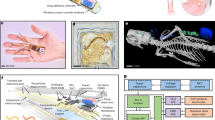
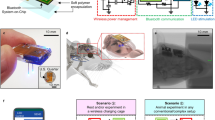
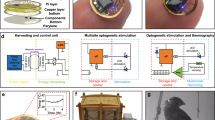
Abstract
Tethered and battery-powered devices that interface with neural tissues can restrict natural motions and prevent social interactions in animal models, thereby limiting the utility of these devices in behavioural neuroscience research. In this Review Article, we discuss recent progress in the development of miniaturized and ultralightweight devices as neuroengineering platforms that are wireless, battery-free and fully implantable, with capabilities that match or exceed those of wired or battery-powered alternatives. Such classes of advanced neural interfaces with optical, electrical or fluidic functionality can also combine recording and stimulation modalities for closed-loop applications in basic studies or in the practical treatment of abnormal physiological processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Rivnay, J., Wang, H., Fenno, L., Delsseroth, K. & Mallaras, G. G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 3, e1601649 (2017).
Hong, G. & Lieber, C. M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 20, 330–345 (2019).
Jeong, J. W. et al. Soft materials in neuroengineering for hard problems in neuroscience. Neuron 86, 175–186 (2015).
Tybrandt, K. et al. High-density stretchable electrode grids for chronic neural recording. Adv. Mater. 30, e1706520 (2018).
Won, S. M. et al. Emerging modalities and implantable techonlogies for neuromodulation. Cell 181, 115–135 (2020).
Azevedo, C. A. & Mammis, A. Neuromodulation therapies for alcohol addiction: a literature review. Neuromodulation 21, 144–148 (2018).
Bouthour, W. et al. Biomarkers for closed-loop deep brain stimulation in Parkinson disease and beyond. Nat. Rev. Neurol. 15, 343–352 (2019).
Fang, J. Y. & Tolleson, C. The role of deep brain stimulation in Parkinson’s disease: an overview and update on new developments. Neuropsychiatr. Dis. Treat. 13, 723–732 (2017).
Kuo, C. H., White-Dzuro, G. A. & Ko, A. L. Approaches to closed-loop deep brain stimulation for movement disorders. Neurosurg. Focus 45, E2 (2018).
Wellman, S. M. et al. A materials roadmap to functional neural interface design. Adv. Funct. Mater. 28, 1701269 (2018).
Robinson, J. T., Jorgolli, M. & Park, H. Nanowire electrodes for high-density stimulation and measurement of neural circuits. Front. Neural Circuits 7, 38 (2013).
Fan, B. & Li, W. Miniaturized optogenetic neural implants: a review. Lab Chip 15, 3838–3855 (2015).
Fu, R. et al. Implantable and biodegradable poly(l-lactic acid) fibers for optical neural interfaces. Adv. Opt. Mater. 6, e1700941 (2018).
Gutruf, P. & Rogers, J. A. Implantable, wireless device platforms for neuroscience research. Curr. Opin. Neurobiol. 50, 42–49 (2018).
Park, S., Loke, G., Fink, Y. & Anikeeva, P. Flexible fiber-based optoelectronics for neural interfaces. Chem. Soc. Rev. 48, 1826–1852 (2019).
Jeong, J. W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Pons-Faudoa, F. P., Ballerini, A., Sakamoto, J. & Grattoni, A. Advanced implantable drug delivery technologies: transforming the clinical landscape of therapeutics for chronic diseases. Biomed. Microdevices 21, 47 (2019).
Sanjay, S. T. et al. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev. 128, 3–28 (2018).
Sim, J. Y., Haney, M. P., Park, S. I., McCall, J. G. & Jeong, J. W. Microfluidic neural probes: in vivo tools for advancing neuroscience. Lab Chip 17, 1406–1435 (2017).
Liu, Y. et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 3, 58–68 (2019).
Zhang, Y. et al. Climbing-inspired twining electrodes using shape memory for peripheral nerve stimulation and recording. Sci. Adv. 5, eaaw1066 (2019).
Opie, N. L. et al. Focal stimulation of the sheep motor cortex with a chronically implanted minimally invasive electrode array mounted on an endovascular stent. Nat. Biomed. Eng. 2, 907–914 (2018).
Yan, Z. et al. Three-dimensional mesostructures as high-temperature growth templates, electronic cellular scaffolds, and self-propelled microrobots. Proc. Natl Acad. Sci. USA 114, E9455–E9464 (2017).
All, A. H. et al. Expanding the toolbox of upconversion nanoparticles for in vivo optogenetics and neuromodulation. Adv. Mater. 31, e1803474 (2019).
Chen, S. et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 359, 679–684 (2018).
