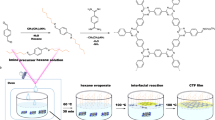
Abstract
Enhancing the sustainability of catalysts is crucial for the practical application of piezo-photocatalytic degradation of sewage. This study introduces a novel approach by fabricating highly stretchable piezoelectric composite nanofiber films through electrospinning TPU/g-C3N4 mixture. The tight integration of TPU nanofibers with g-C3N4 few-layers pre-stresses g-C3N4 and strengthens the mechanical properties of the composite films, achieving a maximum tensile strain and stress of 862% and 6.90 MPa, respectively. With the assistance of 300 W ultrasound, the photocatalytic capability of the TPU/0.2g g-C3N4 composite nanofiber film is enhanced by 43% and maintains nearly 100% of its initial performance after 12 repeated experiments. The electronic, piezoelectric, and optical properties of uniaxial-strained monolayer g-C3N4 are studied by first-principles calculations, revealing that stretching in the armchair direction can double the in-plane piezoelectric coefficient, while compression in the armchair direction simultaneously alters the charge distribution within the heptazine rings and modulates the adsorption sites and energy for oxygen molecule. Therefore, ultrasound-induced dynamic strains can significantly enhance the photocatalytic effect. The degradation of electronic industrial wastewater demonstrates the practical application potential of the catalytic composite nanofiber film. This research offers a pioneering strategy for the development of efficient photocatalytic systems for sewage treatment.
Similar content being viewed by others
Introduction
Piezo-photocatalysis has garnered significant attention in sewage remediation, offering a novel approach to improve photocatalytic capability through harnessing mechanical energy1,2,3. Piezo-photocatalysis can modulate the effective Schottky barrier by forming polarized charges on the material surface through the piezoelectric potential4. Piezo-photocatalysis can modulate the effective Schottky barrier by forming polarized charges on the material surface through the piezoelectric potential5. When a non-centrosymmetric semiconductor is subjected to ultrasonication, an internal electric field is generated through piezoelectric effect, facilitating the separation of electrons-holes pairs excited by light irradiation. This process is crucial for enhancing the photocatalytic efficiency in wastewater treatment and air purification4,5,6,7. Despite these promising developments, piezo-photocatalysis is still hindered by the limitations of low mechanical energy utilization and poor recyclability, which makes it less viable for widespread practical applications8,9,10,11.
Graphitic carbon nitride (g-C3N4), renowned for being a metal-free polymer with tunable bandgap and highly delocalized π-conjugated system, has emerged as a focal point in the piezocatalytic and photocatalytic field12,13,14,15,16,17. In 2013, Zelisko et al. discussed the piezoelectricity of g-C3N4 using theoretical calculation and discovered that the e11 of 2D g-C3N4 is about 2.18 × 1010 C·m−1 (see ref. 18), which is higher than that of h-BN and similar to monolayer WS2. In 2021, Lin et al. fabricated g-C3N4-based piezoelectric nanogenerators for mechanical energy harvesting and adjusted the intrinsic lattice strain and crystallinity of g-C3N4 for further improving output performance19. These discoveries have positioned g-C3N4 as an emerging research hotspot for piezo-photocatalytic applications20,21,22,23,24. However, g-C3N4 also has some limitations, such as easy aggregation, insufficient charge separation, and low mechanical energy utilization, posing a significant challenge to its sustainability. These inherent limitations underscore the urgent need for innovative strategies to enhance its piezoelectric response and improve its recyclability, thereby ensuring its viability in practical applications.
Electrospinning is an advanced technique for fabricating composite nanofiber films with the advantages of small pore size, large specific surface area, and interconnected pore structure, which has been employed to synthesize several nanofiber photocatalysts25,26,27. This technique involves the use of a high-voltage electric field to draw charged threads of polymer solutions into ultrafine fibers, which can be easily collected on the metal collector to form a flexible nanofiber film28,29,30. Appropriate post-treatment processes, such as calcining or carbonizing the polymer component in the nanofibers, result in an inorganic nanofiber film that can effectively inhibit photocatalyst aggregation and improve recovery efficiency. Recently, researchers have found that electrospun organic−inorganic composite nanofiber films can be directly used for piezo-photocatalysis without any further treatment and exhibit better piezoelectric performance with a longer lifespan31,32,33,34,35,36,37,38. Liu et al. developed a flexible nanofiber film composed of silk fibroin nanofibers doped with piezoelectric LiNbO3 and g-C3N4 nanoparticles through electrospinning, which exhibited good disinfection effect under visible light irradiation34. Zhong et al. fabricated hybrid g-C3N4/Pt-PVDF electrospun nanofiber films for enhancing hydrogen evolution by water-flow-induced piezoelectricity38. However, these nanofiber films typically use PVDF, PAN, or silk fibroin as the substrate, lacking the crucial property of stretchability. Stretchability is essential for enhancing effectiveness in responding to mechanical stimuli and recyclability. The absence of stretchability limits their ability to efficiently harvest mechanical energy from water flow and ultrasound, posing challenges for their practical applications. For stretchable piezo-photocatalytic composite films, the complex relationship between stretchability, nanofiber structure, piezoelectricity, and catalytic capability needs further research.
In this work, a highly stretchable piezo-photocatalytic composite nanofiber film has been fabricated using electrospinning, with thermoplastic polyurethanes (TPU) as the substrate and g-C3N4 as the active catalyst. The high stretchability and mechanical stability of TPU are combined with the few-layered and porous nature of g-C3N4 to achieve a tight integration of TPU/g-C3N4 components within the nanofibers, enhancing structural stability. The porous g-C3N4 few-layers are characterized to confirm the visible light catalytic capability and piezoelectric property. Various amounts of g-C3N4 are incorporated into TPU to investigate the effects of g-C3N4 content on the structure, mechanical property, and piezo-photocatalytic performance of the nanofiber films. The g-C3N4 few-layers are pretensioned by the contraction of TPU during solvent evaporation in the electrospinning process, and theoretical calculations are employed to study the pretension-enhanced piezoelectricity of g-C3N4. The piezo-photocatalytic capability and stability of pure g-C3N4 powder and TPU/g-C3N4 nanofiber films are compared, revealing that although the photocatalytic performance experiences a certain decline due to the encapsulation of g-C3N4 by TPU, the composite nanofiber films still maintain excellent piezo-photocatalytic activity and stability. To further elucidate the mechanism of piezo-photocatalysis, density functional theory (DFT) is used to calculate the effects of uniaxial compressive or tensile strain in different directions on the electronic structure of g-C3N4. The mechanism by which strain enhances photocatalytic effects is studied from the perspectives of band structure, charge separation, light absorption capability, and oxygen adsorption characteristics. It is found that compressive strain in the armchair direction predominantly contributes to the piezo-photocatalytic effect.
