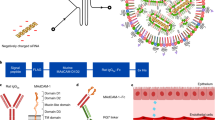
Abstract
Tolerogenic antigen-presenting cells (APCs) are promising as therapeutics for suppressing T cell activation in autoimmune diseases. However, the isolation and ex vivo manipulation of autologous APCs is costly, and the process is customized for each patient. Here we show that tolerogenic APCs can be generated in vivo by delivering, via lipid nanoparticles, messenger RNA coding for the inhibitory protein programmed death ligand 1. We optimized a lipid-nanoparticle formulation to minimize its immunogenicity by reducing the molar ratio of nitrogen atoms on the ionizable lipid and the phosphate groups on the encapsulated mRNA. In mouse models of rheumatoid arthritis and ulcerative colitis, subcutaneous delivery of nanoparticles encapsulating mRNA encoding programmed death ligand 1 reduced the fraction of activated T cells, promoted the induction of regulatory T cells and effectively prevented disease progression. The method may allow for the engineering of APCs that target specific autoantigens or that integrate additional inhibitory molecules.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Fugger, L., Jensen, L. T. & Rossjohn, J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell 181, 63–80 (2020).
Conrad, N. et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet 401, 1878–1890 (2023).
McKinney, E. F., Lee, J. C., Jayne, D. R., Lyons, P. A. & Smith, K. G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523, 612–616 (2015).
Cully, M. T cell-regulating therapies for autoimmune diseases take FDA rejection in stride. Nat. Rev. Drug Discov. 20, 655–657 (2021).
Mullard, A. PD1 agonist antibody passes first phase II trial for autoimmune disease. Nat. Rev. Drug Discov. 22, 526 (2023).
Zhang, B. et al. Site-specific PEGylation of interleukin-2 enhances immunosuppression via the sustained activation of regulatory T cells. Nat. Biomed. Eng. 5, 1288–1305 (2021).
Edner, N. M., Carlesso, G., Rush, J. S. & Walker, L. S. K. Targeting co-stimulatory molecules in autoimmune disease. Nat. Rev. Drug Discov. 19, 860–883 (2020).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346, 1692–1698 (2002).
Cifuentes-Rius, A., Desai, A., Yuen, D., Johnston, A. P. R. & Voelcker, N. H. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat. Nanotechnol. 16, 37–46 (2021).
Audiger, C., Rahman, M. J., Yun, T. J., Tarbell, K. V. & Lesage, S. The importance of dendritic cells in maintaining immune tolerance. J. Immunol. 198, 2223–2231 (2017).
Brown, C. C. & Rudensky, A. Y. Spatiotemporal regulation of peripheral T cell tolerance. Science 380, 472–478 (2023).
Kenison, J. E., Stevens, N. A. & Quintana, F. J. Therapeutic induction of antigen-specific immune tolerance. Nat. Rev. Immunol. 24, 338–357 (2024).
Sugiura, D. et al. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science 364, 558–566 (2019).
Oh, S. A. et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer 1, 681–691 (2020).
Giannoukakis, N., Phillips, B., Finegold, D., Harnaha, J. & Trucco, M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 34, 2026–2032 (2011).
Morante-Palacios, O., Fondelli, F., Ballestar, E. & Martínez-Cáceres, E. M. Tolerogenic dendritic cells in autoimmunity and inflammatory diseases. Trends Immunol. 42, 59–75 (2021).
Zubizarreta, I. et al. Immune tolerance in multiple sclerosis and neuromyelitis optica with peptide-loaded tolerogenic dendritic cells in a phase 1b trial. Proc. Natl Acad. Sci. USA 116, 8463–8470 (2019).
Benham, H. et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci. Transl. Med. 7, 290ra87 (2015).
Passeri, L., Marta, F., Bassi, V. & Gregori, S. Tolerogenic dendritic cell-based approaches in autoimmunity. Int. J. Mol. Sci. 22, 8415 (2021).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022).
Sahin, U., Karikó, K. & Türeci, Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Hassett, K. J. et al. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J. Control. Release 335, 237–246 (2021).
Pardi, N. et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543, 248–251 (2017).
