Abstract
Current methods for the precise integration of DNA sequences into the genome of human T cells predominantly target exonic regions, which limits the choice of integration site and requires complex cell-selection strategies. Here we show that non-viral intron knock-ins for incorporating synthetic exons into endogenous introns enable efficient gene targeting and selective gene knockout in successfully edited cells. In primary human T cells, the knock-in of a chimaeric antigen receptor (CAR) into the T-cell receptor alpha constant locus facilitated the purification of more than 90% CAR+ T cells via the negative selection of T-cell-receptor-negative cells. The method is scalable, applicable across intronic sites, as we show for introns within four distinct endogenous surface-receptor genes, and supports the integration of large synthetic exons (longer than 5 kb), of alternative splicing architectures that preserve endogenous gene expression, and of synthetic promoters allowing for endogenous or user-defined gene regulation. Non-viral intron knock-ins expand the range of targetable genomic sites and provide a simplified and high-throughput strategy for selecting edited primary human T cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and its supplementary tables. An annotated list of all gRNA sequences is available in Supplementary Table 1. An annotated list of all DNA constructs, as well as full DNA sequences for all constructs, is available in Supplementary Table 2. Annotated genebank files for all DNA constructs used in the study are available from the corresponding authors on request.
References
Wang, J. Y. & Doudna, J. A. CRISPR technology: a decade of genome editing is only the beginning. Science 379, eadd8643 (2023).
Makarova, K. S. et al. Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18, 67–83 (2020).
Maeder, M. L. & Gersbach, C. A. Genome-editing technologies for gene and cell therapy. Mol. Ther. 24, 430–446 (2016).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Roth, T. L. et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018).
Shy, B. R. et al. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01418-8 (2022).
Mikkelsen, N. S. & Bak, R. O. Enrichment strategies to enhance genome editing. J. Biomed. Sci. 30, 51 (2023).
Allen, A. G. et al. A highly efficient transgene knock-in technology in clinically relevant cell types. Nat. Biotechnol.42, 458–469 (2024). (2024).
Wiebking, V. et al. Metabolic engineering generates a transgene-free safety switch for cell therapy. Nat. Biotechnol. 38, 1141–1450 (2020).
Levesque, S., Carleton, G., Duque, V. & Goupil, C. Pharmacological control of CAR T cells through CRISPR- driven rapamycin resistance. Preprint at bioRxiv https://doi.org/10.1101/2023.09.14.557485 (2024).
Chavez, M., Rane, D. A., Chen, X. & Qi, L. S. Stable expression of large transgenes via the knock-in of an integrase-deficient lentivirus. Nat. Biomed. Eng. 7, 661–671 (2023).
Mosti, L. et al. Targeted multi-epitope switching enables straightforward positive/negative selection of CAR T cells. Gene Ther. 28, 602–612 (2021).
Yin, H., Kauffman, K. J. & Anderson, D. G. Delivery technologies for genome editing. Nat. Rev. Drug Discov. 16, 387–399 (2017).
Schober, K. et al. Orthotopic replacement of T-cell receptor α- and β-chains with preservation of near-physiological T-cell function. Nat. Biomed. Eng. 3, 974–984 (2019).
Odé, Z., Condori, J., Peterson, N., Zhou, S. & Krenciute, G. CRISPR-mediated non-viral site-specific gene integration and expression in T cells: protocol and application for T-cell therapy. Cancers 12, 1704 (2020).
Bennardo, N., Cheng, A., Huang, N. & Stark, J. M. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLOS Genet. 4, e1000110 (2008).
Chang, H. H. Y., Pannunzio, N. R., Adachi, N. & Lieber, M. R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 18, 495–506 (2017).
Burma, S., Chen, B. P. C. & Chen, D. J. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair 5, 1042–1048 (2006).
Simeonov, D. R. et al. A large CRISPR-induced bystander mutation causes immune dysregulation. Commun. Biol. 2, 70 (2019).
Kosicki, M., Tomberg, K. & Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 36, 765–771 (2018).
Adikusuma, F. et al. Large deletions induced by Cas9 cleavage. Nature 560, E8–E9 (2018).
Stanford, W. L., Cohn, J. B. & Cordes, S. P. Gene-trap mutagenesis: past, present and beyond. Nat. Rev. Genet. 2, 756–768 (2001).
Serebrenik, Y. V., Sansbury, S. E., Kumar, S. S., Henao-Mejia, J. & Shalem, O. Efficient and flexible tagging of endogenous genes by homology-independent intron targeting. Genome Res. 29, 1322–1328 (2019).
