Abstract
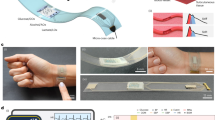
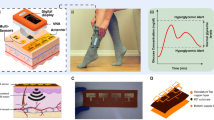
Monitoring the effects of daily activities on the physiological responses of the body calls for wearable devices that can simultaneously track metabolic and haemodynamic parameters. Here we describe a non-invasive skin-worn device for the simultaneous monitoring of blood pressure and heart rate via ultrasonic transducers and of multiple biomarkers via electrochemical sensors. We optimized the integrated device so that it provides mechanical resiliency and flexibility while conforming to curved skin surfaces, and to ensure reliable sensing of glucose in interstitial fluid and of lactate, caffeine and alcohol in sweat, without crosstalk between the individual sensors. In human volunteers, the device captured physiological effects of food intake and exercise, in particular the production of glucose after food digestion, the consumption of glucose via glycolysis, and increases in blood pressure and heart rate compensating for oxygen depletion and lactate generation. Continuous and simultaneous acoustic and electrochemical sensing via integrated wearable devices should enrich the understanding of the body’s response to daily activities, and could facilitate the early prediction of abnormal physiological changes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data generated and analysed during the study are included in the paper and its Supplementary Information.
Code availability
The LabView code used for processing the transducer raw signals into blood-pressure waveforms is available at https://doi.org/10.5281/zenodo.4433220.
References
Kim, J., Campbell, A. S., de Ávila, B. E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Yu, Y., Nyein, H. Y. Y., Gao, W. & Javey, A. Flexible electrochemical bioelectronics: the rise of in situ bioanalysis. Adv. Mater. 32, 1902083 (2020).
Khan, Y., Ostfeld, A. E., Lochner, C. M., Pierre, A. & Arias, A. C. Monitoring of vital signs with flexible and wearable medical devices. Adv. Mater. 28, 4373–4395 (2016).
Wang, C., Wang, C., Huang, Z. & Xu, S. Materials and structures toward soft electronics. Adv. Mater. 30, 1801368 (2018).
Lee, S. P. et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digit. Med. 1, 2 (2018).
Lee, Y.-D. & Chung, W.-Y. Wireless sensor network based wearable smart shirt for ubiquitous health and activity monitoring. Sens. Actuators B 140, 390–395 (2009).
Yapici, M. K., Alkhidir, T., Samad, Y. A. & Liao, K. Graphene-clad textile electrodes for electrocardiogram monitoring. Sens. Actuators B 221, 1469–1474 (2015).
Luo, N. et al. Flexible piezoresistive sensor patch enabling ultralow power cuffless blood pressure measurement. Adv. Funct. Mater. 26, 1178–1187 (2016).
Dagdeviren, C. et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 5, 4496 (2014).
Wang, C. et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2, 687–695 (2018).
Bandodkar, A. J. et al. Tattoo-based noninvasive glucose monitoring: a proof-of-concept study. Anal. Chem. https://doi.org/10.1021/ac504300n (2015).
Lee, H. et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 3, e1601314 (2017).
Kim, J., Campbell, A. S. & Wang, J. Wearable non-invasive epidermal glucose sensors: a review. Talanta 177, 163–170 (2018).
Lee, H., Hong, Y. J., Baik, S., Hyeon, T. & Kim, D. H. Enzyme-based glucose sensor: from invasive to wearable device. Adv. Healthc. Mater. https://doi.org/10.1002/adhm.201701150 (2018).
Kim, J. et al. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. https://doi.org/10.1002/advs.201800880 (2018).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Imani, S. et al. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 7, 11650 (2016).
Bruno, G., Anastasova, S. & Z. Yang, G. A smart wireless ear-worn device for cardiovascular and sweat parameter monitoring during physical exercise: design and performance results. Sensors 19, 1616 (2019).
Hong, Y. J. et al. Multifunctional wearable system that integrates sweat-based sensing and vital-sign monitoring to estimate pre-/post-exercise glucose levels. Adv. Funct. Mater. 28, 1805754 (2018).
