Abstract
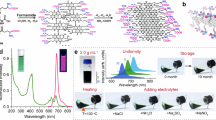
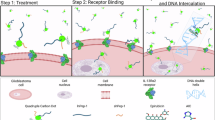
Strategies for selectively imaging and delivering drugs to tumours typically leverage differentially upregulated surface molecules on cancer cells. Here, we show that intravenously injected carbon quantum dots, functionalized with multiple paired α-carboxyl and amino groups that bind to the large neutral amino acid transporter 1 (which is expressed in most tumours), selectively accumulate in human tumour xenografts in mice and in an orthotopic mouse model of human glioma. The functionalized quantum dots, which structurally mimic large amino acids and can be loaded with aromatic drugs through π–π stacking interactions, enabled—in the absence of detectable toxicity—near-infrared fluorescence and photoacoustic imaging of the tumours and a reduction in tumour burden after the targeted delivery of chemotherapeutics to the tumours. The versatility of functionalization and high tumour selectivity of the quantum dots make them broadly suitable for tumour-specific imaging and drug delivery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the Article and its Supplementary Information. The raw and analysed datasets that were generated during the study are too large to be publicly shared, but they are available for research purposes from the corresponding authors on reasonable request.
Change history
04 May 2020
Editor’s note: The editors of Nature Biomedical Engineering have been notified about issues with a few images in this Article. Two micrographs within Fig. 4 and Supplementary Figs. 20, 25 and 61 are duplicated. In addition, a few of the mice panels in Figs. 2c and Supplementary Figs. 29, 31, 37 and 56 appear to be duplicates. The authors have informed us that these are inadvertent errors and believe that the overall data and conclusions are solid, and that they need to retrieve data from the Cell Imaging Facility and Animal Imaging Facility at Beijing Normal University before they can fully clarify the image issues. They have also informed us that access to the data will be delayed because of COVID-19 access restrictions.
21 January 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41551-022-00845-x
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 66, 7–30 (2016).
Kim, S. M., Faix, P. H. & Schnitzer, J. E. Overcoming key biological barriers to cancer drug delivery and efficacy. J. Control. Release 267, 15–30 (2017).
Tringale, K. R., Pang, J. & Nguyen, Q. T. Image-guided surgery in cancer: a strategy to reduce incidence of positive surgical margins. Wiley Interdiscip. Rev. Syst. Biol. Med. 10, e1412 (2018).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Park, K. Facing the truth about nanotechnology in drug delivery. ACS Nano 7, 7442–7447 (2013).
Belfiore, L. et al. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: challenges and opportunities. J. Control. Release 277, 1–13 (2018).
Bae, Y. H. & Park, K. Targeted drug delivery to tumors: myths, reality and possibility. J. Control. Release 153, 198–205 (2011).
Zhou, J., Atsina, K. B., Himes, B. T., Strohbehn, G. W. & Saltzman, W. M. Novel delivery strategies for glioblastoma. Cancer J. 18, 89–99 (2012).
Deeken, J. F. & Loscher, W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin. Cancer Res. 13, 1663–1674 (2007).
Patel, T., Zhou, J., Piepmeier, J. M. & Saltzman, W. M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev. 64, 701–705 (2012).
Nakanishi, T. & Tamai, I. Solute carrier transporters as targets for drug delivery and pharmacological intervention for chemotherapy. J. Pharm. Sci. 100, 3731–3750 (2011).
Liu, R. et al. GLUT1-mediated selective tumor targeting with fluorine containing platinum(II) glycoconjugates. Oncotarget 8, 39476–39496 (2017).
Jin, S. E., Jin, H. E. & Hong, S. S. Targeting l-type amino acid transporter 1 for anticancer therapy: clinical impact from diagnostics to therapeutics. Expert Opin. Ther. Targets. 19, 1319–1337 (2015).
Bodoy, S., Fotiadis, D., Stoeger, C., Kanai, Y. & Palacin, M. The small SLC43 family: facilitator system l amino acid transporters and the orphan EEG1. Mol. Asp. Med. 34, 638–645 (2013).
Hayashi, K. & Anzai, N. Novel therapeutic approaches targeting l-type amino acid transporters for cancer treatment. World J. Gastrointest. Oncol. 9, 21–29 (2017).
Wu, W. et al. Aspartate-modified doxorubicin on its N-terminal increases drug accumulation in LAT1-overexpressing tumors. Cancer Sci. 106, 747–756 (2015).