Fu, J. et al. Improved and flexible HDR editing by targeting introns in iPSCs. Stem Cell Rev. Rep. 18, 1822–1833 (2022).
Li, J. et al. Gene replacements and insertions in rice by intron targeting using CRISPR–Cas9. Nat. Plants 2, 16139 (2016).
Sigal, A. et al. Dynamic proteomics in individual human cells uncovers widespread cell-cycle dependence of nuclear proteins. Nat. Methods 3, 525–531 (2006).
Foy, S. P. et al. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature 615, 687–696 (2023).
Zhang, J. et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature 609, 369–374 (2022).
Orentas, R. J. A positive take on negative selection for CAR-T manufacturing. Mol. Ther. Methods Clin. Dev. 32, 101218 (2024).
Zhong, H. et al. High-fidelity, efficient, and reversible labeling of endogenous proteins using CRISPR-based designer exon insertion. eLife 10, e64911 (2021).
Liu, H. X., Zhang, M. & Krainer, A. R. Identification of functional exonic splicing enhancer motifs recognized by individual SR proteins. Genes Dev. 12, 1998–2012 (1998).
Cáceres, E. F. & Hurst, L. D. The evolution, impact and properties of exonic splice enhancers. Genome Biol. 14, R143 (2013).
Ke, S. et al. Quantitative evaluation of all hexamers as exonic splicing elements. Genome Res. 21, 1360–1374 (2011).
Kluesner, M. G. et al. CRISPR-Cas9 cytidine and adenosine base editing of splice-sites mediates highly-efficient disruption of proteins in primary and immortalized cells. Nat. Commun. 12, 2437 (2021).
Tsunekawa, Y. et al. Developing a de novo targeted knock-in method based on in utero electroporation into the mammalian brain. Development 143, 3216–3222 (2016).
Auer, T. O., Duroure, K., De Cian, A., Concordet, J.-P. & Del Bene, F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 24, 142–153 (2014).
Dunbar, C. E. et al. Gene therapy comes of age. Science 359, eaan4672 (2018).
Smith, C. J. et al. Enabling large-scale genome editing at repetitive elements by reducing DNA nicking. Nucleic Acids Res. 48, 5183–5195 (2020).
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21, 145–161 (2021).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Durrant, M. G. et al. Bridge RNAs direct programmable recombination of target and donor DNA. Nature 630, 984–993 (2024).
Durrant, M. G. et al. Systematic discovery of recombinases for efficient integration of large DNA sequences into the human genome. Nat. Biotechnol. 41, 488–499 (2023).
Vo, P. L. H. et al. CRISPR RNA-guided integrases for high-efficiency, multiplexed bacterial genome engineering. Nat. Biotechnol. 39, 480–489 (2021).
Rezazade Bazaz, M., Ghahramani Seno, M. M. & Dehghani, H. Transposase-CRISPR mediated targeted integration (TransCRISTI) in the human genome. Sci. Rep. 12, 3390 (2022).
Tou, C. J., Orr, B. & Kleinstiver, B. P. Precise cut-and-paste DNA insertion using engineered type V-K CRISPR-associated transposases. Nat. Biotechnol. 41, 968–979 (2023).
De Silva, D. et al. Robust T cell activation requires an eIF3-driven burst in T cell receptor translation. eLife 10, e74272 (2021).
Padovan, E. et al. Expression of two T cell receptor α chains: dual receptor T cells. Science 262, 422–424 (1993).
Acknowledgements
We thank the members of the Satpathy lab for stimulating discussions. This work was supported by a Career Award for Medical Scientists from the Burroughs Wellcome Fund (A.T.S.), a Lloyd J. Old STAR Award from the Cancer Research Institute (A.T.S.), the Parker Institute for Cancer Immunotherapy (A.T.S.), a CRISPR Cures for Cancer Award (A.T.S.), and the California Institute for Regenerative Medicine (DISC2–13212, A.T.S.).
Author information
Authors and Affiliations
Contributions
T.L.R. and A.T.S. conceptualized the study. T.L.R. and A.T.S. wrote and edited the paper and all authors reviewed and provided comments on the paper. T.L.R., J.L., A.M., C.K., and O.T.-N. performed experiments and analysed data. T.L.R. and A.T.S. guided experiments and data analysis.
