl, whereas the degradation percentage was 98.93% for 12 min of dye treatment with the addition of 5 g/L NaCl. The higher the concentration of added inorganic salts, the greater the degradation of dyes within the same time period, aligning with the pattern of degradation rates shown in the figure. Specifically, the degradation rate increased from 0.0894 min−1 to 0.4984 min−1 as the NaCl concentration rose from 0 g/L to 20 g/L. Beyond this concentration, further increases in NaCl did not enhance the degradation rate.
The dye degradation properties may be attributed to the production of Cl₂ and HOCl as described in reaction Eqs. (1) to (4). This apparent promotion suggests that Cl− is highly reactive within the Co@MXene system used in this study26.
To verify the suitability of the system for different reactive dyes, three dyes (C.I. Reactive Red 218, C.I. Reactive Red 195, and C.I. Reactive Black 5) were selected for degradation experiments. The results are presented in Fig. 3f. The experimental conditions were: PMS at 3 g/L, Co@MXene at 1.0 g/L, pH = 7, and treated at 25 oC for 0–36 min. The results indicate that the degradation of monochlorotriazine, monochlorotriazine-vinyl sulfone, and bis-vinyl sulfone dyes can all be achieved under these experimental conditions. The degradation percentage of C.I. Reactive Red 218, C.I. Reactive Red 195, and C.I. Reactive Black 5 was 97.02%, 97.12%, and 97.33%, respectively, and the degradation rates were 0.0894 min−1, 0.0905 min−1, and 0.0821 min−1. The degradation rate of C.I. Reactive Red 195 was greater than that of C.I. Reactive Black 5, and the percentage of degradation was slightly greater than that of C.I. Reactive Red 218. The possible reason for this is that the side chains in the structure of azo dyes greatly affect the efficiency of dye degradation27. While the degradation rates varied slightly among the different dyes, the final degradation levels were similar, demonstrating that the proposed catalytic degradation system is broadly applicable.
Identification of active components
During PMS activation, free radicals such as sulfate radicals (SO4•−), hydroxyl radicals (•OH), and singlet oxygen (1O2) are typically generated. To identify the active species involved in the degradation process, free radical scavenging experiments were performed. Methanol (MeOH) was used as a typical quenching agent for SO4•− and •OH, tertiary butyl alcohol (TBA) was used specifically for •OH28, and furfuryl alcohol (FFA) was used for singlet oxygen (1O2)29. The degradation effects of the dyes in these different systems are illustrated in Fig. 4a.
As shown in Fig. 4a, the final degradation percentage of dyes decreased to 84.34%, 40.2%, and 17.2% after the addition of methanol, tert-butanol, and furfuryl alcohol, respectively. These reductions of 11.83%, 56%, and 78.95% indicate that the addition of these scavengers inhibited dye degradation percentage. This suggests that radicals such as SO4•− and •OH were generated during the activation of PMS by Co@MXene. In the presence of excess methanol, non-radical oxidation of PMS/Co@MXene occurred, indicating that in addition to SO4•− and •OH, PMS activation also tended to produce 1O2 or involve electron transfer reactions30. When furfuryl alcohol was used to quench singlet oxygen (1O2), little degradation of wastewater, confirmed the generation of 1O2. As shown in Fig. 4b, the degradation rate of dyes decreased to 0.0587 min−1, 0.008 min−1, and 0.0022 min−1, after the addition of methanol, tert-butanol, and furfuryl alcohol. Free radical contribution calculated from degradation rate constants, the data show that the contribution of singlet oxygen (1O2) is 66.71%, SO4•− + 1O2 is 8.94%, and •OH + SO4•− is 2.46%. The mechanism of degradation of RR195 may be the opening of the –N=N– and C–N groups in the dye, forming intermediates containing benzene rings, naphthalene rings, and triazine structures, respectively. These intermediates were decomposed into small molecules and organic compounds by the synergistic action of SO4•−, •OH, and 1O231. The dye degradation mechanism is shown in Fig. 4c.
