Abstract
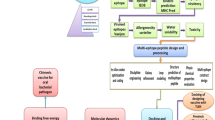
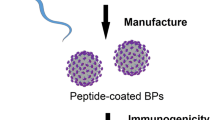
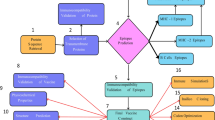
Most bacterial vaccines work for a subset of bacterial strains or require the modification of the antigen or isolation of the pathogen before vaccine development. Here we report injectable biomaterial vaccines that trigger potent humoral and T-cell responses to bacterial antigens by recruiting, reprogramming and releasing dendritic cells. The vaccines are assembled from regulatorily approved products and consist of a scaffold with absorbed granulocyte-macrophage colony-stimulating factor and CpG-rich oligonucleotides incorporating superparamagnetic microbeads coated with the broad-spectrum opsonin Fc-mannose-binding lectin for the magnetic capture of pathogen-associated molecular patterns from inactivated bacterial-cell-wall lysates. The vaccines protect mice against skin infection with methicillin-resistant Staphylococcus aureus, mice and pigs against septic shock from a lethal Escherichia coli challenge and, when loaded with pathogen-associated molecular patterns isolated from infected animals, uninfected animals against a challenge with different E. coli serotypes. The strong immunogenicity and low incidence of adverse events, a modular manufacturing process, and the use of components compatible with current good manufacturing practice could make this vaccine technology suitable for responding to bacterial pandemics and biothreats.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The proteomics data are available on the PRIDE repository under the accession code PXD023763 and can also be accessed via the MassIVE data storage at https://doi.org/10.25345/C5XB70.
References
Rice, L. B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197, 1079–1081 (2008).
Spellberg, B. et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46, 155–164 (2008).
Adalja, A. A. Biothreat agents and emerging infectious disease in the emergency department. Emerg. Med. Clin. North Am. 36, 823–834 (2018).
Messner, P., Schaffer, C. & Kosma, P. Bacterial cell-envelope glycoconjugates. Adv. Carbohydr. Chem. Biochem. 69, 209–272 (2013).
Haji-Ghassemi, O., Blackler, R. J., Martin Young, N. & Evans, S. V. Antibody recognition of carbohydrate epitopesdagger. Glycobiology 25, 920–952 (2015).
Nuttall, J. J. & Eley, B. S. BCG vaccination in HIV-infected children. Tuberc. Res. Treat. 2011, 712736 (2011).
Wilk, M. M. et al. Immunization with whole cell but not acellular pertussis vaccines primes CD4 TRM cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerg. Microbes Infect. 8, 169–185 (2019).
Liang, J. L. et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 67, 1–44 (2018).
Jaffe, J. et al. Effects of conformational changes in peptide-CRM197 conjugate vaccines. Bioconjug. Chem. 30, 47–53 (2019).
Moginger, U. et al. Cross Reactive Material 197 glycoconjugate vaccines contain privileged conjugation sites. Sci. Rep. 6, 20488 (2016).
Zhang, F., Lu, Y. J. & Malley, R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc. Natl Acad. Sci. USA 110, 13564–13569 (2013).
Kang, J. H. et al. An extracorporeal blood-cleansing device for sepsis therapy. Nat. Med. 20, 1211–1216 (2014).
Cartwright, M. et al. A broad-spectrum infection diagnostic that detects pathogen-associated molecular patterns (PAMPs) in whole blood. EBioMedicine 9, 217–227 (2016).
Seiler, B. T. et al. Broad-spectrum capture of clinical pathogens using engineered Fc-mannose-binding lectin enhanced by antibiotic treatment. F1000Res 8, 108 (2019).
Janeway, C. A. Jr & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Kim, J. et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat. Biotechnol. 33, 64–72 (2015).
Ali, O. A., Huebsch, N., Cao, L., Dranoff, G. & Mooney, D. J. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 8, 151–158 (2009).
Ali, O. A. & Mooney, D. J. Immunologically active biomaterials for cancer therapy. Curr. Top. Microbiol Immunol. 344, 279–297 (2011).
Wibowo, D. et al. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials 268, 120597 (2021).
Ke, X. et al. Physical and chemical profiles of nanoparticles for lymphatic targeting. Adv. Drug Deliv. Rev. 151–152, 72–93 (2019).
Xu, W., Riikonen, J. & Lehto, V. P. Mesoporous systems for poorly soluble drugs. Int. J. Pharm. 453, 181–197 (2013).
Ali, O. A. et al. Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants. Cancer Res. 74, 1670–1681 (2014).
Waterhouse A., et al. Modified clinical monitoring assessment criteria for multi-organ failure during bacteremia and sepsis progression in a pig model. Advan. Crit. Care Med. 1, 002 (2018).