Corresponding authors
Ethics declarations
Competing interests
T.L.R. is a founder of Arsenal Biosciences. A.T.S. is a founder of Immunai, Cartography Biosciences, Santa Ana Bio, and Prox Biosciences, an advisor to Zafrens and Wing Venture Capital, and receives research funding from Merck Research Laboratories and Astellas Pharma. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Xiao-Bing Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Efficient knock-in of a synthetic exon across TRAC intronic sites.
a, Observed efficiency of TCR knockout (measured by flow cytometric surface staining for CD3) after gene editing with Cas9 or Cas12a RNPs containing gRNAs targeting TRAC exon 15 or 18 distinct targets within TRAC intron 1 (avoiding a highly repetitive region within the intron). TCR knockout was highly efficient for the exon targeting gRNA, but significantly lower for intronic guides. b, Percentage of total cells expressing the knock-in gene cassette (GFP + ) following CD3 depletion (removing TCR positive cells) after intron knock-in at 18 unique sites throughout TRAC intron 1. n = 2-4 unique donors. c, TCR knockout (measured by flow cytometric surface staining for CD3) after gene editing with Cas12a RNPs containing gRNAs targeting TRAC intron 2. d, Knock-in efficiency at four unique sites within TRAC intron 2. e, Percentage of total cells expressing the knock-in gene cassette (GFP + ) following CD3 depletion (removing TCR positive cells) after intron targeting at 4 unique sites throughout TRAC intron 2. f, Across four TRAC intron 1 target sites targeted with Cas9 RNPs, no major difference in knock-in efficiency and successful protein expression from the integrated synthetic exon was observed when using the endogenous splice acceptor and donor sites from the adjacent endogenous TRAC exons compared to synthetic consensus splice acceptor and donor sites. a-f, n = 2-4 unique primary human T cell donors.
Extended Data Fig. 2 Viability, editing, and activation metrics with non-viral intron editing and negative selection in human T cells.
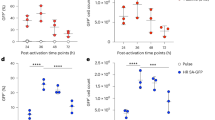
a, Timeline of primary human T cell editing using non-viral intron knock-ins, followed by negative selection. T cells are isolated and activated on Day 0, followed by electroporation based non-viral intron editing on Day 2. CD3 Negative selection was performed on Day 8, six days followed editing. b, Total T cell counts on Day 1 and Day 4 post electroporation relative to no electroporation controls following non-viral intron knock-ins by electroporation of a TRAC intron targeting CRISPR-Cas9 RNP along with a DNA Homology Directed Repair Template. Approximately half of the cell loss observed in the intron knock-in condition (“RNP + DNA”) is due to electroporation itself, and half due to DNA toxicity, as observed previously for non-viral exon knock-ins using electroporation in primary human T cells5,14. n = 3 donors with 3 technical replicates each. c, The percentage of successfully edited intron knock-in cells across timepoints, beginning three days after editing (Day 5 post activation). Across n = 3 donors, editing percentages were stable in culture during post-editing expansion. Error bars represent standard error of the mean. d, At Day 8 post activation, TRAC intron knock-in T cells were negatively selected by binding magnetic beads to the CD3 complex. Compared to the input population prior to selection, post-negative selection intron knock-in T cells did not show any decrease in viability as measured by Tryphan Blue staining and Live/Dead dye flow cytometric staining. The observed increase in viability after selection is likely due to washing steps removing dying cells present in the input culture. e, After either negative selection or no selection, the activation status of TRAC intron knock-in CD19-28z CAR T cells was assessed through in vitro activation by Nalm6 target cells at a 1:4 Effector:Target cell ratio. 24 hours post co-incubation, CD69 and CD25 expression levels in CAR positive (tNGFR + ) and CAR negative (tNGFR-) cells was assayed by flow cytometry. n = 2 unique donors with 3 technical replicates. ns = not significant, Two-sided paired t test.
Extended Data Fig. 3 Direct comparison of common edited primary human T cell selection methods.