Christiansen, M. G., Senko, A. W. & Anikeeva, P. Magnetic strategies for nervous system control. Annu. Rev. Neurosci. 42, 271–293 (2019).
Roet, M. et al. Progress in neuromodulation of the brain: a role for magnetic nanoparticles? Prog. Neurobiol. 177, 1–14 (2019).
Chen, R., Romero, G., Christiansen, M. G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 347, 1477–1480 (2015).
Chu, P. C. et al. Neuromodulation accompanying focused ultrasound-induced blood–brain barrier opening. Sci. Rep. 5, 15477 (2015).
Blackmore, J., Shrivastava, S., Sallet, J., Butler, C. R. & Cleveland, R. O. Ultrasound neuromodulation: a review of results, mechanisms and safety. Ultrasound Med. Biol. 45, 1509–1536 (2019).
Farra, R. et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci. Transl Med. 4, 122ra121 (2012).
Abraham, W. T. et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 377, 658–666 (2011).
Stingl, K. et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc. Biol. Sci. 280, 20130077 (2013).
Borton, D. A., Yin, M., Aceros, J. & Nurmikko, A. An implantable wireless neural interface for recording cortical circuit dynamics in moving primates. J. Neural Eng. 10, 026010 (2013).
Finkelstein, A. et al. Three-dimensional head-direction coding in the bat brain. Nature 517, 159–164 (2015).
Liu, X., Lu, Y., Iseri, E., Shi, Y. & Kuzum, D. A compact closed-loop optogenetics system based on artifact-free transparent graphene electrodes. Front. Neurosci. 12, 132 (2018).
Martinez, D. et al. Adaptive quantization of local field potentials for wireless implants in freely moving animals: an open-source neural recording device. J. Neural Eng. 15, 025001 (2018).
Matsushita, K. et al. A fully implantable wireless ECoG 128-channel recording device for human brain–machine interfaces: W-HERBS. Front. Neurosci. 12, 511 (2018).
Wentz, C. T. et al. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. J. Neural Eng. 8, 046021 (2011).
Gutruf, P. et al. Wireless, battery-free fully implantable multimodal and multisite pace makers for applications in small animal models. Nat. Commun. 10, 5742 (2019).
Burton, A. et al. Wireless battery-free subdermally implantable photometry systems for chronic recording of neural dynamics. Proc. Natl Acad. Sci. USA 117, 2835–2845 (2020).
Ferguson, J. E. & Redish, A. D. Wireless communication with implanted medical devices using the conductive properties of the body. Expert Rev. Med. Devices 8, 427–433 (2011).
Ledet, E. H., Liddle, B., Kradinova, K. & Harper, S. Smart implants in orthopedic surgery, improving patient outcomes: a review. Innov. Entrep. Health 5, 41–51 (2018).
Yang, W., Khan, W., Wu, J. & Li, W. Single-channel opto-neurostimulators: a review. J. Micromech. Microeng. 29, 043001 (2019).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Deisseroth, K. Optogenetics. Nat. Methods 8, 26–29 (2011).
Pisanello, F. et al. Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber. Nat. Neurosci. 20, 1180–1188 (2017).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014).
Kim, C. K. et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat. Methods 13, 325–328 (2016).
Piatkevich, K. D. et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol. 14, 352–360 (2018).
Pisano, F. et al. Depth-resolved fiber photometry with a single tapered optical fiber implant. Nat. Methods 16, 1185–1192 (2019).
Wu, F. et al. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 88, 1136–1148 (2015).
Ghosh, K. K. et al. Miniaturized integration of a fluorescence microscope. Nat. Methods 8, 871–878 (2011).
Resendez, S. L. et al. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat. Protoc. 11, 566–597 (2016).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Gilletti, A. & Muthuswamy, J. Brain micromotion around implants in the rodent somatosensory cortex. J. Neural Eng. 3, 189–195 (2006).
Sridharan, A., Rajan, S. D. & Muthuswamy, J. Long-term changes in the material properties of brain tissue at the implant–tissue interface. J. Neural Eng. 10, 066001 (2013).
Fan, D. et al. A wireless multi-channel recording system for freely behaving mice and rats. PLoS ONE 6, e22033 (2011).
Lu, L. et al. Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain. Proc. Natl Acad. Sci. USA 115, E1374–E1383 (2018).
Zhou, A. et al. A wireless and artefact-free 128-channel neuromodulation device for closed-loop stimulation and recording in non-human primates. Nat. Biomed. Eng. 3, 15–26 (2018).
Zhang, Y. et al. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc. Natl Acad. Sci. USA 116, 21427–21437 (2019).
Zhang, W. & Yartsev, M. M. Correlated neural activity across the brains of socially interacting bats. Cell 178, 413–428.e22 (2019).
Jurgens, U. & Hage, S. R. Telemetric recording of neuronal activity. Methods 38, 195–201 (2006).