Enhanced redox cycle of Co3+ and Co2+
Figure 5a shows the investigated scanning spectra of Co@MXene, indicating the presence of C, N, Ti, O, and Co. The high-resolution Co 2p spectra reveal peaks corresponding to various oxidation states and forms of cobalt: metallic Co with a binding energy of 785.2 eV, Co3+ 2p3/2 with a binding energy of 779.5 eV, Co2+ 2p3/2 with binding energies of 781.3 eV, Co3+ 2p1/2 with a binding energy of 795.0 eV, Co2+ 2p1/2 with a binding energy of 796.6 eV, and satellite peaks at 786.5 eV and 802.2 eV32. These results indicate the successful loading of cobalt and the presence of multiple oxidation states. The area of the Co3+ 2p1/2 peak at a binding energy of 795.0 eV was significantly reduced, while the area of the Co2+ 2p1/2 peak at 796.6 eV increased after used Co@MXene (Fig. 5c). This suggests that Co3+ was reduced to Co2+ by MXene.
In the O 1s XPS spectra (Fig. 5d–f), the three O 1s peaks at 529.3 eV, 531.1 eV, and 532.3 eV correspond to Ti-O, C-Ti-O, and Ti-OH bonds, respectively. The peak intensity of the C-Ti-O bond in Co@MXene was significantly reduced compared to that in MXene. This reduction can be attributed to the introduction of Co, which altered the oxidation state and chemical environment of the MXene surface. The positions of the peaks did not change significantly after the incorporation of Co@MXene.
In the Ti 2p XPS spectra (Fig. 5g–i), there are two prominent peaks at 457.8 eV and 463.5 eV, corresponding to Ti 2p3/2 and Ti 2p1/2, respectively33. Some of these peaks are significantly reduced compared to MXene, suggesting that the preparation of Co@MXene has a strong scavenging effect on the Ti-O bond during the process.
Catalyst and recycling performance of treated wastewater
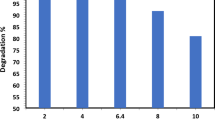
To evaluate the recycling performance of the catalyst, degradation experiments were conducted by adding 0.06 g/L RR195 and 3 g/L PMS to the treated dye solution. After the dye degradation was completed, Co@MXene was recovered and subjected to cycling experiments. The residual dye percentage and the degradation rate are shown in Fig. 6a. As the number of Co@MXene cycles increased from 0 to 10, the residual dye rate increased from 2.88% to 6.47%, indicating a slight decline in dye degradation efficiency. Correspondingly, the dye degradation rate decreased from 0.0973 min−1 to 0.0744 min−1. Compared to cycle 0, the dye degradation percentage decreased by 1.78% and 3.59% at the 5th and 10th cycles, respectively, with a corresponding decrease in the degradation percentage of 11.51% and 23.54%. Despite this decline, the dye degradation percentage at the 10th cycle remained high at 93.53%, confirming that Co@MXene possesses good recycling performance and can be effectively reused multiple times.
To investigate the difference between the dyeing performance of recycled wastewater and deionized water, reuse of reactive dyeing wastewater experiments with RR195 were conducted. The uptake and fixation rates during dyeing are shown in Fig. 6b. When using deionized water (0th cycle), the dyeing rate was 51.54% at 15 min and increased to 87.45% after 75 min. Dyeing with reclaimed water for the same duration resulted in a dyeing percentage increase of 1.29–7.34% compared to the conventional process. This indicates that dye molecules were more readily absorbed by the fibers in reused water. This enhancement is attributed to the retention of cations in the dye solution after filtration, which act as dye promoters by weakening the electrostatic repulsion between the dye and fibers34. Increasing the number of dyeing cycles did not alter the overall trend of dye uptake. With more cycles, the dyeing percentage corresponding to the same time increased, although the color fixation rate remained essentially unchanged.
As displayed in Table 1, compared with the color parameters of fabrics dyed with conventional dyeing, the brightness (L*) of fabrics dyed with recycled wastewater decreased by 0.3–0.57, the red-green component (a*) increased by 0.04 to 0.38, and the yellow-blue component (b*) and the apparent color depth (K/S) increased by 0.06–0.45 and 0.18–0.49, respectively. The results indicate that the color of the cycle-dyed fabric is darker, with a reddish hue and less blue tint. The fabric exhibits a deeper apparent color and improved dyeing fastness. Although the color parameters showed slight variations, these can be corrected by fine-tuning the dyeing process parameters. This demonstrates the feasibility of treating reactive dye wastewater with a treatment agent and using the treated water for reactive dye recycling.