Verbeke, R., Hogan, M. J., Loré, K. & Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 55, 1993–2005 (2022).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Wang, C., Zhao, C., Wang, W., Liu, X. & Deng, H. Biomimetic noncationic lipid nanoparticles for mRNA delivery. Proc. Natl Acad. Sci. USA 120, e2311276120 (2023).
Kenjo, E. et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat. Commun. 12, 7101 (2021).
Krienke, C. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145–153 (2021).
Wilson, E. et al. Efficacy and safety of an mRNA-based RSV PreF vaccine in older adults. N. Engl. J. Med. 389, 2233–2244 (2023).
Kauffman, K. J. et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 15, 7300–7306 (2015).
Zhao, P. et al. Depletion of PD-1-positive cells ameliorates autoimmune disease. Nat. Biomed. Eng. 3, 292–305 (2019).
Wu, Y. et al. Omicron-specific mRNA vaccine elicits potent immune responses in mice, hamsters, and nonhuman primates. Cell Res. 32, 949–952 (2022).
Peng, Q. et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 11, 4835 (2020).
O’Shea, J. J., Laurence, A. & McInnes, I. B. Back to the future: oral targeted therapy for RA and other autoimmune diseases. Nat. Rev. Rheumatol. 9, 173–182 (2013).
Kingsmore, K. M., Grammer, A. C. & Lipsky, P. E. Drug repurposing to improve treatment of rheumatic autoimmune inflammatory diseases. Nat. Rev. Rheumatol. 16, 32–52 (2020).
Brand, D. D., Latham, K. A. & Rosloniec, E. F. Collagen-induced arthritis. Nat. Protoc. 2, 1269–1275 (2007).
Wu, J. et al. TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat. Commun. 13, 676 (2022).
Wirtz, S. et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 12, 1295–1309 (2017).
Tang, C. et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat. Immunol. 19, 755–765 (2018).
Van Assche, G. et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 125, 1025–1031 (2003).
Sharpe, A. H. & Pauken, K. E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 18, 153–167 (2018).
Breda, L. et al. In vivo hematopoietic stem cell modification by mRNA delivery. Science 381, 436–443 (2023).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Serra, P. & Santamaria, P. Antigen-specific therapeutic approaches for autoimmunity. Nat. Biotechnol. 37, 238–251 (2019).
Miller, S. D., Turley, D. M. & Podojil, J. R. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol. 7, 665–677 (2007).
Kurochkina, Y. et al. SAT0212 The safety and tolerability of intra-articular injection of tolerogenic dendritic cells in patients with rheumatoid arthritis: the preliminary results. Ann. Rheum. Dis. 77, 966–967 (2018).
Jauregui-Amezaga, A. et al. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn’s disease: a phase I study. J. Crohns Colitis 9, 1071–1078 (2015).
Dong, S. et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight 6, e147474 (2021).
Raffin, C., Vo, L. T. & Bluestone, J. A. Treg cell-based therapies: challenges and perspectives. Nat. Rev. Immunol. 20, 158–172 (2020).
Hirai, T. et al. Selective expansion of regulatory T cells using an orthogonal IL-2/IL-2 receptor system facilitates transplantation tolerance. J. Clin. Invest. 131, e139991 (2021).
Bluestone, J. A. & Tang, Q. Treg cells—the next frontier of cell therapy. Science 362, 154–155 (2018).
Murray, J. A. et al. Safety and tolerability of KAN-101, a liver-targeted immune tolerance therapy, in patients with coeliac disease (ACeD): a phase 1 trial. Lancet Gastroenterol. Hepatol. 8, 735–747 (2023).
Tremain, A. C. et al. Synthetically glycosylated antigens for the antigen-specific suppression of established immune responses. Nat. Biomed. Eng. 7, 1142–1155 (2023).
Kelly, C. P. et al. TAK-101 nanoparticles induce gluten-specific tolerance in celiac disease: a randomized, double-blind, placebo-controlled study. Gastroenterology 161, 66–80.e8 (2021).
Tsai, S. et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 32, 568–580 (2010).