Gomez, H. & Kellum, J. A. Lactate in sepsis. JAMA 313, 194–195 (2015).
Zanella, M. T., Kohlmann, O. & Ribeiro, A. B. Treatment of obesity hypertension and diabetes syndrome. Hypertension https://doi.org/10.1161/01.hyp.38.3.705 (2001).
Cheung, B. M. Y. & Li, C. Diabetes and hypertension: is there a common metabolic pathway? Curr. Atheroscler. Rep. 14, 160–166 (2012).
Epstein, M. & Sowers, J. R. Diabetes mellitus and hypertension. Hypertension 19, 403–418 (1992).
Deedwania, P. Hypertension, dyslipidemia, and insulin resistance in patients with diabetes mellitus or the cardiometabolic syndrome: benefits of vasodilating β-blockers. J. Clin. Hypertens. 13, 52–59 (2011).
Murković, I., Steinberg, M. D. & Murković, B. Sensors in neonatal monitoring: current practice and future trends. Technol. Health Care 11, 399–412 (2003).
Ho, K. K. Y. et al. Evaluation of an anti-thrombotic continuous lactate and blood pressure monitoring catheter in an in vivo piglet model undergoing open-heart surgery with cardiopulmonary bypass. Chemosensors 8, 56 (2020).
Chung, H. U. et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019).
Yang, J. et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 94, 91–95 (2020).
Sakharov, D. A. et al. Relationship between lactate concentrations in active muscle sweat and whole blood. Bull. Exp. Biol. Med. 150, 83–85 (2010).
Halliwill, J. R. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc. Sport Sci. Rev. 29, 65–70 (2001).
Carter, J. R., Stream, S. F., Durocher, J. J. & Larson, R. A. Influence of acute alcohol ingestion on sympathetic neural responses to orthostatic stress in humans. Am. J. Physiol. Metab. 300, E771–E778 (2011).
Roerecke, M. et al. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. Lancet Public Health 2, e108–e120 (2017).
Chen, L., Davey Smith, G., Harbord, R. M. & Lewis, S. J. Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Med. 5, e52 (2008).
Maheswaran, R., Gill, J. S., Davies, P. & Beevers, D. G. High blood pressure due to alcohol. A rapidly reversible effect. Hypertension 17, 787–792 (1991).
Ohira, T. et al. Effects of habitual alcohol intake on ambulatory blood pressure, heart rate, and its variability among Japanese men. Hypertension 53, 13–19 (2009).
Hauke, A. et al. Complete validation of a continuous and blood-correlated sweat biosensing device with integrated sweat stimulation. Lab Chip 18, 3750–3759 (2018).
Monte-Moreno, E. Non-invasive estimate of blood glucose and blood pressure from a photoplethysmograph by means of machine learning techniques. Artif. Intell. Med. 53, 127–138 (2011).
Çinar, Y. Blood viscosity and blood pressure: role of temperature and hyperglycemia. Am. J. Hypertens. 14, 433–438 (2001).
Filipovsky, J. et al. The relationship of blood pressure with glucose, insulin, heart rate, free fatty acids and plasma cortisol levels according to degree of obesity in middle-aged men. J. Hypertens. 14, 229–235 (1996).
Mort, J. R. & Kruse, H. R. Timing of blood pressure measurement related to caffeine consumption. Ann. Pharmacother. 42, 105–110 (2008).
Nurminen, M.-L., Niittynen, L., Korpela, R. & Vapaatalo, H. Coffee, caffeine and blood pressure: a critical review. Eur. J. Clin. Nutr. 53, 831–839 (1999).
Tai, L.-C. et al. Methylxanthine drug monitoring with wearable sweat sensors. Adv. Mater. 30, 1707442 (2018).
Ciui, B. et al. Chemical sensing at the robot fingertips: toward automated taste discrimination in food samples. ACS Sens. 3, 2375–2380 (2018).
Asano, R. Y. Acute effects of physical exercise in type 2 diabetes: a review. World J. Diabetes 5, 659–665 (2014).