Discussion
In this study, the Co@MXene catalyst was prepared by molten metal salt method and used in combination with peroxymonosulfate to degrade dyeing wastewater. The degradation was used as the evaluation metric to assess its catalytic performance. Additionally, the recycling performance of the catalyst-degraded wastewater and the dyeing performance of the reused water were investigated. The results demonstrated that the degradation percentage could reach 97.1% for 0.06 g/L of C.I. Reactive Red 195 dye solution using 1.0 g/L of Co@MXene and 3 g/L of PMS treated for 36 min at 25 oC with an initial pH of 7. The effect of NaCl on the dye degradation rate was significant. In this system SO4•−, •OH, and 1O2 act together. Utilizing the strong reducing properties of the MXene carrier, Co3+/Co2+ cycling can be triggered to accelerate the activation of PMS. The Co@MXene catalyst prepared in this experiment was cycled 10 times, resulting in a residual dye rate of only 6.47%, demonstrating high reusability. The dyeing residue treated in this manner can be effectively used to dye cotton fabrics with reactive dyes. Compared to the conventional process, dyeing with the wastewater medium obtained from the treatment results in a faster dyeing rate. The dyed fabrics are darker and exhibit a more reddish hue with less bluish tint, while the differences in fixation and color fastness are not significant. This experiment provides a feasible strategy for efficient catalytic degradation of dyeing wastewater and using it for dyeing purposes, which lays a foundation for energy saving, emission reduction, and sustainable utilization in the printing and dyeing industry.
Methods
Materials
Ti2AlN MAX ceramic (400 mesh), sodium hydroxide (NaOH, AR), sodium chloride (NaCl, AR), sodium carbonate (Na2CO3, AR), hydrochloric acid (HCl, 36%), ethanol (EtOH, C2H6O, AR), methanol (MeOH, CH3OH, AR), tert-butanol (TBA, C4H10O, AR) and furfuryl alcohol (FFA, C5H6O2, AR) were obtained from Shanghai Macklin Biochemical Technology Co., Ltd (Shanghai, China). PMS (2KHSO5•KHSO4•K2SO4, AR) and cobalt chloride (CoCl2, AR) were obtained from Aladdin (Shanghai, China). All chemicals were used without further purification. C.I. Reactive Red 218 (RR218), C.I. Reactive Red 195 (RR195), and C.I. Reactive Black 5 (RB5) for salt-free inkjet were purchased from Jiangsu Shenxin Dyestuff Chemical Co., Ltd.
Preparation of Co@MXene
Co@MXene catalysts were prepared by the molten salt method. First, Ti2AlN powder and CoCl2 were ground in an agate mortar at a molar ratio of 1:2. Then, the resulting mixture was transferred to a ceramic boat and heated in a muffle furnace (XH4-14, Zhengzhou Xinhan Instrument Co., Ltd) at 1000 oC for 7 h. After cooling to room temperature, the powder containing CoCl2 was soaked in 3 M HCl solution (200 mL) for 4 h to remove the Co particles. Subsequently, the mixture was centrifuged at 3500 rpm for 15 min. The centrifuged sediment was washed with ultrapure water until the pH of the supernatant exceeded 6.5. Finally, the Co@MXene catalyst was obtained by drying at −20 oC for 48 h in a freezing dryer (LGJ-22D, Beijing Sihuan Scientific Instrument Factory Co., Ltd).
Degradation of wastewater
All degradation experiments were conducted using a digital thermostatic water bath (HH-S4, Changzhou Guoyu Instrument Manufacturing Co., Ltd.). In the experiments, 100 mL of 0.06 g/L reactive dyes simulated wastewater was used. 1–5 g/L of PMS, and 0.1–2.0 g/L of Co@MXene were added to the solution. The degradation experiments were carried out at 25 oC. The absorbance values at the maximum absorption wavelength of the dye were measured (RR195 = 542 nm, RR218 = 547 nm, RB5 = 604 nm), and the degradation of the reactive dye was calculated based on the absorbance of the solution.
