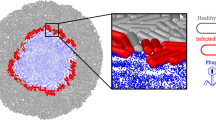
Abstract
The efficacy of bacteriophages in treating bacterial infections largely depends on the phages’ vitality, which is impaired when they are naturally released from their hosts, as well as by culture media, manufacturing processes and other insults. Here, by wrapping phage-invaded bacteria individually with a polymeric nanoscale coating to preserve the microenvironment on phage-induced bacterial lysis, we show that, compared with naturally released phages, which have severely degraded proteins in their tail, the vitality of phages isolated from polymer-coated bacteria is maintained. Such latent phages could also be better amplified, and they more efficiently bound and lysed bacteria when clearing bacterial biofilms. In mice with bacterially induced enteritis and associated arthritis, latent phages released from orally administered bacteria coated with a polymer that dissolves at neutral pH had higher bioavailability and led to substantially better therapeutic outcomes than the administration of uncoated phages.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions described in the study are provided within the paper and its Supplementary Information. The proteomics data are available via ProteomeXchange with identifier PXD054755. The raw and analysed datasets generated during the study are available for research purposes from the corresponding author on reasonable request. Source data are provided with this paper.
References
Gómez-Ochoa, S. A. et al. Efficacy of phage therapy in preclinical models of bacterial infection: a systematic review and meta-analysis. Lancet Microbe 3, 956–968 (2022).
Abedon, S. T. Use of phage therapy to treat long-standing, persistent, or chronic bacterial infections. Adv. Drug Deliv. Rev. 145, 18–39 (2019).
Petrovic Fabijan, A. et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 5, 465–472 (2020).
Ferry, T. et al. Personalized bacteriophage therapy to treat pandrug-resistant spinal Pseudomonas aeruginosa infection. Nat. Commun. 13, 4239 (2022).
Federici, S. et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185, 2879–2898 (2022).
Fernández, L. et al. Gram-positive pneumonia: possibilities offered by phage therapy. Antibiotics 10, 1000 (2021).
Hitchcock, N. M. et al. Current clinical landscape and global potential of bacteriophage therapy. Viruses 15, 1020 (2023).
Xu, Z. et al. Shelf-life prediction and storage stability of Aeromonas bacteriophage vB_AsM_ZHF. Virus Res. 323, 198997 (2023).
Luong, T., Salabarria, A. C., Edwards, R. A. & Roach, D. R. Standardized bacteriophage purification for personalized phage therapy. Nat. Protoc. 15, 2867–2890 (2020).
Jault, P. et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 19, 35–45 (2019).
Duyvejonck, H. et al. Evaluation of the stability of bacteriophages in different solutions suitable for the production of magistral preparations in Belgium. Viruses 13, 865 (2021).
Bourdin, G. et al. Amplification and purification of T4-like Escherichia coli phages for phage therapy: from laboratory to pilot scale. Appl. Environ. Microbiol. 80, 1469–1476 (2014).
Jończyk-Matysiak, E. et al. Factors determining phage stability/activity: challenges in practical phage application. Expert Rev. Anti Infect. Ther. 17, 583–606 (2019).
Jończyk, E., Kłak, M., Międzybrodzki, R. & Górski, A. The influence of external factors on bacteriophages—review. Folia Microbiol. 56, 191–200 (2011).
Górski, A., Borysowski, J. & Międzybrodzki, R. Phage therapy: towards a successful clinical trial. Antibiotics 9, 827 (2020).
Sarker, S. A. et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine 4, 124–137 (2016).
Vinner, G. K. et al. Microencapsulation of Salmonella-specific bacteriophage Felix O1 using spray-drying in a pH-responsive formulation and direct compression tableting of powders into a solid oral dosage form. Pharmaceuticals 12, 43 (2019).
Richards, K. & Malik, D. J. Microencapsulation of bacteriophages using membrane emulsification in different pH-triggered controlled release formulations for oral administration. Pharmaceuticals 14, 424 (2021).
Malik, D. J. et al. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 249, 100–133 (2017).
Mourosi, J. T. et al. Understanding bacteriophage tail fiber interaction with host surface receptor: the key “Blueprint” for reprogramming phage host range. Int. J. Mol. Sci. 23, 12146 (2022).
Bonachela, J. A., Choua, M. & Heath, M. R. Unconstrained coevolution of bacterial size and the latent period of plastic phage. PLoS ONE 17, e0268596 (2022).
Abedon, S. T. Selection for bacteriophage latent period length by bacterial density: a theoretical examination. Microb. Ecol. 18, 79–88 (1989).
Sheikh, A. et al. Enterotoxigenic Escherichia coli heat-labile toxin drives enteropathic changes in small intestinal epithelia. Nat. Commun. 13, 6886 (2022).
Rogers, A. P., Mileto, S. J. & Lyras, D. Impact of enteric bacterial infections at and beyond the epithelial barrier. Nat. Rev. Microbiol. 21, 260–274 (2023).
Duan, Y., Young, R. & Schnabl, B. Bacteriophages and their potential for treatment of gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 19, 135–144 (2022).