a, Intron knock-in of a tNGFR-CAR-PuroR synthetic exon enabled a single population of edited cells to be compatible with four common selection methods. Representative flow cytometric plots of TRAC intron CAR T cells after purification with four distinct selection methods. Knock-in of a synthetic exon containing a tNGFR-CAR-PuroR multicistronic cassette to TRAC intron 1 enabled successfully edited cells to be negatively selected by CD3 Depletion (removal of TCR positive cells), positively selected by streptavidin magnetic bead enrichment after binding of anti-tNGFR biotinylated antibodies, fluorescence-activated cell sorting after binding of anti-tNGFR fluorescent antibodies, or drug selection after culture in puromycin. Negative selection by CD3 depletion yields predominantly a successfully edited CAR + T cell population without the endogenous TCR, although rarer TCR negative / knock-in negative cells are present (likely due to the RNP induced double stranded break within the intron causing a large deletion that included a portion of one or both adjacent exons, instead of the more common NHEJ repair outcome of smaller indels). Positive selection, sorting, and drug selection in contrast remove all knock-in negative cells, but retain a population of TCR positive / knock-in positive cells (likely due to successful HDR mediated knock-in to one TRAC allele with either no editing or a small indel removed during mRNA splicing on the second TRAC allele; while in some T cells one TCRα loci is silenced, numerous T cells express functional TCRα chains from both alleles48). Only negative selections do not require additional genetic material (for example no selection marker or resistance gene necessary) and leave cells untouched post-selection. b, Percentage of residual TCR positive cells following four different selection methods. TRAC intron knock-in followed by CD3 Negative Selection (Blue) leaves almost no detectable TCR+ cells remaining. c, In vitro killing of Nalm6 target cells by TRAC intron knock-in CD19-28z CAR T cells following negative, positive, sorting, or drug selection, measured 48 hours post co-incubation. n = 4 unique donors. ns = not significant, * = P < 0.05, Two-sided paired t test. d, In vitro proliferation of TRAC intron knock-in CD19-28z CAR T cells following negative, positive, sorting, or drug selection after co-culture with Nalm6 target cells. Error bars represent standard error of the mean from n = 4 unique donors. ns = not significant, * = P < 0.05, Two-sided Mann-Whitney test. e, Puromycin dose titrations reveal a tradeoff between purity and cell yield when performing drug selections. Increasing doses of puromycin yielded greater T cell purity, but overall edited cell yield began to decline with increasing puromycin concentrations. Beginning 6 days post editing, 100,000 bulk edited T cells were treated with indicated doses of puromycin for 48 hours. A dose of 5 ug/mL was used for experiments in Fig. 2b-c to balance purity and yield. n = 2 donors with 4 technical replicates each.
Extended Data Fig. 4 Variable 5’ and 3’ splicing architectures enable control of alternative splicing of synthetic exons.
a, Correlation between observed cellular protein phenotypes by flow cytometry with inferred splicing behaviour. Cells expressing both the protein encoded by the synthetic exon (for example GFP) and the endogenous protein (for example TCR) must be undergoing both synthetic splicing and endogenous splicing. These two splicing outcomes could be occurring from the same pre-mRNA transcript from a single allele by alternative splicing (single pre-mRNA transcript spliced into two different mature mRNA transcripts, one encoding the synthetic gene and a second encoding the endogenous gene), or the two splicing outcomes could be occurring within two separate pre-mRNA transcripts expressed from two different alleles (for example one allele with successful intron knock-in of the synthetic exon, with the second allele being either unedited or possessing small indels from NHEJ repair that do not interfere with endogenous gene expression). Biallelic intron knock-in experiments (Fig. 5) support that the majority, but not all, dual positive cells result from alternative splicing of the same pre-mRNA from a single allele. b, Variable 3’ synthetic exon splicing architectures lead to controllable degrees of alternative splicing. Balanced splicing (2A-SD, far left) results in the majority of knock-in (GFP) cells being negative for the endogenous gene (TCR-), with the residual GFP + TCR+ cells likely due to one knock-in allele and one WT or NHEJ edited allele. Creation of unbalanced splicing by replacement of the splice donor with a polyA (either SV40, WPRE, or TRAC’s endogenous polyA47) resulted in an increase in dual positive cells, with varying levels of endogenous gene expression depending on the polyA sequence used (SV40 > WPRE or TRAC). The differing levels of endogenous gene expression observed were potentially due to variable efficiency of RNA transcription termination by the polyA sequences, with the shorter SV40 polyA sequence less efficient at stopping transcription, enabling more mRNA transcripts to continue to the endogenous exons downstream from the knocked in synthetic exon and thus be capable of alternative splicing. Indeed, addition of a splice donor sequence following the polyA tails decreased the expression levels of the endogenous gene