After the dye degradation was completed, the system was filtered using a 0.22 μm filter membrane. The solid particles were then collected after each reaction and washed by centrifugation (4500 rpm, 10 min) until the pH of the supernatant was neutral. The particles were dried under vacuum for 24 h. This cyclic experimental process was repeated to further evaluate the catalyst performance.
Quenching of free radicals
In the degradation experiments, methanol was used to quench SO4•− and •OH radicals, tert-butanol was used to quench •OH radicals and furfuryl alcohol was used to quench singlet oxygen (1O2). Control experiments were performed without adding any radical quenching agents. The pH of the solution was adjusted with 0.01 mol/L H2SO4 and 0.01 mol/L NaOH. The free radical contribution was calculated using Eq. (5).
Where RROS is the contribution of free radicals, k is the rate constant under the condition without the addition of the bursting agent, and kROS is the reaction system with the addition of different bursting agents.
Reuse of treated dyeing wastewater
Dyeing was performed using an HT-B-24 dyeing machine with a capacity of 150 mL (Guangdong Heshan Hongfa Printing and Dyeing Machinery Manufacturing Co., Ltd.). A 2.0 g sample of 100% bleached cotton fabric was weighed and immersed in a dye bath containing 60 mL of treated dyeing wastewater or deionized water (bath ratio 30:1) with the dye concentration of 0.5%(o.w.f) RR195. Dyeing at 25 oC and maintained for 15 min. Subsequently, 60 g/L of sodium chloride was added to the dyeing machine and mixed for an additional 15 min. The dyeing temperature was further raised to 60 oC for 10 min, followed by the addition of 20 g/L of Na2CO3 to the dyeing solution. Finally, dyed fabrics were fixed at 60 °C for 30 min. The dyeing residue was degraded and the treated dyeing wastewater was recovered for ten cycles of dyeing experiments. The dyeing process is shown in Fig. 731.
Degradation evaluations
The absorbance values corresponding to 300–800 nm were determined using an ultraviolet-visible spectrophotometer. Degradation percentage of reactive dyes was calculated from the absorbance values at the maximum absorption wavelengths (RR195 = 542 nm, RR218 = 547 nm, RB5 = 604 nm). A degradation percentage curve was then plotted. The degradation percentage was calculated using Eq. (6).
Where S is the dye degradation percentage, At is the dye absorbance at time t, and A0 is the initial dye absorbance.
Degradation rate of the dyeing solution was calculated according to Eq. (7). Take the reaction time as the horizontal coordinate and ln(At/A0) as the vertical coordinate to obtain the linear equation of the reaction rate. The slope of this equation represents the kinetic reaction rate constant.
Where At is the dye absorbance at time t, A0 is the starting dye absorbance, t is the time, and kobs is the reaction rate constant.
Dyeing evaluations
The exhaustion rate of dye was calculated using Eq. (8).
Where E is the exhaustion rate, A0 and At indicate the initial and final absorbance of the dyeing process, respectively.
The soaping residue, dyeing residue, and original dyeing solution were obtained and tested directly for absorbance. The fixation rate of reactive dye is calculated using Eq. (9).
Where F represents the fixation rate, A0 is the absorbance of dye solution at 0 min, A1 and A2 are the absorbance of solutions at the end of the dyeing and soaping process.
The Color i5 colorimeter was utilized to measure the brightness (L*), red-green light (a*), yellow-blue light (b*), and apparent color depth (K/S value) of the fabrics35. According to the national standard textile dyeing fastness test (GB/T 3921-2020) to test the color fastness to rubbing of dyed cotton fabrics.
Methods of analysis
The surface micromorphology and elemental content of the samples were tested using a scanning electron microscope (SEM, Hitachi S4800, Japan) and an X-ray energy dispersive spectroscopy (EDS). Analyze the crystalline state of the sample using X-ray diffraction (XRD, Bruker D8 Advance, Germany), the elemental composition and valence information were determined using X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, USA). The functional groups of the samples were characterized using Fourier Transform Infrared Spectroscopy (FTIR, Thermo Nicolet Nexus670FTIR, USA) to determine the type of functional groups present in the catalyst material by scanning and analyzing the characteristic absorption peaks.