Zhang, Y., Sharma, S., Tom, L., Liao, Y.-T. & Wu, V. C. H. Gut phageome—an insight into the role and impact of gut microbiome and their correlation with mammal health and diseases. Microorganisms 11, 2454 (2023).
Gutiérrez, B. & Domingo-Calap, P. Phage therapy in gastrointestinal diseases. Microorganisms 8, 1420 (2020).
Hsu, B. B. et al. In situ reprogramming of gut bacteria by oral delivery. Nat. Commun. 11, 5030 (2020).
Yin, H. et al. Microencapsulated phages show prolonged stability in gastrointestinal environments and high therapeutic efficiency to treat Escherichia coli O157:H7 infection. Vet. Res. 52, 118 (2021).
Meng, L. et al. Nanocapping-enabled charge reversal generates cell-enterable endosomal-escapable bacteriophages for intracellular pathogen inhibition. Sci. Adv. 8, eabq2005 (2022).
Feng, P., Cao, Z., Wang, X., Li, J. & Liu, J. On-demand bacterial reactivation by restraining within a triggerable nanocoating. Adv. Mater. 32, 2002406 (2020).
Mahmood, S. et al. Synthesis, characterization and safety profiling of eudragit-based pH-responsive hydrogels: a promising platform for colonic delivery of losartan potassium. Curr. Drug Deliv. 16, 548–564 (2019).
Moustafine, R. I. et al. Structural transformations during swelling of polycomplex matrices based on countercharged (meth) acrylate copolymers (EudragitR EPO/EudragitR L 100-55). J. Pharm. Sci. 100, 874–885 (2011).
Sakhno, T. V., Barashkov, N. N., Irgibaeva, I. S., Pustovit, S. V. & Sakhno, Y. E. Polymer coatings for protection of wood and wood-based materials. Adv. Chem. Eng. 6, 93–110 (2016).
Emslander, Q. et al. Cell-free production of personalized therapeutic phages targeting multidrug-resistant bacteria. Cell Chem. Biol. 29, 1434–1445.e7 (2022).
Stone, E., Campbell, K., Grant, I. & McAuliffe, O. Understanding and exploiting phage–host interactions. Viruses 11, 567 (2019).
Kortright, K. E., Chan, B. K., Koff, J. L. & Turner, P. E. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25, 219–232 (2019).
Choi, V., Rohn, J. L., Stoodley, P., Carugo, D. & Stride, E. Drug delivery strategies for antibiofilm therapy. Nat. Rev. Microbiol. 21, 555–572 (2023).
Arciola, C. R., Campoccia, D. & Montanaro, L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 16, 397–409 (2018).
Chegini, Z. et al. Bacteriophage therapy against Pseudomonas aeruginosa biofilms: a review. Ann. Clin. Microbiol. Antimicrob. 19, 45 (2020).
Winstanley, C., O’Brien, S. & Brockhurst, M. A. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 24, 327–337 (2016).
Ly-Chatain, M. H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 5, 51 (2014).
Hassan, A. Y., Lin, J. T., Ricker, N. & Anany, H. The age of phage: friend or foe in the new dawn of therapeutic and biocontrol applications? Pharmaceuticals 14, 199 (2021).
Dąbrowska, K. Phage therapy: what factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 39, 2000–2025 (2019).
Colom, J. et al. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 81, 4841–4849 (2015).
Kauffman, K. M. et al. Resolving the structure of phage–bacteria interactions in the context of natural diversity. Nat. Commun. 13, 372 (2022).
Zampara, A. et al. Exploiting phage receptor binding proteins to enable endolysins to kill Gram-negative bacteria. Sci. Rep. 10, 12087 (2020).
Brzozowska, E., Bazan, J. & Gamian, A. The functions of bacteriophage proteins. Postepy Hig. Med. Dosw. (Online) 65, 167–176 (2011).
Crosby, J. & Crump, M. P. The structural role of the carrier protein – active controller or passive carrier. Nat. Prod. Rep. 29, 1111–1137 (2012).
Kreitz, J. et al. Programmable protein delivery with a bacterial contractile injection system. Nature 616, 357–364 (2023).
Bernal, P. et al. A novel stabilization mechanism for the type VI secretion system sheath. Proc. Natl Acad. Sci. USA 118, e2008500118 (2021).
Rodwell, E. V. et al. Isolation and characterisation of bacteriophages with activity against invasive non-typhoidal Salmonella causing bloodstream infection in Malawi. Viruses 13, 478 (2021).
Paradiso, R. et al. Complete genome sequences of three Siphoviridae bacteriophages infecting Salmonella enterica serovar enteritidis. Genome Announc. 4, e00939-16 (2016).
Wang, X. et al. Versatility of bacterial outer membrane vesicles in regulating intestinal homeostasis. Sci. Adv. 9, eade5079 (2023).
Chriswell, M. E. et al. Clonal IgA and IgG autoantibodies from individuals at risk for rheumatoid arthritis identify an arthritogenic strain of Subdoligranulum. Sci. Transl. Med. 14, eabn5166 (2022).