Stoodley, C. J. et al. Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat. Neurosci. 20, 1744–1751 (2017).
Wyart, C. et al. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 461, 407–410 (2009).
Endo, H. et al. Wireless enzyme sensor system for real-time monitoring of blood glucose levels in fish. Biosens. Bioelectron. 24, 1417–1423 (2009).
Michael, Y. M. & Ulanovsky, N. Representation of three-dimensional space in the hippocampus of flying bats. Science 340, 367–372 (2013).
Roberts, T. F., Gobes, S. M., Murugan, M., Olveczky, B. P. & Mooney, R. Motor circuits are required to encode a sensory model for imitative learning. Nat. Neurosci. 15, 1454–1459 (2012).
Kouhani, M. H. M., Luo, R., Madi, F., Weber, A. J. & Li, W. A wireless smartphone controlled, battery powered, head mounted light delivery system for optogenetic stimulation. In Proc. 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 3366–3369 (IEEE, 2018).
Gagnon-Turcotte, G. et al. A wireless headstage for combined optogenetics and multichannel electrophysiological recording. IEEE Trans. Biomed. Circuits Syst. 11, 1–14 (2017).
Gutruf, P., Good, C. H. & Rogers, J. A. Perspective: implantable optical systems for neuroscience research in behaving animal models—current approaches and future directions. APL Photonics 3, 120901 (2018).
Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 1, 16063 (2016).
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2, 16093 (2017).
Kozai, T. D. Y., Jaquins-Gerstl, A. S., Vazquez, A. L., Michael, A. C. & Cui, X. T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 6, 48–67 (2015).
Salatino, J. W., Ludwig, K. A., Kozai, T. D. Y. & Purcell, E. K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 1, 862–877 (2017).
Rogers, J. A., Someya, T. & Huang, Y. G. Materials and mechanics for stretchable electronics. Science 327, 1603–1607 (2010).
Veiseh, O. et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 14, 643–651 (2015).
Schiavone, G. & Lacour, S. P. Conformable bioelectronic interfaces: mapping the road ahead. Sci. Transl. Med. 11, eaaw5858 (2019).
Vitale, F. et al. Fluidic microactuation of flexible electrodes for neural recording. Nano Lett. 18, 326–335 (2018).
Du, Z. J. et al. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 53, 46–58 (2017).
Luan, L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 3, e1601966 (2017).
Sekitani, T. & Someya, T. Stretchable, large‐area organic electronics. Adv. Mater. 22, 2228–2246 (2010).
Kim, D. H., Xiao, J. L., Song, J. Z., Huang, Y. G. & Rogers, J. A. Stretchable, curvilinear electronics based on inorganic materials. Adv. Mater. 22, 2108–2124 (2010).
Oh, J. Y. et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 539, 411–415 (2016).
Kaltenbrunner, M. et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 499, 458–463 (2013).
Jang, H. et al. Graphene-based flexible and stretchable electronics. Adv. Mater. 28, 4184–4202 (2016).
Wang, S. H. et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 555, 83–88 (2018).
Sun, Y. G., Choi, W. M., Jiang, H. Q., Huang, Y. G. Y. & Rogers, J. A. Controlled buckling of semiconductor nanoribbons for stretchable electronics. Nat. Nanotechnol. 1, 201–207 (2006).
Zhang, Y. H. et al. Mechanics of ultra-stretchable self-similar serpentine interconnects. Acta Mater. 61, 7816–7827 (2013).
Qiang, Y. et al. Transparent arrays of bilayer-nanomesh microelectrodes for simultaneous electrophysiology and two-photon imaging in the brain. Sci. Adv. 4, eaat0626 (2018).
de la Oliva, N., Mueller, M., Stieglitz, T., Navarro, X. & del Valle, J. On the use of parylene C polymer as substrate for peripheral nerve electrodes. Sci. Rep. 8, 5965 (2018).
Chiang, C. H. et al. Development of a neural interface for high-definition, long-term recording in rodents and nonhuman primates. Sci. Transl. Med. 12, eaay4682 (2020).
Viventi, J. et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 14, 1599–1605 (2011).
Xu, S. et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 344, 70–74 (2014).
Kim, D.-H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Lee, S. et al. Ultrasoft electronics to monitor dynamically pulsing cardiomyocytes. Nat. Nanotechnol. 14, 156–160 (2019).
Park, S. I. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 33, 1280–1286 (2015).
Kim, T. I. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Shin, G. et al. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron 93, 509–521 (2017).
Park, S. I. et al. Stretchable multichannel antennas in soft wireless optoelectronic implants for optogenetics. Proc. Natl Acad. Sci. USA 113, E8169–E8177 (2016).
Gutruf, P. et al. Fully implantable optoelectronic systems for battery-free, multimodal operation in neuroscience research. Nat. Electron. 1, 652–660 (2018).