Data availability
The authors confirm the data supporting the findings of the study are found within the main article. Additional data pertaining to the study may be requested from the authors.
References
Ewuzie, U. et al. A review on treatment technologies for printing and dyeing wastewater (PDW). J. Water Process. Eng. 50, 103273 (2022).
Liu, Z., Khan, T. A., Islam, M. A. & Tabrez, U. A review on the treatment of dyes in printing and dyeing wastewater by plant biomass carbon. Bioresour. Technol. 354, 127168 (2022).
Raees, A. et al. Adsorption potential of Schizophyllum commune white rot fungus for degradation of reactive dye and condition optimization: a thermodynamic and kinetic study. Adsorpt. Sci. Technol. 2023, 4725710 (2023).
Nidheesh, P. V., Divyapriya, G., Titchou, F. E. & Hamdani, M. Treatment of textile wastewater by sulfate radical based advanced oxidation processes. Sep. Purif. Technol. 293, 121115 (2022).
Shu, D. et al. Sustainable cotton dyeing with reactive dyes in the recycled dyeing wastewater. Pigm. Resin. Technol. https://doi.org/10.1108/PRT-09-2023-0082 (2024).
Ji, Y., Dong, C., Kong, D., Lu, J. & Zhou, Q. Heat-activated persulfate oxidation of atrazine: implications for remediation of groundwater contaminated by herbicides. Chem. Eng. J. 263, 45–54 (2015).
Fang, G., Gao, J., Dionysiou, D. D., Liu, C. & Zhou, D. Activation of persulfate by quinones: free radical reactions and implication for the degradation of PCBs. Environ. Sci. Technol. 47, 4605–4611 (2013).
Song, H. et al. Electrochemically activated PMS and PDS: radical oxidation versus nonradical oxidation. Chem. Eng. J. 391, 123560 (2020).
Guan, Y.-H., Ma, J., Li, X.-C., Fang, J.-Y. & Chen, L.-W. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ. Sci. Technol. 45, 9308–9314 (2011).
Qin, F. et al. Catalyst‐free photochemical activation of peroxymonosulfate in xanthene‐rich systems for fenton‐like synergistic decontamination: efficacy of proton transfer process. Chem. Int. Ed. 135, e202300256 (2023).
Anipsitakis, G. P., Stathatos, E. & Dionysiou, D. D. Heterogeneous activation of oxone using Co3O4. Indian. J. Chem. A. 109, 13052–13055 (2005).
Gao, Y., Chen, Z., Zhu, Y., Li, T. & Hu, C. New insights into the generation of singlet oxygen in the metal-free peroxymonosulfate activation process: important role of electron-deficient carbon atoms. Environ. Sci. Technol. 54, 1232–1241 (2019).
Pang, L. et al. Preparation of Co-C/N flower-like single-atom catalysts via TCPP coordination confinement for enhanced activation of peroxymonosulfate. J. Alloy. Compd. 972, 172856 (2024).
Bouzayani, B., Rosales, E., Pazos, M., Elaoud, S. C. & Sanromán, M. A. Homogeneous and heterogeneous peroxymonosulfate activation by transition metals for the degradation of industrial leather dye. J. Clean. Prod. 228, 222–230 (2019).
Naguib, M. et al. Two-dimensional transition metal carbides. Acs. Nano. 6, 1322–1331 (2012).
Bai, X. & Guan, J. Applications of MXene‐based single‐atom catalysts. Small. Struct. 4, 2200354 (2023).
Vasyukova, I. A., Zakharova, O. V., Kuznetsov, D. V. & Gusev, A. A. Synthesis, toxicity assessment, environmental and biomedical applications of MXenes: a review. Nanomaterials 12, 1797 (2022).
Wenyang, L., Jiangbo, Z., Tingting, C. & Meijun, H. Tribological research of MAX metalmatrix self-lubricating composite. Nonferrous Met. Sci. Eng. 8, 61–67 (2017).
Li, S., Gu, M., Huang, J., Wang, Y. & Yu, G. Oligolayered Co@MXene with a Co···SO3 cation-π bridge for ultra-rapid catalytic oxidation of a novel “forever chemical” OBS. Appl. Catal. B-Environ. 311, 121364 (2022).