Results
Characterizations of porous g-C3N4 few-layers
Figure 1a displays the scanning electron microscopy (SEM) image of the synthesized g-C3N4 nanostructures, revealing a distinctive layered morphology. The transmission electron microscopy (TEM) image further confirms the layered stacking nature of g-C3N4 (Fig. 1b), which is crucial for facilitating efficient charge separation and enhancing photocatalytic activity. Moreover, the high-resolution TEM (HRTEM) image highlights the presence of numerous nanopores distributed throughout the g-C3N4 few-layers (Fig. 1c). These nanopores play an important role in increasing the material’s surface area and are expected to improve the overall photocatalytic efficiency by enhancing light absorption, providing active sites, and facilitating the diffusion of reactants. KOH is a strong alkaline activator for synthesizing porous carbon materials39,40,41. Under high temperature condition, KOH will etch with carbon material, form K2CO3, and release gas, thus forming pores in the carbon material. Further insights into the structure properties of porous g-C3N4 few-layers are obtained through various spectroscopic analyses. Figure 1d presents the electron paramagnetic resonance (EPR) spectrum of g-C3N4, showing a single Lorentzian line at g = 2.001. This type of paramagnetic species originates from unpaired electrons in the localized π-conjugated structure, as opposed to electron pairs in σ-type bonds and the 2pz sub-orbit of bridging nitride atoms in g-C3N4, indicating the presence of numerous defects created during the formation of the nanopores42. X-ray photoelectron spectroscopy (XPS) spectra of g-C3N4 are shown in Fig. 1e−i. Figure 1e shows the survey spectrum, revealing the presence of C, N, and O elements. The C 1 s fine spectrum (Fig. 1f) indicates the presence of N–C = N, C–(N)3, and C–C bonds, which are further confirmed by the N 1 s fine spectrum (Fig. 1g)42,43. The peak at 288.6 eV corresponds to the C–O bond44. The O 1 s fine spectrum (Fig. 1h) is fitted into two peaks at 531.4 and 532.1 eV, which are ascribed to the C = O and C–OH, respectively41,45. It can be concluded from the high content of C–OH that KOH under hear treatment can release –OH groups to extract protons from the carrier by breaking the C–N bond and form C–OH at defect sites41. These results suggest the incorporation of oxygen due to surface oxidation induced by nanopores. Calculated from VB-XPS spectrum (Fig. 1i), the valence band maximum (VBM) is determined to be 1.83 eV, providing insights into the band structure of g-C3N4. Additionally, Fig. 1j shows the absorption edge of g-C3N4 is around 440 nm, indicating its capability to absorb visible light. Figure 1k exhibits the Tauc plot (derived from Fig. 1j), estimating a bandgap value of ~2.897 eV, which is suitable for visible light-driven photocatalysis. Fig. 1l demonstrates the band structure of g-C3N4 relative to the RHE. The conduction band minimum (CBM) of g-C3N4 is more negative than the potential of \({O}_{2}/{\,\cdot\, O}_{2}^{-}\), showing the ability to convert the adsorbed oxygen molecule into superoxide radicals. In contrast, the VBM is not more positive than the potential of \({{OH}}^{-}/\,\cdot\, {OH}\), thus preventing the direct generation of hydroxyl radicals. When subjected to environmental stimuli, the equilibrium state of g-C3N4 is disrupted, exhibiting varying catalytic capabilities (Fig. S1). Under light illumination (Fig. S1a), electrons in the valence band are excited to the conduction band to generate superoxide radicals. The separation of electron−hole pairs is slow, leading to rapid recombination. During ultrasonic treatment (Fig. S1b), the ultrasound-induced dynamic piezoelectric field significantly affects the surface band structure. The potential of the negatively polarized surface shifts in the positive direction, causing the holes in the valence band to combine with hydroxyl groups in water for producing hydroxyl radicals. Under the combined effects of ultrasound and light irradiation (Fig. S1c), the generation, separation, and migration of photogenerated carriers are enhanced, enabling the simultaneous production of large amounts of superoxide and hydroxyl radicals. These comprehensive characterizations confirm the successful synthesis of porous g-C3N4 with a layered structure, abundant defects, and a suitable electronic configuration for enhanced photocatalytic activity, highlighting the potential of porous g-C3N4 few-layer as an advanced material for environmental applications.
The topographical and piezoelectric properties of g-C3N4 are obtained through atomic force microscopy (AFM) and piezoresponse force microscopy (PFM) analyses, as shown in Fig. 2. Figure 2a, b shows the 3D and 2D topographical images of g-C3N4, respectively, demonstrating significant undulations and numerous wrinkles with a maximum height fluctuation of ~220 nm within the 4 μm2 range. These surface features could contribute to the high surface area, tighten the combination of g-C3N4 and TPU, and further boost the photocatalytic efficiency. During the PFM test, a reversible electric field is vertically applied under contact mode to create a local vibration on the specimen for piezoelectric response of g-C3N4. The amplitude image (Fig. 2c) and phase image (Fig. 2d) show an uneven distribution of piezoelectric response across the g-C3N4 sheet. The brightness in the amplitude and phase images corresponds to the intensity and direction of the piezoelectric field, respectively. The regions with higher piezoelectric response correlate with areas of larger strain, suggesting that the piezoelectric characteristics of g-C3N4 are significantly influenced by mechanical deformation. The amplitude-voltage and phase reversal curves are obtained under a bias electric field scanning from −10 to 10 V (Fig. 2e). The characteristic butterfly curve and phase reversal curve further confirm that the few-layer g-C3N4 exhibits robust ferroelectric behavior46,47, which is beneficial for applications that involve mechanical−electrical energy conversion. As a result, the effective piezoelectric coefficient (d11) of g-C3N4 few-layers is calculated to be ~1.8 nm V−1 (Fig. 2f)19,48. The unique wrinkle structure and substantial piezoelectric response suggest the potential application of porous g-C3N4 few-layers in the field of advanced piezoelectric photocatalytic applications.
Characterizations of TPU/g-C3N4 composite nanofiber films
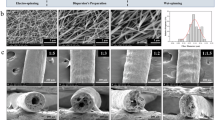
To overcome the challenge of difficult recovery of g-C3N4 powders and improve their practical applicability in piezo-photocatalysis, porous g-C3N4 few-layers are incorporated into TPU nanofibers by electrospinning (Fig. 3a). To demonstrate the stretchability of TPU-based nanofiber films, the stress–strain curves of nanofiber films are provided (Fig. 3b). The electrospinning pure TPU film exhibits a strain of 333% under a stress of 1.66 MPa, demonstrating typical stretchability. After doping with different proportions of g-C3N4 few-layers, both the tolerable stress and strain of the composite films show significant improvement, with the maximum tensile stress and strain reaching 6.90 MPa (for TPU/0.2g g-C3N4) and 862% (for TPU/0.3g g-C3N4), respectively. The full stress−strain curves can be divided into three regions: the elastic region, the plastic region, and the elongation at break49. In the latter part of the plastic region, the TPU/0.3g g-C3N4 composite nanofiber film generates greatest strain under the same stress, thereby inducing a large electric field within the g-C3N4 and resulting in a strong piezoelectric-enhance effect. The TPU/0.2g g-C3N4 composite film withstands the strongest tensile forces and exhibits the most robust response in the elastic region, meeting the requirements for long-term work under high-pressure induced by ultrasonic cavitation effect during piezo-photocatalytic process. These indicate that g-C3N4 plays a crucial role in enhancing the stretchability and stress tolerance of TPU nanofiber films, thereby improving their suitability and reusability as piezo-photocatalysts. It can be inferred that the incorporation of g-C3N4 significantly affects the diameter and distribution of nanofibers, thus modulating the tensile elongation and strength of the nanofiber films, as illuminated by the SEM images (Fig. 3c−f). Viscosity is one of the key factors affecting the electrospinning process and fiber quality38. The viscosity of the precursor solutions with different g-C3N4 concentrations is shown in Fig. S2, indicating that the viscosity decreases with increasing g-C3N4 concentration, which is a primary reason for the reduction in fiber diameter. Furthermore, several beads are observed in the composite films, which are induced by the reduced viscosity and the size difference between g-C3N4 and TPU nanofibers. The g-C3N4 embedded beads not only introduce pretension along the nanofibers50, but also acts as connection points for fiber cross-linking51, which significantly improve the mechanical properties of the composite nanofiber films. In the precursor solution, the TPU polymer can easily infiltrate in the g-C3N4 interlayers benefiting from unique porous/wrinkled structure of g-C3N4 few-layers, forming hydrogen bond interaction between the g-C3N4, TPU, and dimethylacetamide (DMF)51. After the ultrasonic treatment, the average size of g-C3N4 was reduced, which greatly prevent aggregation and ensure homogeneity in their spin dope solution. Owing to the abundant hydrogen bonds between the surface groups of g-C3N4 few-layers, TPU, and DMF, g-C3N4 few-layers can be well dispersed in the precursor solution, leading to the embedded few-layers within the fiber during electrospinning51. The uniform distribution and strong interfacial adhesion between the TPU matrix and g-C3N4 few-layers facilitate effective stress transfer during mechanical deformation, boosting the tensile strength and mechanical stability of the nanofiber films. During the electrospinning process, the rapid evaporation of the solvent and the fast rotation rate of collector induce shear deformation in the TPU52,53,54, which pre-stretches the g-C3N4 few-layers. This pretension may enhance the piezoelectric properties of g-C3N4, further contributing to the overall mechanical robustness of the nanofiber films. Figure 3g displays the actual stretchability and recoverability of the TPU/0.2g g-C3N4 composite nanofiber film. After being stretched beyond 50% and then released, the composite film easily returns to its original size without exhibiting noticeable plastic deformation.