Singha, S. et al. Peptide-MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat. Nanotechnol. 12, 701–710 (2017).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Katakura, K. et al. Toll-like receptor 9–induced type I IFN protects mice from experimental colitis. J. Clin. Invest. 115, 695–702 (2005).
Moskowitz, R. W. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr. Cartil. 14, 13–29 (2006).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52025036 to Y.W., 82173390 to M.L. and 52495014 to Y.W.), the National Key R&D Program of China (2020YFA0710700 and 2022YFC2303300 to Y.W.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0490000 and XDB0940303 to Y.W.), the Anhui Provincial Key Research and Development Project (2023s07020019 to Y.W.), the Anhui Provincial Major Science and Technology Project (202303a07020010 to Y.W.), the Anhui Provincial Natural Science Foundation (2408085J042 to M.L.), the project of collaborative innovation for colleges of Anhui province (GXXT-2022-063 to M.L.) and the USTC Research Funds of the Double First-Class Initiative (YD9100002054 to Y.W. and YD9110002021 to M.L.). This work was partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication. This work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China.
Author information
Authors and Affiliations
Contributions
Y.W., M.L., Y.L. and Q.L. conceptualized and designed the research. Y.L., Q.L., B.Z., S.C., Y.S., Z.L., J.Z. and Y.Y. performed the experiments. S.C. provided help in designing LNP formulations. Y.L., Q.L. and B.Z. analysed the experimental data. Y.L., M.L., Q.L., B.Z. and Y.W. prepared the figures and wrote the paper. Y.W. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Jeffrey Hubbell, Tianmeng Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 In vivo-produced tol-APCs inhibit RA progression.
a, Statistical data of OARSI score. b, c, The percentage of IFN-γ+ (b) and TNF-α+ (c) area per FOV. d, Representative images of CD4, CD8, and Foxp3 staining from the knee joint of one mouse in a group of four. Scale bar = 200 µm. Arrows refer to Foxp3+ cells. e-g, Number of CD4+ (e), CD8+ (f) and Foxp3+ (g) cells per FOV. RA mice were subcutaneously treated with PBS, LNPs, or LNPs/mPDL1 (5 μg mRNA) at the lower right back. Mice treated with iTNF-α served as the positive control group. Normal group comprises healthy mice. n = 4 biologically independent mice per group for data in a-c and e-g. Data are expressed as the mean ± s.e.m. Statistical significances were determined using one-way ANOVA with Dunnett’s post hoc test. Comparisons were performed between the LNPs/mPDL1 group and each of the other groups. N.S. is P ≥ 0.05, and significant P values are displayed.
Extended Data Fig. 2 In vivo-produced tol-APCs mediate potent therapeutic effects in DSS-induced UC mice.
a, Representative images of CD8, Foxp3, IFN-γ, and TNF-α staining from the colon of one mouse in a group of four. Arrows refer to Foxp3+ cells. Scale bar = 200 µm. b-e, The number of CD8+ (b) and Foxp3+ (c) cells and the percentage of IFN-γ+ (d) and TNF-α+ (e) area per FOV. Mice were treated with PBS, LNPs, or LNPs/mPDL1 (5 μg mRNA) via subcutaneous injection at the lower right back. Mice treated with cyclosporine served as the positive control group. Normal group comprises healthy mice. n = 4 biologically independent mice per group for data in b-e. Data are expressed as the mean ± s.e.m. Statistical significances were determined using one-way ANOVA with Dunnett’s post hoc test. Comparisons were performed between the LNPs/mPDL1 group and each of the other groups. N.S. is P ≥ 0.05, and significant P values are displayed.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Supplementary data
Source data for the supplementary figures.
Source data
Source Data Figs. 2–7 and Extended Data Figs. 1 and 2
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Liu, Q., Zhang, B. et al. Generation of tolerogenic antigen-presenting cells in vivo via the delivery of mRNA encoding PDL1 within lipid nanoparticles. Nat. Biomed. Eng 9, 1320–1334 (2025). https://doi.org/10.1038/s41551-025-01373-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01373-0
This article is cited by
-
Lipid nanoparticles with PDL1-encoding mRNA spread tolerance
Nature Reviews Rheumatology (2025)