Fan, Z., Zhou, S., Garcia, C., Fan, L. & Zhou, J. pH-responsive fluorescent graphene quantum dots for fluorescence-guided cancer surgery and diagnosis. Nanoscale 9, 4928–4933 (2017).
Yuan, F. et al. Shining carbon dots: synthesis and biomedical and optoelectronic applications. Nanotoday 11, 565–586 (2016).
Yuan, F. et al. Nitrogen-rich D-π-A structural carbon quantum dots with a bright two-photon fluorescence for deep-tissue imaging. ACS Appl. Bio. Mater. 1, 853–858 (2018).
Peng, J. et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 12, 844–849 (2012).
Kendall, P. A. Use of ninhydrin reaction for quantitative estimation of amino groups in insoluble specimens. Nature 197, 1305–1306 (1963).
Fan, Z. T. et al. Surrounding media sensitive photoluminescence of boron-doped graphene quantum dots for highly fluorescent dyed crystals, chemical sensing and bioimaging. Carbon 70, 149–156 (2014).
Li, S. et al. Exceptionally high payload of the IR780 Iodide on folic acid-functionalized graphene quantum dots for targeted photothermal therapy. ACS Appl. Mater. Inter. 9, 22332–22341 (2017).
Zhang, M. et al. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J. Mater. Chem. 22, 7461–7467 (2012).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Weber, J., Beard, P. C. & Bohndiek, S. E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 13, 639–650 (2016).
Zhou, J. et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl Acad. Sci. USA 104, 16158–16163 (2007).
Xiao, Y. et al. Ex vivo dynamics of human glioblastoma cells in a microvasculature-on-a-chip system correlates with tumor heterogeneity and subtypes. Adv. Sci. 6, 1801531 (2019).
Zhou, J. et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc. Natl Acad. Sci. USA 110, 11751–11756 (2013).
Ren, D., Kratz, F. & Wang, S. W. Engineered drug-protein nanoparticle complexes for folate receptor targeting. Biochem. Eng. J. 89, 33–41 (2014).
Gillies, R. J. The tumour microenvironment: causes and consequences of hypoxia and acidity. Introduction. Novartis Found. Symp. 240, 1–6 (2001).
Vaupel, P., Kallinowski, F. & Okunieff, P. Blood-flow, oxygen and nutrient supply, and metabolic microenvironment of human-tumors—a review. Cancer Res. 49, 6449–6465 (1989).
Uchino, H. et al. Transport of amino acid-related compounds mediated by l-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol. Pharm. 61, 729–737 (2002).
Yan, R., Zhao, X., Lei, J. & Zhou, Q. Structure of the human LAT1–4F2hc heteromeric amino acid transporter complex. Nature 568, 127–130 (2019).
dos Santos, T., Varela, J., Lynch, I., Salvati, A. & Dawson, K. A. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS ONE 6, e24438 (2011).
Ganbold, T., Han, S., Hasi, A. & Baigude, H. Receptor-mediated delivery of therapeutic RNA by peptide functionalized curdlan nanoparticles. Int. J. Biol. Macromol. 126, 633–640 (2019).
Poirier, C., van Effenterre, D., Delord, B., Johannes, L. & Roux, D. Specific adsorption of functionalized colloids at the surface of living cells: a quantitative kinetic analysis of the receptor-mediated binding. Biochim. Biophys. Acta 1778, 2450–2457 (2008).
Cheung-Ong, K., Giaever, G. & Nislow, C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem. Biol. 20, 648–659 (2013).
Yin, J., Deng, X. D., Zhang, J. & Lin, J. Current understanding of interactions between nanoparticles and ABC transporters in cancer cells. Curr. Med. Chem. 25, 5930–5944 (2018).
Yuan, Y. L. et al. Nanoparticle delivery of anticancer drugs overcomes multidrug resistance in breast cancer. Drug Deliv. 23, 3350–3357 (2016).
Bulbake, U., Doppalapudi, S., Kommineni, N. & Khan, W. Liposomal formulations in clinical use: an updated review. Pharmaceutics 9, 12 (2017).
Geier, E. G. et al. Structure-based ligand discovery for the large-neutral amino acid transporter 1, LAT-1. Proc. Natl Acad. Sci. USA 110, 5480–5485 (2013).
Li, G. et al. Permeability of endothelial and astrocyte cocultures: in vitro blood-brain barrier models for drug delivery studies. Ann. Biomed. Eng. 38, 2499–2511 (2010).