a Schematic diagram of the electrospinning method. b The stress–strain curves. c–f The film SEM image of TPU (c), TPU/0.1g g-C3N4 (d), TPU/0.2g g-C3N4 (e), and TPU/0.3g g-C3N4 (f). g The photographs of TPU/0.2g g-C3N4 composite film in different strains. h FTIR spectra. i XRD patterns. j PL spectra. k Raman spectra.
To further investigate the composition and structure of the TPU/g-C3N4 composite nanofiber films, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction spectroscopy (XRD), Photoluminescence (PL), and Raman analyses are conducted. As shown in the FTIR spectra (Fig. 3h), the absorption peak of g-C3N4 at 810 cm−1 is attributed to the bending vibration of the triazine heterocyclic ring and the characteristic peaks in the range of 1200–1600 cm−1 correspond to the typical stretching vibration of C–N heterocyclic ring55,56,57. The broadband observed in the 3100–3300 cm−1 region is attributed to N–H components of residual amino groups and O–H bonds of adsorbed water58. Pure TPU nanofiber film exhibits characteristic absorption bands at around 3330 cm−1 (O–H or N–H stretching), 1726 cm−1 (C = O stretching), 1528 cm−1 (N–H bending), and 1100 cm−1 (C–O–C stretching)59,60. In the spectra of TPU/g-C3N4 composite nanofiber films, the characteristic bands of both TPU and g-C3N4 are clearly observed, indicating successful incorporation of g-C3N4 into the TPU nanofibers. The XRD spectrum (Fig. 3i) of g-C3N4 powder shows a distinct diffraction peak at 27.34°, corresponding to (002) crystal plane of the inter-planar stacking of the aromatic system61,62. The pure TPU nanofiber film exhibits a broad amorphous peak centered at about 20.52°. In the XRD spectra of the TPU/g-C3N4 composite nanofiber films, the characteristic peak of g-C3N4 is superimposed on the amorphous background of TPU, confirming the dispersion of g-C3N4 within the TPU nanofibers without altering the amorphous nature of TPU. The PL spectra further provide insights into the photoelectric properties of these samples (Fig. 3j). The g-C3N4 powder displays a strong emission peak around 440 nm, indicative of its bandgap energy63. While the pure TPU film shows negligible emission, being non-photoluminescent. The TPU/g-C3N4 composite films also exhibit a notable emission peak around 440 nm, confirming the emission peaks are derived from g-C3N4. Raman spectra (Fig. 3k) reveal the characteristic peaks corresponding to their molecular structures. The g-C3N4 powder shows a spectrum consistent with the results reported in the literature64. The pure TPU nanofiber film exhibits sharp characteristic bands at 1622 and 2787–3076 cm−1, relating to its polymeric structure65. In the Raman spectra of TPU/g-C3N4 composite films, peaks corresponding to both TPU and g-C3N4 are evident. With the increase of g-C3N4 content, the Raman characteristic peaks of TPU are gradually covered by the characteristic peaks of g-C3N4. Based on these results, it is evident that g-C3N4 has been successfully incorporated into the TPU nanofiber films. The characteristic spectral features of both g-C3N4 and TPU are present in the composite films, indicating successful blending and maintenance of the distinctive properties of each component.
Piezo-photocatalysis of TPU/g-C3N4 composite nanofiber films
To investigate the piezo-photocatalytic performance of various TPU/g-C3N4 composite nanofiber films under high stress, the photocatalytic capabilities for methyl blue (MB) degradation are tested under 300 W UV light irradiation alone or in combination with 900 W ultrasound (Fig. S3), highlighting the substantial contribution of the piezoelectric effect in enhancing photocatalytic activity. To thoroughly study the effective piezoelectric photocatalysis of TPU/g-C3N4 nanofiber films, a comparison of the catalytic performance between TPU/0.2g g-C3N4 nanofiber films (21 cm2 in area, containing ~10 mg of g-C3N4) and 10 mg of g-C3N4 powder is conducted. The MB adsorption/desorption equilibrium is achieved within 30 min during the dark process involving nanofiber film (Fig. 4a). The absorbance remains constant over the next 90 min, indicating that MB does not undergo self-degradation by the composite film in the absence of light and ultrasound. Figure 4b compares the photocatalytic, piezocatalytic, and piezo-photocatalytic degradation performances of the composite nanofiber film, and the fitted degradation rate curves are shown in Fig. 4c. Under 300 W ultrasound for 150 min, the MB degradation rate is only 32.19%, demonstrating that the composite film is ineffective for individual piezocatalysis. Under the light irradiation with the same power, the photocatalytic degradation rate is significantly higher (86.04%). When UV light and ultrasound are applied simultaneously, the degradation rate further increases to 93.72%, clearly demonstrating that combined light irradiation and ultrasound can significantly enhance catalytic efficiency. The corresponding reaction rate constants for piezocatalysis, photocatalysis, and piezo-photocatalytic are 0.00192, 0.01309, and 0.01874 min−1, respectively. The g-C3N4 powder is also tested under the same conditions as a control group (Fig. 4d−f). To improve the dispersion of g-C3N4 during the photocatalytic reaction, the solution is mechanically stirred with a rotation speed of 300 rpm. The stirring speed is sufficiently low to avoid significantly affecting the catalytic activity of g-C3N4 powder. The g-C3N4 powder also reaches adsorption equilibrium within 30 min and remains stable for the following 90 min (Fig. 4d). The adsorption capability of g-C3N4 powder is much higher than that of composite film due to the sufficiently exposed surfaces for providing MB adsorption sites. Under ultrasound, light, and combined ultrasound-light conditions for 60 min, the MB degradation rates of g-C3N4 powder are 14.97%, 75.29%, and 81.91%, respectively (Fig. 4e). These degradation rates are higher than those of the nanofiber films, potentially because some active sites on the g-C3N4 within the nanofiber film are covered by TPU matrix. However, the reaction rate constant for piezo-photocatalytic degradation of g-C3N4 powder (0.0285 min−1) is only 22% higher than photocatalysis (0.0233 min−1) (Fig. 4f). In contrast, the ultrasound-induced enhancement of TPU/0.2g g-C3N4 composite film is 43%, suggesting that ultrasound contributes more significantly to the catalytic performance in the nanofiber film.
a–c MB adsorption (a), catalytic degradation (b), and reaction rate constant (c) of the composite film. d–f MB adsorption (d), catalytic degradation (e), and reaction rate constant (f) of porous g-C3N4 few-layers. g–i EPR signal for the generation of superoxide radical (g), hydroxyl radical (h), and singlet oxygen (i) under different conditions.