Xu, S. et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 4, 1543 (2013).
Kim, J. et al. Epidermal electronics with advanced capabilities in near-field communication. Small 11, 906–912 (2015).
Kim, D. H., Ghaffari, R., Lu, N. S. & Rogers, J. A. Flexible and stretchable electronics for biointegrated devices. Annu. Rev. Biomed. Eng. 14, 113–128 (2012).
Kang, S. K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Scholten, K. & Meng, E. A review of implantable biosensors for closed-loop glucose control and other drug delivery applications. Int. J. Pharm. 544, 319–334 (2018).
Scholten, K. & Meng, E. Materials for microfabricated implantable devices: a review. Lab Chip 15, 4256–4272 (2015).
Hassler, C., Boretius, T. & Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Polym. Phys. 49, 18–33 (2011).
Joung, Y. H. Development of implantable medical devices: from an engineering perspective. Int. Neurourol. J. 17, 98–106 (2013).
Fang, H. et al. Ultrathin, transferred layers of thermally grown silicon dioxide as biofluid barriers for biointegrated flexible electronic systems. Proc. Natl Acad. Sci. USA 113, 11682–11687 (2016).
Song, E., Li, J. & Rogers, J. A. Barrier materials for flexible bioelectronic implants with chronic stability—current approaches and future directions. APL Mater. 7, 050902 (2019).
Hashimoto, M., Hata, A., Miyata, T. & Hirase, H. Programmable wireless light-emitting diode stimulator for chronic stimulation of optogenetic molecules in freely moving mice. Neurophotonics 1, 011002 (2014).
Gagnon-Turcotte, G. et al. A wireless optogenetic headstage with multichannel electrophysiological recording capability. Sensors 15, 22776–22797 (2015).
Mei, H. & Irazoqui, P. P. Miniaturizing wireless implants. Nat. Biotechnol. 32, 1008–1010 (2014).
Ben Amar, A., Kouki, A. B. & Cao, H. Power approaches for implantable medical devices. Sensors 15, 28889–28914 (2015).
Wang, J., He, T. & Lee, C. Development of neural interfaces and energy harvesters towards self-powered implantable systems for healthcare monitoring and rehabilitation purposes. Nano Energy 65, 104039 (2019).
Hwang, G.-T. et al. Self-powered deep brain stimulation via a flexible PIMNT energy harvester. Energy Environ. Sci. 8, 2677–2684 (2015).
Yao, G. et al. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat. Commun. 9, 5349 (2018).
Bullen, R. A., Arnot, T. C., Lakeman, J. B. & Walsh, F. C. Biofuel cells and their development. Biosens. Bioelectron. 21, 2015–2045 (2006).
Settaluri, K. T., Lo, H. & Ram, R. J. Thin thermoelectric generator system for body energy harvesting. J. Electron. Mater. 41, 984–988 (2012).
Montgomery, K. L. et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969–974 (2015).
Lee, S. et al. A 250 μm × 57 μm microscale opto-electronically transduced electrodes (MOTEs) for neural recording. IEEE Trans. Biomed. Circuits Syst. 12, 1256–1266 (2018).
Ding, H. et al. Microscale optoelectronic infrared-to-visible upconversion devices and their use as injectable light sources. Proc. Natl Acad. Sci. USA 115, 6632–6637 (2018).
Charthad, J., Weber, M. J., Chang, T. C. & Arbabian, A. A mm-sized implantable medical device (IMD) with ultrasonic power transfer and a hybrid bi-directional data link. IEEE J. Solid State Circuits 50, 1741–1753 (2015).
Seo, D. et al. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron 91, 529–539 (2016).
Rezaei, M., Maghsoudloo, E., Bories, C., De Koninck, Y. & Gosselin, B. A low-power current-reuse analog front-end for high-density neural recording implants. IEEE Trans. Biomed. Circ. Sys. 12, 271–280 (2018).
Park, S. Y., Cho, J., Lee, K. & Yoon, E. Dynamic power reduction in scalable neural recording interface using spatiotemporal correlation and temporal sparsity of neural signals. IEEE J. Solid State Circuits 53, 1102–1114 (2018).
Wu, X. et al. A 0.04 mm3 16 NW wireless and batteryless sensor system with integrated Cortex-M0+ processor and optical communication for cellular temperature measurement. In Proc. 2018 IEEE Symposium on VLSI Circuits 191–192 (IEEE, 2018).
Schwarz, D. A. et al. Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat. Methods 11, 670–676 (2014).
Burton, A. et al. Wireless, battery-free subdermally implantable photometry systems for chronic recording of neural dynamics. Proc. Natl Acad. Sci. USA 117, 2835–2845 (2020).