for all three polyA sequences, returning completely to baseline (2A-SD construct, far left) with the WPRE and TRAC polyA, and lowering the TCR expression level for the SV40 polyA, supporting the interpretation that unbalanced splice sites in the polyA only constructs lead to greater alternative splicing. SV40 polyA flow plot reproduced from Fig. 3b for comparisons. c, Alternative splicing can also be controlled by varying the synthetic exon’s 5’ splicing architecture. In intron knock-in constructs containing both an SV40 or TRAC endogenous polyA sequences at their 3’ end, inclusion of Exonic Splicing Silencer (ESS) DNA sequences adjacent to the synthetic exon’s 5’ splice acceptor resulted in increased numbers of dual positive cells showing evidence of alternative splicing. The short 6-8 bp ESS sequences were introduced into the 2 A multicistronic element at the 5’ end of the synthetic exon (necessary to separate the new synthetic gene’s protein translation from the translation of the preceding endogenous exon) using degenerate bases to maximize the number and strength of the ESS sequences present33. In pre-mRNA transcripts containing unbalanced splice sites due to the 3’ polyA, inclusion of ESS sequences at the synthetic exon’s 5’ end reduces binding by the SR proteins that mediate splicing and increases the chance that the preceding endogenous exon will splice with the downstream endogenous exon rather than the synthetic exon. Both splicing outcomes occur with alternative splicing, resulting in expression of both the endogenous gene and the new synthetic gene. In contrast, similar optimization of the 2 A elements degenerate bases to include Exonic Splicing Enhancer elements (or insertion of in-frame ESE elements prior to the unaltered 2 A sequence) largely prevented alternative splicing, returning the levels of dual positive cells back to amount seen with synthetic exons containing balanced splice sites (for example the amount of dual positive cells seen due to one knock-in allele and one unedited/NHEJ allele). ESS-2A – SV40 polyA flow plot reproduced from Fig. 3d for comparisons. d, Summary heatmap of tested combinations of 5’ and 3’ splicing architectures. For applications requiring both endogenous and synthetic splicing of a synthetic exon integrated into an endogenous intronic region, an Exonic Splicing Silencer 5’ architecture paired with a short SV40 polyA 3’ architecture proved optimal. For applications requiring predominantly synthetic splicing, an Exonic Splicing Enhancer (ESE) 5’ architecture paired with any of the tested polyA 3’ architectures showed approximately equivalent degrees of synthetic splicing.
Extended Data Fig. 5 Generalized endogenous gene targeting with exon and intron knock-ins.
a, A new synthetic gene can be introduced under endogenous regulatory control of an existing gene without also loosing expression of the existing gene only be integration at the N terminus (immediately before the Start codon) or C terminus (immediately before the Stop codon) of the targeted gene. This limitation in target sites means that efficient gRNAs for gene knock-in may not be present for many genes, as observed in previous studies6. b, Intron knock-ins offer greater flexibility for placing a synthetic gene under endogenous regulatory control without disrupting the endogenous gene. Using optimized 5’ and 3’ splicing architectures to induce alternative splicing, a synthetic exon can be introduced throughout the intronic regions of the endogenous gene, with alternative splicing resulting in two separate mature mRNA transcripts, one encoding the new synthetic protein and a second encoding the endogenous gene. Orders of magnitude more gRNA target sites are available within intronic regions than only at the very N or C terminus of a gene. c, Exonic targeting of a new synthetic gene within the coding sequence of an endogenous gene disrupts the endogenous gene’s sequence, resulting in a multicistronic mRNA transcript encoding the new synthetic protein and two partial fragments of the endogenous protein, causing knockout of the endogenous gene. d, Intron targeting with a synthetic exon containing optimized 5’ and 3’ splicing architectures to induce synthetic splicing only also results in endogenous gene knockout. However unlike in exonic targeting, where alleles with unsuccessful knock-ins largely have NHEJ induced indels resulting in frameshift mutations and protein knockout, with intron targeting NHEJ induced indels reside within an intronic sequence that is largely tolerant of short DNA changes which will be spliced out of the final mRNA transcript (more rare but detectable larger deletions that include parts of the surrounding exons induced by double stranded breaks can still result in endogenous protein knockout). If the targeted endogenous gene is a surface receptor, then intron knock-ins offer the unique ability to perform negative selections to purify successfully edited cells.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roth, T.L., Lu, J., McClellan, A. et al. Non-viral intron knock-ins for targeted gene integration into human T cells and for T-cell selection. Nat. Biomed. Eng 9, 1309–1319 (2025). https://doi.org/10.1038/s41551-025-01372-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01372-1
This article is cited by
-
The hidden risks of CRISPR/Cas: structural variations and genome integrity
Nature Communications (2025)