To elucidate the role of ultrasound-induced piezoelectric field in enhancing the photocatalytic performance of TPU/g-C3N4 composite films, EPR technology is employed to measure the generation of reactive oxygen species (ROSs) of superoxide (\({\cdot O}_{2}^{-}\)), hydroxyl (\(\cdot {OH}\)), and singlet oxygen (1O2) radicals under different conditions (Fig. 4g−i). No radicals are generated under dark conditions, consistent with the previously observed MB degradation performance. Under light irradiation, a significant amount of superoxide radicals is generated (Fig. 4g), which is known to play a crucial role in the photocatalytic degradation process. The hydroxyl radicals are not directly produced (Fig. 4h), and the production of singlet oxygen is relatively minor (Fig. 4i), which is attributed to the fact that the insufficiently positive VBM of g-C3N4 is not effective for the direct oxidation of water to hydroxyl radicals. When ultrasound is applied, a moderate amount of superoxide, hydroxyl, and singlet oxygen radicals are produced, which is attributed to the piezoelectric field induced by the cavitation effect, where the collapse of microbubbles generates localized high temperature and pressure on the nanofibers, facilitating the formation of reactive oxygen species. Under combined light and ultrasound conditions, all three types of radicals are generated in large quantities. The combined application of light and ultrasound significantly enhances the production of reactive oxygen species, substantially boosting overall photocatalytic activity. This synergistic effect can be attributed to the piezoelectric fields generated by the TPU/g-C3N4 composite films under ultrasonic excitation, which facilitates the separation and migration of photogenerated charge carriers, enhancing the generation of radicals. These results underscore the potential of leveraging the synergistic effects of light irradiation and ultrasound to achieve superior catalytic performance in environmental remediation with TPU/g-C3N4 composite nanofiber films.
The piezocatalytic and piezo-photocatalytic efficiencies are highly dependent on the magnitude of the piezoelectric field. Continuous ultrasonic irradiation generates numerous cavitation bubbles, whose collapse exerts significant pressure on the piezoelectric material, thereby inducing a periodic built-in electric field5. The increase of ultrasonic power can significantly increase the piezoelectric field inside g-C3N4, which is conducive to the piezoelectric-related catalytic reaction. To investigate the influence of ultrasonic power on the piezoelectric effect, piezocatalytic tests are conducted at ultrasonic power of 300, 500, and 700 W, as shown in Fig. 5a. The piezocatalytic degradation efficiency of MB increases with ultrasound power due to the stronger cavitation effects and more intense thermal excitation. The reaction rate constants for the three conditions are calculated to be 0.00192, 0.00422, and 0.00584 min−1, respectively (Fig. 5b), which are substantially lower than those observed for low-power photocatalysis. Applying 300 W light irradiation along with ultrasound on the composite nanofiber films significantly enhances degradation efficiency (Fig. 5c). Under the coupling effect of 300 W light irradiation and 700 W ultrasound, the degradation rate of MB reaches 93.31% within 60 min, achieving deep purification of MB sewage. The reaction rate constants correspondingly increase to 0.01874, 0.02729, and 0.04507 min−1 (Fig. 5d), which are 9.7, 6.4, and 7.7 times higher than those without UV light, respectively. This suggests that when the power levels of light and ultrasound are comparable, the enhancement in photocatalytic performance is more pronounced.
The reusability of photocatalysts is an important factor in evaluating their potential for practical applications. Cycling piezo-photocatalytic experiments for MB degradation are carried out to investigate the reusability of the prepared composite films (Fig. 5e). After 12 cycles, the TPU/g-C3N4 composite film maintains a deep-degradation efficiency of nearly 100% under the synergistic effect of 300 W light irradiation and 900 W ultrasound for 120 min, demonstrating the excellent stability and reusability of the TPU/g-C3N4 composite film. To further investigate the stability, FTIR, XRD, Raman, and PL analyses are conducted on the post-experimental TPU/g-C3N4 composite film, confirming that the TPU/g-C3N4 composite nanofiber film remains virtually unchanged after the experiment (Fig. S4).
DFT calculations
Strain strategy is an effective method to modulate the electronic and piezoelectric properties of two-dimensional materials66. To reveal the mechanism of strain-enhanced photocatalytic activity, strained g-C3N4 is investigated by first-principles calculation. Stolbov et al. indicated that the electronic structure of g-C3N4 is predominantly determined by strong in-plane C–N covalent bonds, with interlayer van der Waals (vdW) interactions having minimal impact67. Therefore, to reduce computational load, monolayer g-C3N4 is selected for the calculations. Figure S5a illustrates the top and side views of the optimized structures of pristine monolayer g-C3N4, consisting of six C atoms and seven N atoms arranged in a heptazine-based network and forming a planar and porous structure with a stable hexagonal pattern. Based on the symmetry, there are three nonequivalent nitrogen atoms and two nonequivalent carbon atoms, labelled N1, N2, N3, C1, and C2, respectively. The bond lengths and bond angles are presented in Tables S1 and S2, respectively, which are closely consistent with the theoretical results68,69,70. Figure S5b−d demonstrates the atomic structure of monolayer g-C3N4 under deformation of X1.04, X0.96, and Y0.96, respectively. Under the above deformation conditions, the bond lengths and bond angles slightly change without destroying the symmetry, indicating the crystal structure remains stable. It is noticed that strain in both the zigzag and armchair directions does not induce out-of-plane displacement, allowing for the consideration of only in-plane effects in subsequent calculations.
As a non-centrosymmetric 2D material with hexagonal symmetry and point group 6̅m2, only in-plane strain components are considered using Voigt notation, thus the elastic tensor (Cijk), strain tensor (dijk), and piezoelectric stress tensor (eijk) are simplified to second-rank matrix (Cij, dij, and eij) as Eq. (1),
suggestion that there is only one piezoelectric strain constant (d11) along the zigzag direction, as derived by Eq. (2)71.
The piezoelectric characteristics of monolayer g-C3N4 under different strains are calculated, as shown in Table S3. It is clearly demonstrated that the tensile strain along armchair direction undergoes a significant enhancement of the in-plane piezoelectric coefficient. When the tensile strain reaches 4%, the piezoelectric coefficient is twice that of the unstrained state. This indicates that pretension induced by electrospinning can enhance photocatalytic activity by improving the piezoelectric electric field.
The strain-induced changes in atomic position can affect the coupling strength of orbital coupling, thereby leading to changes in the bandgap of g-C3N4. Figure S6a shows the band structures of the monolayer g-C3N4 without strain. The calculated band structure of pristine monolayer g-C3N4 reveals an indirect bandgap of ~1.24 eV, with the VBM located at the Γ point and the CBM positioned between the Γ and M points. The calculated bandgap is much lower than the experimental value (2.897 eV in Fig. 1k), which results from the limitation of GGA functional and does not affect the accuracy of the electronic structure calculations. Figure S6b−d show the band structures of g-C3N4 under different uniaxial strains. The deformation along different directions induces significant variation in the band structure. Under X1.04 strain, the edge states of the valence band at the Γ point shift towards lower energy and the VBM moves to near K point, resulting in a larger indirect bandgap (Fig. S6b). Conversely, applying a 4% compressive strain in either the X-axis (Fig. S6c) or Y-axis (Fig. S6d), the conduction band edge at Γ point moves closer to Fermi level, leading to a decreased bandgap value. The VBM remains stationary, while the CBM shifts towards Γ point, reaching a near-direct bandgap at the Γ−Γ point, facilitating the absorption of visible light and the transition of photogenerated electrons.
The total density of states (TDOS) and projected DOS (PDOS) are investigated near the Fermi level to gain deeper insights into the effects of uniaxial strain on the electronic structure of g-C3N4 (Figs. S7 and S8). Figure S7a indicates that the VBM is primarily composed of N 2p orbitals, while the CBM is mainly formed by a combination of N 2p and C 2p orbitals, confirming the strong covalent bonding between carbon and nitrogen atoms within the pristine g-C3N4 lattice. Strain has a regulatory effect on the distribution of TDOS in valence bands, while impacting little on the orbital contributions of individual atoms (Fig. S7b−d). No impurity energy levels crossing the Fermi level can be observed, indicating that the strained g-C3N4 retains semiconductor property.