Robbins, J. B., Schneerson, R., Horwith, G., Naso, R. & Fattom, A. Staphylococcus aureus types 5 and 8 capsular polysaccharide-protein conjugate vaccines. Am. Heart J. 147, 593–598 (2004).
Frenck, R. W. Jr et al. Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): a phase 2 randomised controlled trial. Lancet Infect. Dis. 19, 631–640 (2019).
Shah, N. J. et al. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat. Biomed. Eng. 4, 40–51 (2020).
Ali, O. A., Tayalia, P., Shvartsman, D., Lewin, S. & Mooney, D. J. Inflammatory cytokines presented from polymer matrices differentially generate and activate DCs in situ. Adv. Funct. Mater. 23, 4621–4628 (2013).
Li, A. W. et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat. Mater. 17, 528–534 (2018).
Dellacherie, M. O., Li, A. W., Lu, B. Y. & Mooney, D. J. Covalent conjugation of peptide antigen to mesoporous silica rods to enhance cellular responses. Bioconjug. Chem. 29, 733–741 (2018).
Cyster, J. G. & Schwab, S. R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 (2012).
Lindquist, R. L. et al. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5, 1243–1250 (2004).
Sun, L., Middleton, D. R., Wantuch, P. L., Ozdilek, A. & Avci, F. Y. Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology 26, 1029–1040 (2016).
Liebermeister, W. et al. Visual account of protein investment in cellular functions. Proc. Natl Acad. Sci. USA 111, 8488–8493 (2014).
Sellars, B. B., Sherrod, D. R. & Chappel-Aiken, L. Using word clouds to analyze qualitative data in clinical settings. Nurs. Manag. 49, 51–53 (2018).
Moran, J. L. & Solomon, P. J. Statistics in review Part I: graphics, data summary and linear models. Crit. Care Resusc. 9, 81–90 (2007).
Acknowledgements
We thank D. Bolgen, A. Nedder, K. Imaizumi and S. Bardales for their assistance with the mouse and pig models. This work was supported by the Wyss Institute for Biologically Inspired Engineering, DARPA (grant no. W911NF-16-C-0050 to D.E.I. and M.S.) and the National Institutes of Health (grant no. 1 R01 CA223255 to D.J.M.).
Author information
Authors and Affiliations
Contributions
M.S., E.J.D. and M.J.C. conceived the project, which was directed by D.E.I. and D.J.M. Vaccines were prepared and analysed by B.T.S., D.A.W., A.G.S., N.D., M.K., C.L.H., S.A.R., M.O.D., A.W.L. and J.M.S. Experiments in the mouse and pig models were conducted by F.L., A.R.G., K.R.L., F.R.U., C.D.Y., A.R.J. and S.L.L. The data were analysed by M.S., F.L., M.J.C., N.D. and V.C. The manuscript was written by M.S., E.J.D., D.E.I. and D.J.M. All authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.J.M. received sponsored research funding from Novartis, and has equity in Lyell and Attivare Therapeutics. D.E.I. is a founder, member of the board of directors and scientific advisory board, and equity holder in Boa Biomedical, Inc. M.S. is a founder and equity holder in BOA Biomedical. E.J.D., F.L. and B.T.S. are founders and have equity in Attivare Therapeutics. Inventors, patent applications: D.J.M., D.E.I., M.S., M.J.C., E.J.D., B.T.S., F.L., A.G.S., A.R.G., J.M.S. and D.A.W. For each patent, the serial number, country and patent number are provided: (1) 15/434,781; US; 10,813,988; (2) 17/015,177; US; (3) 2018-543154; Japan; 6854530; (4) 17753811; EPO; and (5) 202000000000; China. All other authors declare that they have no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Tarek Fahmy, Michael Mitchell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Super, M., Doherty, E.J., Cartwright, M.J. et al. Biomaterial vaccines capturing pathogen-associated molecular patterns protect against bacterial infections and septic shock. Nat Biomed Eng 6, 8–18 (2022). https://doi.org/10.1038/s41551-021-00756-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-021-00756-3
This article is cited by
-
Kinetics of molecular patterns captured by mannose-binding lectin in septic shock correlate with clinical outcome: a monocentric prospective observational study
Intensive Care Medicine Experimental (2025)
-
Chiral polypeptide hydrogels regulating local immune microenvironment and anti-tumor immune response
Nature Communications (2025)
-
Design and translation of injectable biomaterials
Nature Reviews Bioengineering (2024)
-
Cytokine-overexpressing dendritic cells for cancer immunotherapy
Experimental & Molecular Medicine (2024)
-
Multimodal probing of T-cell recognition with hexapod heterostructures
Nature Methods (2024)