Miller, A. L. et al. In vivo synthesis of bacterial amyloid curli contributes to joint inflammation during S. Typhimurium infection. PLoS Pathog. 16, e1008591 (2020).
Mills, K. H. G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 23, 38–54 (2023).
Diard, M. et al. A rationally designed oral vaccine induces immunoglobulin A in the murine gut that directs the evolution of attenuated Salmonella variants. Nat. Microbiol. 6, 830–841 (2021).
Chen, K., Magri, G., Grasset, E. K. & Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 20, 427–441 (2020).
Urrechaga, E., Bóveda, O., Aguirre, U., García, S. & Pulido, E. Neutrophil cell population data biomarkers for acute bacterial infection. J. Pathol. Infect. Dis. 1, 1–7 (2018).
Takayuki, H. et al. Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin. Chim. Acta 457, 46–53 (2016).
Kowalska-Kępczyńska, A., Weronika, K. & Helena, D. Innovative assessment practices of inflammation. Eur. J. Med. Technol. 1, 26 (2020).
Guo, Z. et al. Lymphopenia caused by virus infections and the mechanisms beyond. Viruses 13, 1876 (2021).
Son, B. et al. Isolation and characterization of a Weizmannia coagulans bacteriophage Youna2 and its endolysin PlyYouna2. J. Microbiol. Biotechnol. 33, 67 (2023).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331 (2017).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Fernandez, J. J. & Li, S. TomoAlign: A novel approach to correcting sample motion and 3D CTF in CryoET. J. Struct. Biol. 213, 4 (2021).
Winkler, H. et al. Tomographic subvolume alignment and subvolume classification applied to myosin V and SIV envelope spikes. J. Struct. Biol. 165, 64–77 (2009).
Goddard, T. D. et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Prot. Sci. 27, 14–25 (2018).
Acknowledgements
We thank P. He for offering the phage of S. aureus. This work was financially supported by the National Key Research and Development Program of China (2021YFA0909400, J.L.), the National Natural Science Foundation of China (22425505, J.L.; 22307077, G.X.; 32201144, S.L.; 22375127, Y.P.) and the Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20210700, Y.P.).
Author information
Authors and Affiliations
Contributions
J.L. supervised the project. J.L., S.L. and G.X. designed the experiments. S.L. and G.X. performed and interpreted the experiments. S.L. conducted cellular and animal experiments. J.H. performed the single-particle cryo-TEM experiment. L.M. and Y.P. provided analytical tools, critical comments and suggestions. G.X., S.L. and J.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of the coating on bacteria.
a, Representative TEM images of the sections of S. Typhimurium and copolymer-coated phage-infected S. Typhimurium. Scale bars, 500 nm. The formed nanocoating structure on bacterial surface was highlighted in an enlarged view. Scale bar, 100 nm. b, c, Flow cytometry (b) histograms and (c) scatter plots of S. Typhimurium and copolymer-coated phage-infected S. Typhimurium, respectively. d, Percentage of copolymer-coated phage-infected S. Typhimurium after coating formation. e, MFI of S. Typhimurium and Cy3-labeled copolymer-coated phage-infected S. Typhimurium, respectively. Data are represented as mean ± s.d. (n = 5). P values were determined using two-tailed t-test.
Extended Data Fig. 2 Proteomic analysis of In-phages and Ex-phages.
a, b, The (a) principal component analysis and (b) Venn diagram of the protein expression data in Ex-phages and In-phages. c, Dotrod-heatmap of the proteins of Ex-phages and In-phages. d, A co-occurrence network constructed from the relative abundances of differential proteins in Ex-phages versus In-phages. e–g, Comparative analysis of (e) DNA ligase, (f) DNA helicase, and (g) ribonucleoside-diphosphate reductase. Data are represented as mean ± s.d. (n = 3). P values were determined using two-tail t-test.
Extended Data Fig. 3 Inhibitory effect of phages on the growth of S. Typhimurium and the colonization in the gut.
a, Experimental design for the animal study of S. Typhimurium infection. b, Bacterial counts in the feces. c, Confocal images of the colon sections stained with S. Typhimurium O antibody (green) and DAPI (blue). Scale bar, 50 µm. d, Levels of phages in the feces. e, Abundance of phages in the whole intestine at the end of treatment. Data are represented as mean ± s.d. (n = 5–6). P value was determined using one-way ANOVA. Elements created with BioRender.com.
Supplementary information
Supplementary Information
Supplementary figures.
Supplementary Data
Source data for the supplementary figures.
Source data
Source Data for Figs. 1–8
Statistical source data.
Source Data for Extended Data Figs. 1–3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, S., Xie, G., He, J. et al. Enhancing phage therapy by coating single bacteriophage-infected bacteria with polymer to preserve phage vitality. Nat. Biomed. Eng 9, 1155–1171 (2025). https://doi.org/10.1038/s41551-025-01354-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01354-3
This article is cited by
-
Engineered bacteria for cancer therapy: synergistic innovations in synthetic biology and materials science
Science China Materials (2025)