To facilitate an intuitive understanding of the band edge composition, the partial charge density at CBM and VBM of monolayer g-C3N4 is depicted in Fig. S9. Figure S9a presents the partial charge density profiles of the CBM and VBM of monolayer g-C3N4. It can be observed that the VBM is occupied by all N2 atoms, while the CBM is predominantly localized on the C1 and C2 atoms with moderate distribution over the N1 and N2 atoms. Notably, the tri-coordinated bridging nitrogen atom (N3) does not participate in either the valence band or the conduction band. Under appropriate photon excitation, the electrons can neither be excited from the N3 atom nor be promoted from near atoms to the N3 atom, which also hinders electron transfer between adjacent heptazine units. Consequently, the photogenerated electrons and holes are confined within isolated heptazine units, preventing their effective separation and leading to a high likelihood of recombination. This is another factor that limits the photocatalytic activity of intrinsic g-C3N4. When strain is applied to g-C3N4, there is a significant change in the charge distribution of VBM. The charge distribution changes significantly under strain along the X-axis, particularly showing obvious charge separation at the N3 and C1 atoms (Fig. S9b). Owing to the asymmetric heptazine units along zigzag direction, the positive and negative charge centers of the structure undergo a directional shift, which can even reverse the electric dipole moment by tensile stress (Fig. S9c). Under the armchair strain, the electric dipole moment of one side is depressed, while the other side is strengthened, thereby endowing the structure with favorable piezoelectric properties. This will greatly facilitate charge separation and transfer in g-C3N4, thereby enhancing its photocatalytic performance. The charge distribution changes little when strain is applied along the Y-axis (Fig. S9d), which is ascribed to the symmetric structure along the zigzag direction.
To understand the charge transfer mechanisms during the photocatalytic processes, the strain-modulated work functions (Φ) of monolayer g-C3N4 are calculated by the formula Φ = Evac − EF, where Evac and EF denote the stationary charge energy in vacuum and the Fermi level of the g-C3N4, respectively. The work function of pristine g-C3N4 is ~4.73 eV (Fig. S10a), which is close to the standard hydrogen energy level, indicating that it can effectively participate in superoxide radical or hydrogen evolution reactions. Under strain conditions, the work function experiences slight changes with a difference of less than 0.5 eV (Fig. S11). Dielectric function bridges the inter-band transition and solid electronics. Within the linear response range, the dielectric function is expressed as the formula ε(ω) = ε1(ω) + ε2(ω), where ε1(ω) and ε2(ω) represent the real and imaginary parts of complex dielectric function, correlating with the energy harvesting and light absorption properties of g-C3N4, respectively (Fig. S12). Based on the complex dielectric functions, the light absorption coefficient (α(ω)) can be calculated by Eq. (3).
Figure S10b depicts the wavelength-dependent curves of the optical absorption coefficients of strained g-C3N4. The optical absorption property of pristine g-C3N4 exhibits significant absorption in the UV range and moderate absorption in the VIS range (<540 nm), suggesting an excellent candidate for UV–VIS photocatalytic applications. The absorption spectra of g-C3N4 under compressive strains exhibit red shifts, whereas the shift occurs in the opposite direction under tensile strain, which is consistent with the changes observed in the bandgap.
The generation of free radicals is a crucial step in the photocatalytic process. Due to the unique band structure of g-C3N4, only singlet oxygen and superoxide radicals, but not hydroxyl radicals, can be generated under light irradiation. Consequently, the adsorption of oxygen molecules on g-C3N4 significantly impacts its photocatalytic activity. Experimental methods face challenges in determining the configurations, distances, strengths, and other aspects of adsorption. Therefore, first-principles calculation is employed to investigate the effect of uniaxial strain on the oxygen adsorption on monolayer g-C3N4 (Fig. 6). The O2-adsorption model is built by placing an individual O2 molecule on the surface of g-C3N4 in theoretical simulations. The oxygen adsorption code is initially determined using the adsorption locator module, suggesting the possible adsorption sites for both pristine (Fig. S13) and strained (Fig. S14) g-C3N4. In this case, three sites (center of the heptazine ring, center of the six-fold cavity, and bridge N3 between two heptazine rings) and the horizontal mode are designated for precise adsorption calculations (Fig. S15).
Table S4 shows the calculation results of adsorption energy for all the O2-g-C3N4 adsorption models. All the adsorption energy values are negative, indicating that the adsorption of O2 onto g-C3N4 is exothermic. O2 is most likely to adsorb at site 2 in pristine g-C3N4 (Fig. 6a). However, both the adsorption energy and adsorption site are significantly modulated by strain. Under X1.04 strain, the adsorption energy nearly approaches zero, indicating a weakened adsorption capability (Fig. 6b). In contrast, under compressive strain, the adsorption energy becomes more negative and reaches its minimum at X0.96 strain (Fig. 6c), which suggests easier chemical adsorption and facilitates the generation of superoxide radicals. Interestingly, under compressive strain, the optimal adsorption site shifts from site 2 to site 1, indicating a change in oxygen adsorption from the central carbon atom to the cavity. The adsorption of oxygen does not induce out-of-plane deformation of g-C3N4, except when the adsorption occurs at site 1 and 2 under Y0.96 strain (Fig. 6d).
Practice application in sewage treatment
Based on the excellent performance of electrospun TPU/g-C3N4 composite nanofiber film, we have developed a piezo-photocatalytic sewage treatment system consisting of an ultrasonic cell, a light source array, and a film module (Fig. 7a). The reaction cell with a volume of 13.5 L (30 cm × 30 cm × 15 cm) is supported by six ultrasonic oscillators with 60 W power. A 300 W UV lamp with 324 LEDs and a center wavenumber of 365 nm is placed on top of the ultrasonic cell (Fig. 7b). A piece of TPU/g-C3N4 composite film (24 cm × 32 cm) is fixed at the bottom of reaction cell. By performing piezo-photocatalytic treatment on refractory electronic industrial sewage, the chemical oxygen demand (COD) value decreases from 15,000 to 4300 mg L−1 after 150 min, meeting the standard required for entry into biochemical treatment processes. Energy consumption is one of the critical factors in transitioning new water treatment technologies from laboratory research to practical applications72. Figure 7c compares the energy consumptions of photocatalytic, piezocatalysis and piezo-photocatalysis based on the composite nanofiber film for treating refractory electronic industrial sewage. The energy consumption (EE/O) is calculated by Eq. (4)73,
where W is the total input energy containing light power (300 W) and ultrasound power (60 W), V is the sewage volume (13.5 L), and C0/Ct is the ratio of the initial and final COD value. The results demonstrates that piezo-photocatalysis exhibits lower energy consumption compared to photocatalysis and piezocatalysis. This demonstrates that electrospun TPU/g-C3N4 piezo-photocatalytic composite films have broad application prospects in the treatment of highly challenging wastewater, potentially making a positive contribution to environmental protection and sustainable development.
Discussion
In summary, this study presents a groundbreaking approach to enhancing the efficiency and stability of piezo-photocatalysts by electrospinning highly stretchable TPU/g-C3N4 composite nanofiber films. Employing the pretension of g-C3N4 by TPU matrix, this approach highlights the effectiveness of ultrasound in amplifying piezoelectric effects, which are crucial for driving the enhanced catalytic processes. The TPU/g-C3N4 composite nanofiber films demonstrate excellent reusability and structural integrity, maintaining high catalytic performance over 12 cycles. Such durability underscores their potential for practical environmental applications, offering an efficient and sustainable solution for wastewater treatment. Supported by experimental validations and theoretical calculations, the excellent properties of the nanofiber catalytic film can be attributed to two key aspects: The tight integration of TPU and g-C3N4 enables the composite film to withstand greater stress and experience larger strain, thus enhancing mechanical energy utilization and film stability; Ultrasound-driven dynamic compressive and tensile strains improve photocatalytic activity by enhancing the separation and transfer of photogenerated charges and simultaneously adjusting the adsorption types and sites of oxygen molecules. This study not only advances the understanding of the integration of piezoelectric and photocatalytic effects, but also paves the way for developing next-generation catalytic materials with superior performance and reliability in environmental remediation.
