Abstract
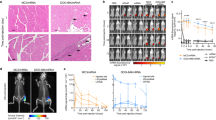
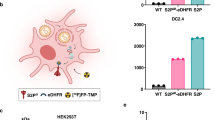
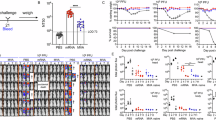
Visualization of the spatio–temporal trafficking of vaccines after their delivery would help evaluate the efficacy of candidate formulations and aid their rational design for preclinical and translational studies. Here, we show that a dual radionuclide–near-infrared probe allows for quantitative, longitudinal and non-invasive monitoring, via positron emission tomography–computed tomography and near-infrared imaging of cynomolgus macaques, of the trafficking dynamics to draining lymph nodes of a model messenger RNA vaccine labelled with the probe. After intramuscular administration of the vaccine to the monkeys, we observed the dynamics of the mRNA vaccine at the injection site and in the draining lymph nodes, performed cellular analyses of the involved tissues using flow cytometry and identified through immunofluorescence that professional antigen-presenting cells are the primary cells containing the injected mRNA and encoding the antigen. This approach may reveal spatio–temporal determinants of vaccine efficacy in preclinical and translational studies employing large mammals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information. The PET–CT-scanner acquisition settings are available on request from the corresponding author.
References
Qin, L., Gilbert, P. B., Corey, L., McElrath, M. J. & Self, S. G. A framework for assessing immunological correlates of protection in vaccine trials. J. Infect. Dis. 196, 1304–1312 (2007).
Gilbert, P. B., Qin, L. & Self, S. G. Evaluating a surrogate endpoint at three levels, with application to vaccine development. Stat. Med. 27, 4758–4778 (2008).
DeFrancesco, L. The ‘anti-hype’ vaccine. Nat. Biotechnol. 35, 193–197 (2017).
Pulendran, B. & Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 12, 509–517 (2011).
Verbeke, R. et al. Co-delivery of nucleoside-modified mRNA and TLR agonists for cancer immunotherapy: restoring the immunogenicity of immunosilent mRNA. J. Control. Release 266, 287–300 (2017).
Fotin-Mleczek, M. et al. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother. 34, 1–15 (2011).
Lonez, C., Vandenbranden, M. & Ruysschaert, J.-M. Cationic lipids activate intracellular signaling pathways. Adv. Drug Deliv. Rev. 64, 1749–1758 (2012).
Pollard, C. et al. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol. Ther. 21, 251–259 (2013).
Petsch, B. et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 30, 1210–1216 (2012).
Pardi, N. et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543, 248–251 (2017).
Benteyn, D., Heirman, C., Bonehill, A., Thielemans, K. & Breckpot, K. mRNA-based dendritic cell vaccines. Expert Rev. Vaccines 14, 161–176 (2015).
Diken, M. et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 18, 702–708 (2011).
Broos, K. et al. Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific T-cell responses despite the induction of type I interferon. Mol. Ther. Nucleic Acids 5, e326 (2016).
Stoll, S., Delon, J., Brotz, T. M. & Germain, R. N. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science 296, 1873–1876 (2002).
van Aalst, S. et al. Routing dependent immune responses after experimental R848-adjuvated vaccination. Vaccine 36, 1405–1413 (2018).
Frey, S. E. et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naive subjects. Vaccine 33, 5225–5234 (2015).
Desigaux, L. et al. Self-assembled lamellar complexes of siRNA with lipidic aminoglycoside derivatives promote efficient siRNA delivery and interference. Proc. Natl Acad. Sci. USA 104, 16534–16539 (2007).
Habrant, D. et al. Design of ionizable lipids to overcome the limiting step of endosomal escape: application in the intracellular delivery of mRNA, DNA, and siRNA. J. Med. Chem. 59, 3046–3062 (2016).
Colombani, T. et al. Self-assembling complexes between binary mixtures of lipids with different linkers and nucleic acids promote universal mRNA, DNA and siRNA delivery. J. Control. Release 249, 131–142 (2017).
Kirschman, J. L. et al. Characterizing exogenous mRNA delivery, trafficking, cytoplasmic release and RNA–protein correlations at the level of single cells. Nucleic Acids Res. 45, e113 (2017).
Alonas, E., Vanover, D., Blanchard, E., Zurla, C. & Santangelo, P. J. Imaging viral RNA using multiply labeled tetravalent RNA imaging probes in live cells. Methods 98, 91–98 (2016).
Bhosle, S. M. et al. Unifying in vitro and in vivo IVT mRNA expression discrepancies in skeletal muscle via mechanotransduction. Biomaterials 159, 189–203 (2018).