Sun, M. et al. Realization of the photostable intrinsic core emission from carbon dots through surface deoxidation by ultraviolet irradiation. J. Phys. Chem. Lett. 10, 3094–3100 (2019).
Yuan, F. et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 9, 2249 (2018).
Yuan, F. et al. Bright multicolor bandgap fluorescent carbon quantum dots for electroluminescent light-emitting diodes. Adv. Mater. 29, 1604436 (2017).
Lim, S. Y., Shen, W. & Gao, Z. Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 44, 362–381 (2015).
Mabray, M. C., Barajas, R. F. Jr & Cha, S. Modern brain tumor imaging. Brain Tumor Res. Treat. 3, 8–23 (2015).
Kim, D. K. et al. Characterization of the system l amino acid transporter in T24 human bladder carcinoma cells. Biochim. Biophys. Acta 1565, 112–121 (2002).
Puris, E., Gynther, M., Huttunen, J., Auriola, S. & Huttunen, K. M. l-type amino acid transporter 1 utilizing prodrugs of ferulic acid revealed structural features supporting the design of prodrugs for brain delivery. Eur. J. Pharm. Sci. 129, 99–109 (2019).
Gynther, M. et al. Large neutral amino acid transporter enables brain drug delivery via prodrugs. J. Med. Chem. 51, 932–936 (2008).
Gynther, M. et al. Brain uptake of ketoprofen-lysine prodrug in rats. Int. J. Pharm. 399, 121–128 (2010).
Huttunen, K. M. et al. l-Type amino acid transporter 1 (LAT1)-mediated targeted delivery of perforin inhibitors. Int. J. Pharm. 498, 205–216 (2016).
Peura, L. et al. Large amino acid transporter 1 (LAT1) prodrugs of valproic acid: new prodrug design ideas for central nervous system delivery. Mol. Pharm. 8, 1857–1866 (2011).
Puris, E., Gynther, M., Huttunen, J., Petsalo, A. & Huttunen, K. M. L-type amino acid transporter 1 utilizing prodrugs: how to achieve effective brain delivery and low systemic exposure of drugs. J. Control. Release 261, 93–104 (2017).
Gynther, M. et al. Amino acid promoieties alter valproic acid pharmacokinetics and enable extended brain exposure. Neurochem. Res. 41, 2797–2809 (2016).
Acknowledgements
This work was supported by NSFC grants (numbers 21573019 and 21872010, to L.F.), a NSFC Major Research Plan grant (number 21233003, to L.F.), a NSFC grant (21773016, to J.Zhu) and NIH grants (NS095817 and NS110721, to J.Zhou).
Author information
Authors and Affiliations
Contributions
L.F., J.Zhu, S.L., W.S., H.W. and J.Zhou designed the experiments. S.L., W.S., H.W., T.Y., C.Y., J.L., G.D., F.Y., S.Z., Y.L., X.L., H.T., A.T.C., F.L. and Y.Z. performed the experiments. All of the authors were involved in the analyses and interpretation of data. S.L., L.F. and J.Zhou wrote the paper, with help from the co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures, tables and references.
Supplementary Video 1
Penetration of LAAM TC-CQDs into HeLa cells.
Supplementary Video 2
Penetration of LAAM TC-CQDs into CCC-ESF-1 cells.
Supplementary Video 3
Three-dimensional reconstruction of FL images of LAAM TC-CQDs in mice bearing HeLa tumours.
Supplementary Video 4
Three-dimensional reconstruction of FL images of G-CQDs in mice bearing HeLa tumours.
Rights and permissions
About this article
Cite this article
Li, S., Su, W., Wu, H. et al. Targeted tumour theranostics in mice via carbon quantum dots structurally mimicking large amino acids. Nat Biomed Eng 4, 704–716 (2020). https://doi.org/10.1038/s41551-020-0540-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-020-0540-y
This article is cited by
-
Carbon quantum dots as multifunctional nanomaterials for sustainable optoelectronic biosensing and green photonics
Communications Materials (2026)
-
Development of a quadruple-conjugated carbon dot nanomodel for targeted glioma therapy
Communications Chemistry (2026)
-
Targeting JAK2 with a multifunctional nanoinhibitor for long-lasting anti-inflammatory effects and bone repair
Journal of Nanobiotechnology (2025)
-
Oxidative stress induced paclitaxel-derived carbon dots inhibit glioblastoma proliferation and EMT process
Journal of Nanobiotechnology (2025)
-
Sterically chained amino acid-rich water-soluble carbon quantum dots as a robust tumor-targeted drug delivery platform
Nature Communications (2025)