Sivaji, V. et al. ReStore: a wireless peripheral nerve stimulation system. J. Neurosci. Methods 320, 26–36 (2019).
Dagdeviren, C. et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl Acad. Sci. USA 111, 1927–1932 (2014).
Ouyang, H. et al. Symbiotic cardiac pacemaker. Nat. Commun. 10, 1821 (2019).
Falk, M., Villarrubia, C. W. N., Babanova, S., Atanassov, P. & Shleev, S. Biofuel cells for biomedical applications: colonizing the animal kingdom. ChemPhysChem 14, 2045–2058 (2013).
Rapoport, B. I., Kedzierski, J. T. & Sarpeshkar, R. A glucose fuel cell for implantable brain–machine interfaces. PLoS ONE 7, e38436 (2012).
Mercier, P. P., Lysaght, A. C., Bandyopadhyay, S., Chandrakasan, A. P. & Stankovic, K. M. Energy extraction from the biologic battery in the inner ear. Nat. Biotechnol. 30, 1240–1243 (2012).
Schroeder, T. B. H. et al. An electric-eel-inspired soft power source from stacked hydrogels. Nature 552, 214–218 (2017).
Nan, K. W. et al. Compliant and stretchable thermoelectric coils for energy harvesting in miniature flexible devices. Sci. Adv. 4, eaau5859 (2018).
Kim, A., Ochoa, M., Rahimi, R. & Ziaie, B. New and emerging energy sources for implantable wireless microdevices. IEEE Access 3, 89–98 (2015).
Mickle, A. D. et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 (2019).
Obaid, S. & Lu, L. Highly efficient microscale gallium arsenide solar cell arrays as optogenetic power options. IEEE Photonics J. 11, 8400108 (2019).
Zhang, H. et al. Wiress, battery-free optoelectronic systems as subdermal implants for local tissue oximetry. Sci. Adv. 5, eaaw0873 (2019).
Zhang, Y. et al. Battery-free, fully implantable optofluidic cuff system for wireless optogenetic and pharmacological neuromodulation of peripheral nerves. Sci. Adv. 5, eaaw5296 (2019).
Jia, Y. Y., Wang, Z. Y., Mirbozorgi, S. A. & Ghovanloo, M. A closed-loop wireless homecage for optogenetic stimulation experiments. In Proc. 2015 IEEE Biomedical Circuits and Systems Conference (BioCAS) 426–429 (2015).
Khalifa, A. et al. The Microbead: a 0.009 mm3 implantable wireless neural stimulator. IEEE Trans. Biomed. Circuits Syst. 13, 971–985 (2019).
Dagdeviren, C. et al. Flexible piezoelectric devices for gastrointestinal motility sensing. Nat. Biomed. Eng. 1, 807–817 (2017).
Dagdeviren, C. et al. Recent progress in flexible and stretchable piezoelectric devices for mechanical energy harvesting, sensing and actuation. Extrem. Mech. Lett. 9, 269–281 (2016).
El Ichi-Ribault, S. et al. Remote wireless control of an enzymatic biofuel cell implanted in a rabbit for 2 months. Electrochim. Acta 269, 360–366 (2018).
Dong, K. et al. Microbial fuel cell as power supply for implantable medical devices: a novel configuration design for simulating colonic environment. Biosens. Bioelectron. 41, 916–919 (2013).
Gonzalez-Solino, C. & Lorenzo, M. D. Enzymatic fuel cells: towards self-powered implantable and wearable diagnostics. Biosensors 8, 11 (2018).
Rasmussen, M., Ritzmann, R. E., Lee, I., Pollack, A. J. & Scherson, D. An implantable biofuel cell for a live insect. J. Am. Chem. Soc. 134, 1458–1460 (2012).
Halamkova, L. et al. Implanted biofuel cell operating in a living snail. J. Am. Chem. Soc. 134, 5040–5043 (2012).
Castorena-Gonzalez, J. A. et al. Biofuel cell operating in vivo in rat. Electroanalysis 25, 1579–1584 (2013).
Zebda, A. et al. Challenges for successful implantation of biofuel cells. Bioelectrochemistry 124, 57–72 (2018).
Yu, X. et al. Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 575, 473–479 (2019).
Merli, F. et al. Design, realization and measurements of a miniature antenna for implantable wireless communication systems. IEEE Trans. Antennas Propag. 59, 3544–3555 (2011).
Park, S. I. et al. Ultraminiaturized photovoltaic and radio frequency powered optoelectronic systems for wireless optogenetics. J. Neural Eng. 12, 056002 (2015).
Xie, Z., Avila, R., Huang, Y. & Rogers, J. A. Flexible and stretchable antennas for biointegrated electronics. Adv. Mater. 32, e1902767 (2019).
Dobkin, D. M. The RF in RFID: UHF RFID in Practice (Newnes, 2012).