Methods
Synthesis of porous g-C3N4 few-layers
The porous g-C3N4 few-layers were synthesized by pyrolysis of urea in air. Typically, 10 g of urea was dissolved in 100 mL of KOH aqueous solution (6 M), heated at 80 °C for 20 min, and dried in oven. Then, the powder was placed inside a sealed alumina crucible in a Muffle furnace (KSL–1100X–S, MTI, China). The temperature slowly increased with a heating rate of 1 °C min−1 and held at 550 °C for 2 h. After cooling down to room temperature, the resulting yellow powder was harvested for subsequent utilization. To pulverize g-C3N4 bulk into g-C3N4 few-layers, 100 mg of yellow powder was dispersed in 100 mL of isopropanol at room temperature and stripped for 11 h using an ultrasonic cell shredder with a power of 1 kW under a controlled temperature of 20 °C. The resulting turbid liquid was centrifuged at 8000 rpm for 10 min in a high-speed centrifuge (HC–3018, Zonkia, China). Finally, the supernatant was reserved and dried in air at 50 °C for 12 h, yielding pale yellow porous g-C3N4 few-layers.
Preparation of TPU/g-C3N4 composite nanofiber film
In total, 3 g of TPU powder was added into 17 mL of DMF dispersed with g-C3N4 few-layers and intensely stirred for 14 h at room temperature to obtain a uniform colloid suspension. To optimize the concentration, 0.1, 0.2, and 0.3 g of g-C3N4 were used, separately. In all, 10 mL of the colloid suspension was filled into a syringe equipped with a metal needle (17-gauge) and secured to a microinjection pump. A metallic rotating collector was positioned 10 cm away from the needle, and a voltage of 20 kV was applied between the needle and the collector. During the electrospinning process, the feed rate of injection pump and the rotation speed of collector were set to 1 mL h−1 and 1500 rpm, respectively. After electrospinning, the TPU/g-C3N4 composite nanofiber film was peeled off from the collector and naturally dried in air for 24 h to fully evaporate the DMF solvent and achieve pretension of g-C3N4.
Characterization
The morphologies of the samples were observed by SEM (Regulus 8230, Hitachi, Japan) and TEM (JEM − F200, JEOL, Japan). The crystal structures were studied by XRD (Miniflex 600, Rigaku, Japan). The recombination rates of photoinduced charge carriers were investigated by a PL (FLS1000, Edinburgh, UK). The functional groups were characterized by FTIR (Nicolet iS20, Thermo Scientific, USA). The elemental analyses were obtained by XPS (ESCALAB 250Xi, Thermo Scientific, USA). Raman spectra were performed by a homemade set with an excitation wavelength of 785 nm. The UV–VIS absorption spectra were acquired by a diffuse reflectance spectrometer (DRS, U4150, Hitachi, Japan). The tensile properties were measured using an electronic universal testing machine (5969, Instron, USA) with a tensile rate of 0.5 mm min−1 at room temperature. For standardized fabrication, the films of uniform thickness were cut into strips with a width of 2 cm and a length of 10 cm. The piezoelectric and ferroelectric properties of the porous g-C3N4 few-layers were investigated using PFM (NT–MDT, Ntegra Spectra, Russia). The radical generation rates under light irradiation, ultrasound, and the combined ultrasound-light excitation were obtained by EPR (200 M, CIQTEK, China).
Piezo-photocatalytic tests
The catalytic capabilities of the nanofiber films were assessed by degrading MB. Typically, 250 mg of nanofiber film (6 cm × 6 cm in area) was immersed in 50 mL of MB aqueous solution (5 g L−1). Prior to the catalytic reaction, the solution was slowly stirred in dark for 30 min to achieve adsorption−desorption equilibrium. For piezo-photocatalysis, light was supplied by a 300 W mercury lamp, and ultrasound was provided by an ultrasonic cell crusher (SCIENTZ–IID, China) at a frequency of 20 kHz with power settings of 300, 500, and 700 W. During the experiments, the solution was continuously exposed to light, ultrasound, or both for photocatalysis, piezocatalysis, and piezo-photocatalysis, respectively. Once the reaction began, 3 mL of MB solution was extracted every 30 min, and the absorbance was measured using a UV–VIS spectrophotometer (M5, MAPADA, China). To raise the data reliability, all the catalytic tests were repeated five times. Reproducibility tests were conducted with each catalytic experiment lasting for 2.5 h. After each experiment, the nanofiber film was thoroughly rinsed with deionized water and dried before repeating the catalytic process.
DFT calculation
The electronic structure of monolayer g-C3N4 was calculated using the VASP software. The interactions between ions and electrons were described using the projected augmented wave (PAW) method. The exchange-correlation potential was characterized using the generalized gradient approximation (GGA) combined with the Perdew−Burke−Ernzerhof (PBE) method. The atomic structure of monolayer g-C3N4 was obtained from the Materials Project (hexagonal, mg-1193580). The first Brillouin zone was sampled using a 14 × 8 × 1 Monkhorst−Pack k-point grid. The BFGS algorithm was employed to optimize the structure until atomic forces were less than 2 × 10−4 Ha, and the plane-wave cutoff energy was set to 700 eV. To eliminate the influence of periodic condition between adjacent layers, the thickness of the vacuum layer was set to 20 Å.
After optimizing the initial structure of monolayer g-C3N4, small uniaxial tensile and compressive strains were applied along the armchair or zigzag directions. The strain (ε) was calculated by the formula ε = (a − a0)/a0, where a and a0 represented the lattice constants under strained and unstrained conditions, respectively. Positive and negative values of strain indicated tensile and compressive strain, respectively. The strain components along the armchair and zigzag direction were denoted as X1+ε and Y1+ε, respectively. Considering the strain effects on phase transition and structural stability, ε = 0.04 was selected as a representative value for subsequent calculations. Therefore, the compressive strain in the armchair direction, the tensile strain in the armchair direction, and the compressive strain in the zigzag direction were denoted as X0.96, X1.04, and Y0.96, respectively.
The calculations of oxygen adsorption on strained monolayer g-C3N4 were implemented in the DMol3 code and the Forcete code. The electronic exchange and correlation effects were described using GGA–PBE, and the double numeric plus polarization (DNP) basis set was employed. An orbital occupation value of 0.005 Ha was used. The convergence tolerances for energy change, maximum force, and maximum displacement were set to 1.0 × 10−5 Ha, 0.002 Ha Å−1, and 0.005 Å, respectively. A 3 × 3 × 1 k-point mesh was employed for Brillouin zone integration based on the Monkhorst−Pack scheme. Regarding the confirmation of adsorption sites, three adsorption configurations were identified by screening in the adsorption location code. These adsorption sites were subsequently reoptimized using the DMol3 code.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhou, X. F., Shen, B., Lyubartsev, A., Zhai, J. W. & Hedin, N. Semiconducting piezoelectric heterostructures for piezo- and piezo-photocatalysis. Nano Energy 96, 107141 (2022).
Wang, M. Y. et al. Remarkably enhanced hydrogen generation of organolead halide perovskites via piezocatalysis and photocatalysis. Adv. Energy Mater. 9, 1901801 (2019).
Liang, Z., Yan, C. F., Rtimi, S. & Bandara, J. Piezoelectric materials for catalytic/photocatalytic removal of pollutants: Recent advances and outlook. Appl. Catal. B-Environ. 241, 256–269 (2019).
Hu, C. et al. Coupling piezocatalysis and photocatalysis in Bi4NbO8X (X = Cl, Br) polar single crystals. Adv. Funct. Mater. 30, 1908168 (2020).
Tu, S. C. et al. Piezocatalysis and piezo-photocatalysis: catalysts classification and modification strategy, reaction mechanism, and practical application. Adv. Funct. Mater. 30, 2005158 (2020).