Ghidiu, M., Lukatskaya, M. R., Zhao, M.-Q., Gogotsi, Y. & Barsoum, M. W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature. 516, 78–81 (2014).
Chen, W., Tang, J., Lin, X., Ai, Y. & Ye, N. Formation mechanism of high-purity Ti2AlN powders under microwave sintering. Materials. 13, 5356 (2020).
Ghanbari, F. & Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants. Chem. Eng. J. 310, 41–62 (2017).
Tian, H., Cui, K., Sun, S., Li, H. & Chen, X. Construction of acidic microenvironments to overcome the pH dependence of iron-based catalysts and application to the degradation of micropollutants by AOPs. Chem. Eng. J. 488, 150934 (2024).
Chen, W. et al. Activation of peroxymonosulfate for degrading ibuprofen via single atom Cu anchored by carbon skeleton and chlorine atom: The radical and non-radical pathways. Sci. Total. Environ. 858, 160097 (2023).
Yang, P., Long, Y., Huang, W. & Liu, D. Single-atom copper embedded in two-dimensional MXene toward peroxymonosulfate activation to generate singlet oxygen with nearly 100% selectivity for enhanced Fenton-like reactions. Appl. Catal. B-Environ. 324, 122245 (2023).
Yang, P. et al. Singlet oxygen-dominated activation of peroxymonosulfate by CuO/MXene nanocomposites for efficient decontamination of carbamazepine under high salinity conditions: performance and singlet oxygen evolution mechanism. Sep. Purif. Technol. 285, 120288 (2022).
Ismail, G. A. & Sakai, H. Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere 291, 132906 (2022).
Zhu, C. et al. Contribution of alcohol radicals to contaminant degradation in quenching studies of persulfate activation process. Water Res. 139, 66–73 (2018).
Zhai, L. et al. Degradation of norfloxacin by sulfur-doped iron-cobalt oxides activated perodisulfate: Synergism between free radicals and singlet oxygen. Chem. Eng. J. 478, 147378 (2023).
Liang, J., Fu, L., Gao, K. & Duan, X. Accelerating radical generation from peroxymonosulfate by confined variable Co species toward ciprofloxacin mineralization: ROS quantification and mechanisms elucidation. Appl. Catal. B-Environ. 315, 121542 (2022).
Shu, D. et al. Cleaner reactive dyeing with the recycled dyeing wastewater. J. Environ. Chem. Eng. 12, 113069 (2024).
Chen, G., Zhang, L., Fan, X. & Wu, H. Interfacial and defect polarization in MXene-like laminated spinel for electromagnetic wave absorption application. J. Colloid Interf. Sci. 588, 813–825 (2021).
Liu, J. et al. Size-regulated Co-doped hetero-interfaced 3D honeycomb MXene as high performance electromagnetic absorber with anti-corrosion performance. Appl. Surf. Sci. 645, 158846 (2024).
Lu, X. et al. Negatively charged hollow crosslinked aromatic polymer fiber membrane for high-efficiency removal of cationic dyes in wastewater. Chem. Eng. J. 433, 133650 (2022).
Hossain, A., Samanta, A., Bhaumik, N., Vankar, P. & Shukla, D. Non-toxic coloration of cotton fabric using non-toxic colorant and nontoxic crosslinker. J. Text. Sci. Eng. 8, 374 (2018).
Acknowledgements
This work was supported by Innovation and Entrepreneurship Training Program for College Students (202410082023) and (X202410082063), Youth Foundation of Hebei Province Department of Education Fund (QN2023090). This work was supported by the Champion Project of Xinjiang Uygur Autonomous Region titled “Salt-Free Dyeing Technology for Cellulose Fibers with Reactive Dyes”. The authors are also very grateful for the help provided by Baijiang Niu during the experimental process.
Author information
Authors and Affiliations
Contributions
D.S. put forward the experimental idea; X.Z. designed and performed the experiments, and drafted the manuscript; B.H. and W.L. performed the characterization; B.W. and C.X. supervised the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shu, D., Zhang, X., Han, B. et al. Enhanced degradation and recycling of reactive dye wastewater using cobalt loaded MXene catalysts. npj Clean Water 7, 88 (2024). https://doi.org/10.1038/s41545-024-00391-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-024-00391-w