Liang, F. et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol. Ther. 25, 2635–2647 (2017).
Shustov, A. V., Mason, P. W. & Frolov, I. Production of pseudoinfectious yellow fever virus with a two-component genome. J. Virol. 81, 11737–11748 (2007).
Op De Beeck, A. et al. Role of the transmembrane domains of prM and E proteins in the formation of yellow fever virus envelope. J. Virol. 77, 813–820 (2003).
Moses, W. W. Fundamental limits of spatial resolution in PET. Nucl. Instrum. Methods Phys. Res. A 648, S236–S240 (2011).
Pauthner, M. et al. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46, 1073–1088 (2017).
Lu, F. & HogenEsch, H. Kinetics of the inflammatory response following intramuscular injection of aluminum adjuvant. Vaccine 31, 3979–3986 (2013).
Calabro, S. et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29, 1812–1823 (2011).
Thomas, S. N., Rohner, N. A. & Edwards, E. E. Implications of lymphatic transport to lymph nodes in immunity and immunotherapy. Annu. Rev. Biomed. Eng. 18, 207–233 (2016).
Wood, K. J., Bushell, A. & Hester, J. Regulatory immune cells in transplantation. Nat. Rev. Immunol. 12, 417–430 (2012).
Ford, M. L., Adams, A. B. & Pearson, T. C. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat. Rev. Nephrol. 10, 14–24 (2014).
Reed, S. G., Orr, M. T. & Fox, C. B. Key roles of adjuvants in modern vaccines. Nat. Med. 19, 1597–1608 (2013).
Deering, R. P., Kommareddy, S., Ulmer, J. B., Brito, L. A. & Geall, A. J. Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines. Expert Opin. Drug Deliv. 11, 885–899 (2014).
Liang, F. & Loré, K. Local innate immune responses in the vaccine adjuvant-injected muscle. Clin. Transl. Immunol. 5, e74 (2016).
Liang, F. et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 9, eaal2094 (2017).
Acknowledgements
The Petit Institute Core at Georgia Tech, particularly S. Durham and A. Shaw, who provided guidance and resources for flow cytometry and confocal microscopy, respectively. This work was supported by the Defense Advanced Research Projects Agency, Sanofi Pasteur and the RNArmorVax Consortium (to P.J.S). The views, opinions and/or findings expressed are those of the author(s) and should not be interpreted as representing the official views or policies of the US Department of Defense or the US Government.
Author information
Authors and Affiliations
Contributions
K.E.L. performed the probe labelling of mRNA, 64Cu-reporter labelling, necropsies, near-IR tissue extraction and processing, tissue imaging and quantification, PET–CT data analysis, and wrote the manuscript. S.M.B. performed in vitro transfections, probe labelling and optimization of mRNA labelling, nanoparticle optimization, tissue staining, imaging and quantification, and wrote the manuscript. C.Z. performed optimization of mRNA labelling with probes, HPLC of RNA, RT–PCR of innate gene expression, preparation of samples for flow cytometry, and paper revision and editing. J.B. analysed the flow cytometry data. K.A.R., P.X. and L.M.S. performed the flow cytometry. D.V. and M.A. performed PET imaging and necropsy. B.P. synthesized the CholK nanoparticles. P.B. synthesized the IVT YF prME mRNA. F.V. designed the experiments, and performed PET imaging, animal care and necropsies. P.J.S. designed the experiments, performed probe labelling of mRNA, PET imaging and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.P. owns stock in In-Cell-Art, which commercializes lipidic aminoglycoside derivatives. P.B. is an employee at CureVac, which commercializes RNA-based vaccines.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Supplementary Video
3D reconstruction of vaccine PET–CT signal at 28 hours post injection in AF093.
Rights and permissions
About this article
Cite this article
Lindsay, K.E., Bhosle, S.M., Zurla, C. et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET–CT and near-infrared imaging. Nat Biomed Eng 3, 371–380 (2019). https://doi.org/10.1038/s41551-019-0378-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-019-0378-3
This article is cited by
-
Intranasal unadjuvanted LcrV boosts parental Yersinia OMV primed lung immunity against pneumonic plague in mice
Nature Communications (2026)
-
Optimal murine CD4+ T cell priming by mRNA-lipid nanoparticle vaccines requires endogenous antigen processing
Nature Communications (2026)
-
Impact of cell line and reporter gene selection on in-vitro transfection evaluation of mRNA lipid nanoparticles
AAPS Open (2025)
-
Monitoring mRNA vaccine antigen expression in vivo using PET/CT
Nature Communications (2025)
-
Progress in cancer vaccines enabled by nanotechnology
Nature Nanotechnology (2025)