Singh, S. & Khare, N. Coupling of piezoelectric, semiconducting and photoexcitation properties in NaNbO3 nanostructures for controlling electrical transport: Realizing an efficient piezo-photoanode and piezo-photocatalyst. Nano Energy 38, 335–341 (2017).
Ren, Q. et al. Adjusting photocatalytic hydrogen production of TiO2 hollow nanospheres through nitrogen-induced shallow trapping levels. J. Adv. Dielectr. 15, 2450005 (2024).
Feng, Y., Li, J. & Yan, H. Synthesis of BiFeO3/ZnO heterjunction with enhanced piezocatalytic performance for highly-efficient degradation of organic pollutant. J. Adv. Dielectr. 15, 2450023 (2025).
Liu, T. et al. Efficient photocatalytic degradation of statin via optimization on ZnIn2S4/Bi2WO6 Z-scheme heterostructure. J. Adv. Dielectr. 14, 2440020 (2024).
Yang, K. et al. Surface structural transformation of Ni2P@C electrocatalysts for overall alkaline water splitting. Prog. Nat. Sci. Mater. Int. 34, 102–107 (2024).
Yu, G. L. et al. S-scheme heterojunction construction of Fe/BiOCl/BiVO4 for enhanced photocatalytic degradation of ciprofloxacin. Prog. Nat. Sci. Mater. Int. 34, 290–303 (2024).
Wang, Y., Wang, X. C. & Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 51, 68–89 (2012).
Zhang, J. S., Chen, Y. & Wang, X. C. Two-dimensional covalent carbon nitride nanosheets: synthesis, functionalization, and applications. Energy Environ. Sci. 8, 3092–3108 (2015).
Ong, W. J., Tan, L. L., Ng, Y. H., Yong, S. T. & Chai, S. P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem. Rev. 116, 7159–7329 (2016).
Wang, J. L. & Wang, S. Z. A critical review on graphitic carbon nitride (g-C3N4)-based materials: preparation, modification and environmental application. Coord. Chem. Rev. 453, 214338 (2022).
Hu, C. et al. Exceptional cocatalyst-free photo-enhanced piezocatalytic hydrogen evolution of carbon nitride nanosheets from strong in-plane polarization. Adv. Mater. 33, 2101751 (2021).
Xu, R. J., Cui, L. F. & Kang, S. F. Countering microplastics pollution with photocatalysis: challenge and prospects. Prog. Nat. Sci. Mater. Int. 33, 251–266 (2023).
Zhang, T. J. et al. The photocatalytic hydrogen evolution of g-C3N4/K0.5Na0.5NbO3 nanofibers heterojunction under visible light. J. Photochem. Photobiol. A 435, 114192 (2023).
Wang, R. C., Lin, Y. C., Chen, H. C. & Lin, W. Y. Energy harvesting from g-C3N4 piezoelectric nanogenerators. Nano Energy 83, 105743 (2021).
Shao, Y. et al. Piezocatalytic performance difference of graphitic carbon nitride (g-C3N4) derived from different precursors. Chem. Phys. Lett. 801, 139748 (2022).
Tang, R. et al. Unique g-C3N4/PDI-g-C3N4 homojunction with synergistic piezo-photocatalytic effect for aquatic contaminant control and H2O2 generation under visible light. Appl. Catal. B-Environ. 303, 120929 (2022).
Vuong, H.-T. et al. Defect engineering of porous g-C3N4 to add multifunctional groups for enhanced production of H2O2 via piezo-photocatalysis. ACS Appl. Nano Mater. 6, 664–676 (2023).
Xu, T. et al. Constructing crystalline g-C3N4/g-C3N4-xSx isotype heterostructure for efficient photocatalytic and piezocatalytic performances. Energy Environ. Mater. 6, e12306 (2023).
Zhai, H. et al. Flexible construction of heteroatom-free g-C3N4/g-C3N4 homojunction with switching charge dynamics toward efficient photo-piezocatalytic performance. Appl. Catal. B-Environ. Energy 349, 123909 (2024).
Xue, J. J., Wu, T., Dai, Y. Q. & Xia, Y. N. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem. Rev. 119, 5298–5415 (2019).
Xue, J. J., Xie, J. W., Liu, W. Y. & Xia, Y. N. Electrospun nanofibers: new concepts, materials, and applications. Acc. Chem. Res. 50, 1976–1987 (2017).
Shi, Q. X. et al. Controllable preparation of MnCo2O4 spinel and catalytic persulfate activation in organic wastewater treatment: experimental and immobilized evaluation. Prog. Nat. Sci. Mater. Int. 34, 776–786 (2024).
Xia, G. B., Song, B. B. & Fang, J. Electrical stimulation enabled via electrospun piezoelectric polymeric nanofibers for tissue regeneration. Research 2022, 9896274 (2022).
Wang, Z. X., Tai, Y. Y., Ye, Z. Y., Nam, J. & Yin, Y. D. Integration of ZnS: Mn2+ microparticles into electrospun PVDF-based nanofibers for enhanced mechanoluminescence. Adv. Funct. Mater. 34, 2410358 (2024).
Chen, S. S., Wang, C. H., Xu, D. Y. & Li, J. S. HKUST-1 derived one-dimensional Cu/N-doped carbon nanofibers for simultaneous detection of acetaminophen and sulfanilamide. Prog. Nat. Sci. Mater. Int. 33, 825–832 (2023).
Ding, D. et al. Piezo-photocatalytic flexible PAN/TiO2 composite nanofibers for environmental remediation. Sci. Total Environ. 824, 153790 (2022).
Verma, S., Sharma, M. & Vaish, R. Photo-piezocatalysis in electrospun PVDF + WS2 membrane. Environ. Sci. Nano 9, 3885–3899 (2022).
Yin, J. Y. et al. Piezoelectric field enhanced photocatalytic efficiency of PVDF/TiO2 core/shell nanofibrous membrane via coaxial electrospinning. J. Environ. Chem. Eng. 11, 110298 (2023).
Wang, Z. K. et al. A versatile silk fibroin based filtration membrane with enhanced mechanical property, disinfection and biodegradability. Chem. Eng. J. 426, 131947 (2021).
Porwal, C., Verma, S., Gaur, A., Chauhan, V. S. & Vaish, R. Bi2ZnB2O7–PVDF electrospun composite membrane for waste water treatment utilizing photo-piezocatalysis. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 298, 116842 (2023).
Wang, Z. C. et al. A novel ZnO/CQDs/PVDF piezoelectric system for efficiently degradation of antibiotics by using water flow energy in pipeline: performance and mechanism. Nano Energy 107, 108162 (2023).
Orudzhev, F. et al. Ultrasound and water flow driven piezo-phototronic effect in self-polarized flexible α-Fe2O3 containing PVDF nanofibers film for enhanced catalytic oxidation. Nano Energy 90, 106586 (2021).
Chen, M. et al. Enhanced hydrogen evolution in porous and hybrid g-C3N4/Pt-PVDF electrospun membranes via piezoelectricity from water flow energy. Adv. Funct. Mater. 34, 2402477 (2024).
Zhang, H. et al. Improved H2O2 photogeneration by KOH-doped g-C3N4 under visible light irradiation due to synergistic effect of N defects and K modification. Appl. Surf. Sci. 527, 146584 (2020).
Shi, S. & Liu, Y. Nitrogen-doped activated carbons derived from microalgae pyrolysis by-products by microwave/KOH activation for CO2 adsorption. Fuel 306, 121762 (2021).
Liang, X., Dong, F., Tang, Z. & Wang, Q. Surface hydroxy functionalized Pt/g-C3N4-CNS for highly efficient methanol electrocatalytic oxidation. Mol. Catal. 530, 112638 (2022).
Xia, P. F., Cheng, B., Jiang, J. Z. & Tang, H. Localized π-conjugated structure and EPR investigation of g-C3N4 photocatalyst. Appl. Surf. Sci. 487, 335–342 (2019).