Ho, J. S. et al. Self-tracking energy transfer for neural stimulation in untethered mice. Phys. Rev. Appl. 4, 024001 (2015).
Elder, J. A. & Cahill, D. F. (eds) Biological Effects of Radiofrequency Radiation (US Environmental Protection Agency, 1984).
Marincic, A. S. Nikola Tesla and the wireless transmission of energy. IEEE Trans. Power Appar. Syst. 101, 4064–4068 (1982).
Tesla, N. Apparatus for transmitting electrical energy. US Patent 1,119,732 (1914).
Das Barman, S., Reza, A. W., Kumar, N., Karim, M. E. & Munir, A. Wireless powering by magnetic resonant coupling: recent trends in wireless power transfer system and its applications. Renew. Sust. Energ. Rev. 51, 1525–1552 (2015).
Sallan, J., Villa, J. L., Llombart, A. & Sanz, J. F. Optimal design of ICPT systems applied to electric vehicle battery charge. IEEE Trans. Ind. Electron. 56, 2140–2149 (2009).
Kalwar, K. A., Aamir, M. & Mekhilef, S. Inductively coupled power transfer (ICPT) for electric vehicle charging—a review. Renew. Sust. Energ. Rev. 47, 462–475 (2015).
Fareq, M., Fitra, M., Irwanto, M., Hasan, S. & Arinal, M. Low wireless power transfer using inductive coupling for mobile phone charger. J. Phys. Conf. Ser. 495, 012019 (2014).
Kurs, A. et al. Wireless power transfer via strongly coupled magnetic resonances. Science 317, 83–86 (2007).
Ho, S. L., Wang, J. H., Fu, W. N. & Sun, M. G. A comparative study between novel witricity and traditional inductive magnetic coupling in wireless charging. IEEE Trans. Magn. 47, 1522–1525 (2011).
Low, Z. N., Chinga, R. A., Tseng, R. & Lin, J. S. Design and test of a high-power high-efficiency loosely coupled planar wireless power transfer system. IEEE Trans. Ind. Electron. 56, 1801–1812 (2009).
Zhang, Y. M., Zhao, Z. M. & Chen, K. N. Frequency decrease analysis of resonant wireless power transfer. IEEE Trans. Power Electron. 29, 1058–1063 (2014).
Yoon, I. J. & Ling, H. Investigation of near-field wireless power transfer in the presence of lossy dielectric materials. IEEE Trans. Antennas Propag. 61, 482–488 (2013).
Poon, A. S. Y., O’Driscoll, S. & Meng, T. H. Optimal frequency for wireless power transmission into dispersive tissue. IEEE Trans. Antennas Propag. 58, 1739–1750 (2010).
Cecil, S. et al. Numerical assessment of specific absorption rate in the human body caused by NFC devices. In Proc. 2010 Second International Workshop on Near Field Communication 65–70 (IEEE, 2010).
Chung, H. U. et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019).
Kim, J. et al. Miniaturized flexible electronic systems with wireless power and near-field communication capabilities. Adv. Funct. Mater. 25, 4761–4767 (2015).
Lazaro, A., Villarino, R. & Girbau, D. A survey of NFC sensors based on energy harvesting for IoT applications. Sensors 18, 3746 (2018).
Ray, T. R. et al. Bio-integrated wearable systems: a comprehensive review. Chem. Rev. 119, 5461–5533 (2019).
Hui, S. Y. R., Zhong, W. & Lee, C. K. A critical review of recent progress in mid-range wireless power transfer. IEEE Trans. Power Electron. 29, 4500–4511 (2014).
Denisov, A. & Yeatman, E. Ultrasonic vs. inductive power delivery for miniature biomedical implants. In Proc. 2010 International Conference on Body Sensor Networks 84–89 (IEEE, 2010).
Podgorski, K. & Ranganathan, G. Brain heating induced by near-infrared lasers during multiphoton microscopy. J. Neurophysiol. 116, 1012–1023 (2016).
Mialhe, P., & Mouhamed, S. & Haydar, A. The solar cell output power dependence on the angle of incident radiation. Renew. Energ. 1, 519–521 (1991).
Johnson, B. C. et al. StimDust: a 6.5 mm3, wireless ultrasonic peripheral nerve stimulator with 82% peak chip efficiency. In Proc. 2018 IEEE Custom Integrated Circuits Conference (CICC) 1–4 (IEEE, 2018).
Piech, D. K. et al. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 4, 207–222 (2020).
Basaeri, H., Christensen, D. B. & Roundy, S. A review of acoustic power transfer for bio-medical implants. Smart Mater. Struct. 25, 123001 (2016).
Seo, D. et al. Ultrasonic beamforming system for interrogating multiple implantable sensors. In Proc. 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2673–2676 (IEEE, 2015).
Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers (US Food and Drug Administration, 2008).
O'Brien, W. D. Jr. Ultrasound-biophysics mechanisms. Prog. Biophys. Mol. Biol. 93, 212–255 (2007).
Bushberg, J. T. & Boone, J. M. The Essential Physics of Medical Imaging (Lippincott Williams & Wilkins, 2011).
Nelson, T. R., Fowlkes, J. B., Abramowicz, J. S. & Church, C. C. Ultrasound biosafety considerations for the practicing sonographer and sonologist. J. Ultrasound Med. 28, 139–150 (2009).
Samineni, V. K. et al. Fully implantable, battery-free wireless optoelectronic devices for spinal optogenetics. Pain 158, 2108–2116 (2017).
Harvey, C. D., Collman, F., Dombeck, D. A. & Tank, D. W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946 (2009).
Tye, K. M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Yilmaz, G. & Dehollain, C. Wireless Power Transfer and Data Communication for Neural Implants. Case Study: Epilepsy Monitoring (Springer, 2017).
Liang, T. & Yuan, Y. J. Wearable medical monitoring systems based on wireless networks: a review. IEEE Sens. J. 16, 8186–8199 (2016).
Zhang, Z. H., Russell, L. E., Packer, A. M., Gauld, O. M. & Hausser, M. Closed-loop all-optical interrogation of neural circuits in vivo. Nat. Methods 15, 1037–1040 (2018).
Mirza, K. B., Golden, C. T., Nikolic, K. & Toumazou, C. Closed-loop implantable therapeutic neuromodulation systems based on neurochemical monitoring. Front. Neurosci. 13, 808 (2019).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Aravanis, A. M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Shin, Y. et al. Characterization of fiber-optic light delivery and light-induced temperature changes in a rodent brain for precise optogenetic neuromodulation. Biomed. Opt. Express 7, 4450–4471 (2016).
Wang, Z., Hu, M., Ai, X., Zhang, Z. & Xing, B. Near‐infrared manipulation of membrane ion channels via upconversion optogenetics. Adv. Biosyst. 3, e1800233 (2019).
Lin, J. Y., Knutsen, P. M., Muller, A., Kleinfeld, D. & Tsien, R. Y. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16, 1499–1508 (2013).
Arrenberg, A. B., Bene, F. D. & Baier, H. Optical control of zebrafish behavior with halorhodopsin. Proc. Natl Acad. Sci. USA 106, 17968–17973 (2009).
Yarmolenko, P. S. et al. Thresholds for thermal damage to normal tissues: an update. Int. J. Hyperth. 27, 320–343 (2011).
Goncalves, S. B. et al. LED optrode with integrated temperature sensing for optogenetics. Micromachines 9, 473 (2018).
Khan, W. et al. Inductively coupled, mm-sized, single channel optical neuro-stimulator with intensity enhancer. Microsyst. Nanoeng. 5, 23 (2019).
Kampasi, K. et al. Fiberless multicolor neural optoelectrode for in vivo circuit analysis. Sci. Rep. 6, 30961 (2016).
Samineni, V. K. et al. Optogenetic silencing of nociceptive primary afferents reduces evoked and ongoing bladder pain. Sci. Rep. 7, 15865 (2017).
Busskamp, V. & Roska, B. Optogenetic approaches to restoring visual function in retinitis pigmentosa. Curr. Opin. Neurobiol. 21, 942–946 (2011).
Busskamp, V., Picaud, S., Sahel, J. A. & Roska, B. Optogenetic therapy for retinitis pigmentosa. Gene. Ther. 19, 169–175 (2012).
Chow, B. Y. & Boyden, E. S. Optogenetics and translational medicine.Sci. Transl. Med. 5, 177ps175 (2013).
Krook-Magnuson, E., Armstrong, C., Oijala, M. & Soltesz, I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 4, 1376 (2013).
Paz, J. T. et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 16, 64–70 (2013).
Nussinovitch, U. & Gepstein, L. Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat. Biotechnol. 33, 750–754 (2015).
Shao, J. et al. Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice. Sci. Transl. Med. 9, eaal2298 (2017).
Bansal, A., Yang, F., Xi, T., Zhang, Y. & Ho, J. S. In vivo wireless photonic photodynamic therapy. Proc. Natl Acad. Sci. USA 115, 1469–1474 (2018).
Kramer, R. H., Mourot, A. & Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci. 16, 816–823 (2013).
Banghart, M. R. & Sabatini, B. L. Photoactivatable neuropeptides for spatiotemporally precise delivery of opioids in neural tissue. Neuron 73, 249–259 (2012).
Jennings, J. H. et al. Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228 (2013).
Stamatakis, A. M. et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 80, 1039–1053 (2013).
McCall, J. G. et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015).