Klaric, D. & Klaric, V. Alcohol-induced coma, hypothermia and hypotension. J. Membr. Sci. Technol. 5, 1000137 (2015).
Wilson, E. & Waring, W. S. Severe hypotension and hypothermia caused by acute ethanol toxicity. Emerg. Med. J. 24, e7 (2007).
Hillbom, M., Saloheimo, P. & Juvela, S. Alcohol consumption, blood pressure, and the risk of stroke. Curr. Hypertens. Rep. 13, 208–213 (2011).
Nystoriak, M. A. & Bhatnagar, A. Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. https://doi.org/10.3389/fcvm.2018.00135 (2018).
Fagard, R. H. Effects of exercise, diet and their combination on blood pressure. J. Hum. Hypertens. 19, S20–S24 (2005).
Jia, W. et al. Epidermal biofuel cells: energy harvesting from human perspiration. Angew. Chem. Int. Ed. 52, 7233–7236 (2013).
MacDonald, J. R. Potential causes, mechanisms, and implications of post exercise hypotension. J. Hum. Hypertens. 16, 225–236 (2002).
Crisafulli, A. et al. Effect of differences in post-exercise lactate accumulation in athletes’ haemodynamics. Appl. Physiol. Nutr. Metab. 31, 423–431 (2006).
Kokkinos, P. Cardiorespiratory fitness, exercise, and blood pressure. Hypertension 64, 1160–1164 (2014).
Ricci, F. & Palleschi, P. Sensor and biosensor preparation, optimization and applications of Prussian Blue modified electrodes. Biosens. Bioelectron. 21, 389–407 (2005).
Zhao, J. et al. A fully integrated and self-powered smartwatch for continuous sweat glucose monitoring. ACS Sens. 4, 1925–1933 (2019).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114, 4625–4630 (2017).
Acknowledgements
This research was supported by the UCSD Center of Wearable Sensors (CWS) and National Institutes of Health (grant no. 1R21EB027303-01A1). J.R.S. acknowledges support from CNPq (grant no. 216981/2014-0). E.D.l.p. acknowledges support from a UC MEXUS–CONACYT collaborative fellowship (2017–2022). A.A.K. acknowledges support from the Fulbright Egyptian Scholar Program (grant no. ay2019-2020). T.S.-a. acknowledges the Royal Golden Jubilee PhD scholarship of the Thailand Research Fund. We thank the Kraton Corporation for providing the SEBS samples.
Author information
Authors and Affiliations
Contributions
J.R.S., M.L. and L.Y. conceived the original project, designed and performed experiments, analysed data, and participated in the figure design and writing of the manuscript. E.D.l.p. performed experiments, analysed data, and participated in the figure design and writing of the manuscript. K.P. and T.S.-a. performed experiments and analysed data. A.N.d.L.S., A.A.K. and F.Z. performed experiments. N.T. fabricated the devices. J.W. and S.X. conceived the original project, designed the experiments, analysed data, participated in the figure design and manuscript writing, and provided guidance to the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures, references and video captions.
Supplementary Video 1
Application of hydrogels on the device.
Supplementary Video 2
Artery movement during cycling.
Rights and permissions
About this article
Cite this article
Sempionatto, J.R., Lin, M., Yin, L. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat Biomed Eng 5, 737–748 (2021). https://doi.org/10.1038/s41551-021-00685-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-021-00685-1
This article is cited by
-
Wearable Ultrasound Devices for Therapeutic Applications
Nano-Micro Letters (2026)
-
Ultra-strong Ionic Hydrogels for Machine Learning Enabled Human Machine Interfaces
Advanced Fiber Materials (2026)
-
Noninvasive On-Skin Biosensors for Monitoring Diabetes Mellitus
Nano-Micro Letters (2026)
-
Setting up an autonomic unit within the neurological department: a position statement of the European Federation of Autonomic Societies
Clinical Autonomic Research (2026)
-
A method for blood pressure hydrostatic pressure correction using wearable inertial sensors and deep learning
npj Biosensing (2025)