Zhu, Y. Q., Wang, T., Xu, T., Li, Y. X. & Wang, C. Y. Size effect of Pt co-catalyst on photocatalytic efficiency of g-C3N4 for hydrogen evolution. Appl. Surf. Sci. 464, 36–42 (2019).
Jiang, Y. et al. Enhancement of photocatalytic hydrogen evolution activity of porous oxygen doped g-C3N4 with nitrogen defects induced by changing electron transition. Appl. Catal. B-Environ. 240, 30–38 (2019).
Yang, Y. & Bian, Z. Oxygen doping through oxidation causes the main active substance in g-C3N4 photocatalysis to change from holes to singlet oxygen. Sci. Total Environ. 753, 141908 (2021).
He, Q. S., Yi, Y. Y., Shi, W. J., Sun, P. F. & Dong, X. P. Determination of the key role to affect the piezocatalytic activity of graphitic carbon nitride for tetracycline hydrochloride degradation in water. Chemosphere 317, 137828 (2023).
Liang, F. J., Chen, Z. W., Lu, Z. Y. & Wang, X. Piezoelectric-enhanced photocatalytic performance of porous carbon nitride nanosheets. J. Colloid Interface Sci. 630, 191–203 (2023).
Wu, T. et al. Enhanced piezo-photocatalytic degradation of organic pollutants by cambered wall lamellar structure of porous tubular g-C3N4. Nano Energy 120, 109137 (2024).
Tong, L. et al. Electrically conductive TPU nanofibrous composite with high stretchability for flexible strain sensor. Nanoscale Res. Lett. 13, 86 (2018).
Lee, Y. et al. Enhanced mechanical properties and pre-tension effects of polyurethane (PU) nanofiber filaments prepared by electrospinning and dry twisting. J. Polym. Res. 19, 9774 (2012).
Liu, L. et al. Mechanically and environmentally robust composite nanofibers with embedded MXene for wearable shielding of electromagnetic wave. Compos. Commun. 30, 101094 (2022).
Le, B. et al. Flexible piezoelectric PVDF/TPU nanofibrous membranes produced by solution blow spinning. J. Mater. Res. Technol. 24, 5032–5041 (2023).
Mi, H.-Y., Jing, X., Jacques, B. R., Turng, L.-S. & Peng, X.-F. Characterization and properties of electrospun thermoplastic polyurethane blend fibers: effect of solution rheological properties on fiber formation. J. Mater. Res. 28, 2339–2350 (2013).
Sun, H. et al. Largely improved toughness of poly(lactic acid) by unique electrospun fiber network structure of thermoplastic polyurethane. Polym. Test. 64, 250–253 (2017).
Yang, J., Song, H. Y., Xu, O. W., Wan, S. Y. & Zhu, X. S. Preparation of g-C3N4@bismuth dihalide oxide heterojunction membrane and its visible light catalytic performance. Appl. Surf. Sci. 583, 152462 (2022).
Naseri, A., Samadi, M., Pourjavadi, A., Ramakrishna, S. & Moshfegh, A. Z. Enhanced photocatalytic activity of ZnO/g-C3N4 nanofibers constituting carbonaceous species under simulated sunlight for organic dye removal. Ceram. Int. 47, 26185–26196 (2021).
Xue, X. L. et al. Facile fabrication of three-dimensional nanofibrous foams of cellulose@g-C3N4@Cu2O with superior visible-light photocatalytic performance. Carbohydr. Polym. 303, 120455 (2023).
Yao, L. T. et al. Enhancement of AFB1 removal efficiency via adsorption/photocatalysis synergy using surface-modified electrospun PCL-g-C3N4/CQDs membranes. Biomolecules 13, 550 (2023).
Cui, Z. X. et al. Electrospinning preparation of TPU/TiO2/PANI fiber membrane with enhanced dye degradation and photocatalytic Cr(VI) reduction. Colloids Surf. A 664, 131111 (2023).
Li, S. J., Zhang, C. D., Yu, Q. Z. & Jia, L. Functional polyaspartic acid fibers hydrogel membrane with enhanced mechanical performance prepared by coaxial electrospinning. Mater. Res. Express 8, 036407 (2021).
Yuan, F. S. et al. Cross stacking faults in Zr(Fe,Cr)2 face-centered cubic Laves phase nanoparticle. Appl. Surf. Sci. 513, 145716 (2020).
Ismael, M. Facile synthesis of NiO-loaded g-C3N4 heterojunction photocatalyst for efficient photocatalytic degradation of 4-nitrophenol under visible light irradiation. J. Photochem. Photobiol. A 439, 114576 (2023).
Chen, Y. X. et al. The collision between g-C3N4 and QDs in the fields of energy and environment: synergistic effects for efficient photocatalysis. Small 19, 2205902 (2023).
Wang, D., Sun, H., Luo, Q., Yang, X. & Yin, R. An efficient visible-light photocatalyst prepared from g-C3N4 and polyvinyl chloride. Appl. Catal. B-Environ. 156, 323–330 (2014).
Xin, G., Rong, Y., Huang, Y. & Wu, C. Process and mechanism of cutting thermoplastic polyurethane (TPU) film by nanosecond ultraviolet laser. J. Mater. Sci. 56, 16167–16180 (2021).
Guo, S. D., Guo, X. S., Zhang, Y. Y. & Luo, K. Small strain induced large piezoelectric coefficient in α-AsP monolayer. J. Alloy. Compd. 822, 153577 (2020).
Stolbov, S. & Zuluaga, S. Sulfur doping effects on the electronic and geometric structures of graphitic carbon nitride photocatalyst: insights from first principles. J. Phys. Condens. Matter 25, 085507 (2013).
Liu, Y. et al. High-efficiency and selective capture of nitric oxide by fluorine-modified carbon nitride: A DFT investigation. Appl. Surf. Sci. 593, 153353 (2022).
Wang, Y., Tian, Y., Yan, L. & Su, Z. DFT study on sulfur-doped g-C3N4 nanosheets as a photocatalyst for CO2 reduction reaction. J. Phys. Chem. C 122, 7712–7719 (2018).
Zhang, S. et al. Enhanced photodegradation of toxic organic pollutants using dual-oxygen-doped porous g-C3N4: mechanism exploration from both experimental and DFT studies. Appl. Catal. B-Environ. 248, 1–10 (2019).
Guo, S. D., Mu, W. Q. & Zhu, Y. T. Biaxial strain enhanced piezoelectric properties in monolayer g-C3N4. J. Phys. Chem. Solids 151, 109896 (2021).
Tsalas, N., Golfinopoulos, S. K., Samios, S., Katsouras, G. & Peroulis, K. Optimization of energy consumption in a wastewater treatment plant: an overview. Energies 17, 2808 (2024).
Zhuang, W. et al. Piezoelectric catalytic process: a genuinely energy-saving approach for water treatment? A critical review. Chem. Eng. J. 499, 155956 (2024).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 62325505, 62020106014, 62175140, 62475138), the Shanxi Province Graduate Student Research Innovation Project (Grant No. 2024KY102), and the Xinzhou Key Research and Development Project (Grant No. 20240102).
Author information
Authors and Affiliations
Contributions
N.S.: formal analysis, investigation, and writing—original draft. Z.R.: methodology and formal analysis. P.C.: investigation. M.Y.: Data curation. J.W.: funding acquisition and formal analysis. Y.F.: conceptualization, writing-review and editing, funding acquisition, and project administration. J.M.: supervision, funding acquisition, and project administration. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, N., Ren, Z., Chen, P. et al. Highly stretchable TPU/g-C3N4 composite nanofiber film for enhancing the piezo-photocatalytic sewage treatment by electrospinning-induced pretension. npj Clean Water 8, 19 (2025). https://doi.org/10.1038/s41545-025-00452-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41545-025-00452-8