McCall, J. G. et al. Preparation and implementation of optofluidic neural probes for in vivo wireless pharmacology and optogenetics. Nat. Protoc. 12, 219–237 (2017).
Creed, M., Pascoli, V. J. & Lüscher, C. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 347, 659–664 (2015).
McCall, J. G. & Jeong, J. W. Minimally invasive probes for programmed microfluidic delivery of molecules in vivo. Curr. Opin. Neurobiol. 36, 78–85 (2017).
Noh, K. N. et al. Miniaturized, battery-free optofluidic systems with potential for wireless pharmacology and optogenetics. Small 14, 1702479 (2018).
Shmuel, A., Augath, M., Oeltermann, A. & Logothetis, N. K. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 9, 569–577 (2006).
Liu, J. N., Bu, W. & Shi, J. Chemical design and synthesis of functionalized probes for imaging and treating tumor hypoxia. Chem. Rev. 117, 6160–6224 (2017).
Hopf, H. W. & Rollins, M. D. Wounds: an overview of the role of oxygen. Antioxid. Redox Signal. 9, 1183–1192 (2007).
Neely, R. M., Piech, D. K., Santacruz, S. R., Maharbiz, M. M. & Carmena, J. M. Recent advances in neural dust: towards a neural interface platform. Curr. Opin. Neurobiol. 50, 64–71 (2018).
Ghanbari, M. M. et al. A sub-mm3 ultrasonic free-floating implant for multi-mote neural recording. IEEE J. Solid State Circuits 54, 3017–3030 (2019).
Ghanbari, M. M. et al. A 0.8 mm3 ultrasonic implantable wireless neural recording system with linear AM backscattering. In Proc. 2019 IEEE International Solid-State Circuits Conference (ISSCC) 284–286 (2019).
Kang, S. K., Koo, J., Lee, Y. K. & Rogers, J. A. Advanced materials and devices for bioresorbable electronics. Acc. Chem. Res. 51, 988–998 (2018).
Won, S. M. et al. Natural wax for transient electronics. Adv. Funct. Mater. 28, e1801819 (2018).
Boutry, C. M. et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 3, 47–57 (2019).
Koo, J. et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 24, 1830–1836 (2018).
Son, D. et al. Bioresorbable electronic stent integrated with therapeutic nanoparticles for endovascular diseases. ACS Nano 9, 5937–5946 (2015).
Pashaie, R. et al. Closed-loop optogenetic brain interface. IEEE Trans. Biomed. Eng. 62, 2327–2337 (2015).
Jonsson, A. et al. Bioelectronic neural pixel: chemical stimulation and electrical sensing at the same site. Proc. Natl Acad. Sci. USA 113, 9440–9445 (2016).
Capogrosso, M. et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539, 284–288 (2016).
Zanos, S. Closed-loop neuromodulation in physiological and translational research. Cold Spring Harb. Perspect. Med. 9, a034314 (2019).
Lo, M. C. & Widge, A. S. Closed-loop neuromodulation systems: next-generation treatments for psychiatric illness. Int. Rev. Psychiatry 29, 191–204 (2017).
Acknowledgements
L.C. and P.G. acknowledge the support of the Improving Health TRIF Project. P.G. acknowledges the support of NIG-NINDS R01NS112535 and start-up funds from the Department of Biomedical Engineering at the University of Arizona. S.M.W. acknowledges the support of the MSIT (Ministry of Science and ICT), Korea, under the ICT Creative Consilience program (IITP-2020-0-01821), supervised by the IITP (Institute for Information & communications Technology Planning & Evaluation). S.M.W. acknowledges support by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP; Ministry of Science, ICT & Future Planning; grant no. 2020R1G1A1101267). S.M.W. acknowledges the support by Nano Material Technology Development Program (2020M3H4A1A03084600) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT of Korea.
Author information
Authors and Affiliations
Contributions
All authors contributed to writing and revising the manuscript, and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Won, S.M., Cai, L., Gutruf, P. et al. Wireless and battery-free technologies for neuroengineering. Nat. Biomed. Eng 7, 405–423 (2023). https://doi.org/10.1038/s41551-021-00683-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41551-021-00683-3
This article is cited by
-
Integrated implantable bioelectronic system based on intrinsically stretchable and conductive nanocomposites
Health Nanotechnology (2025)
-
Self-contracting, battery-free triboelectric wound healing strip with strong wet adhesion
Nature Communications (2025)
-
A wireless optogenetic stimulation system for long-term function evaluation of mice forelimb with sub-nerve resolution
Nature Communications (2025)
-
Wirelessly controlled drug delivery systems for translational medicine
Nature Reviews Electrical Engineering (2025)
-
Challenges and opportunities in next-generation LED therapeutic devices
Light: Science & Applications (2025)