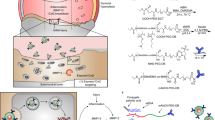
Abstract
Osteoarthritis and rheumatoid arthritis are debilitating joint diseases marked by pain, inflammation and cartilage destruction. Current osteoarthritis treatments only relieve symptoms, while rheumatoid arthritis therapies can cause immune suppression and provide variable efficacy. Here we developed an optimized small interfering RNA targeting matrix metalloproteinase 13 for preferential delivery to arthritic joints. Chemical modifications in a stabilizing ‘zipper’ pattern improved RNA resistance to degradation, and two independent linkers with 18 ethylene glycol repeats connecting to tandem C18 lipids enhanced albumin binding and targeted delivery to inflamed joints following intravenous administration. In preclinical models of post-traumatic osteoarthritis and rheumatoid arthritis, a single intravenous injection of the albumin-binding small interfering RNA achieved long-term joint retention, sustained gene silencing and reduced matrix metalloproteinase 13 activity over 30 days, resulting in decreased cartilage erosion and improved clinical outcomes, including reduced joint swelling and pressure sensitivity. This approach demonstrated superior efficacy over corticosteroids and small-molecule MMP inhibitors, highlighting the therapeutic promise of albumin ‘hitchhiking’ for targeted, systemic delivery of gene-silencing therapeutics to treat osteoarthritis and rheumatoid arthritis.
Similar content being viewed by others
Main
Osteoarthritis (OA) is a degenerative joint disease that can be idiopathic or secondary to injury, in the case of post-traumatic osteoarthritis (PTOA). OA is characterized by cartilage loss, synovial inflammation and formation of osteophytes, all of which contribute to pain and lost joint function1. Patients often develop broader multijoint osteoarthritis (MJOA), which can have debilitating impact on quality of life2. The autoimmune disease rheumatoid arthritis (RA) has independent aetiology and is characterized by severe synovial and systemic inflammation, bone erosion and cartilage damage3,4. Current OA treatment options are palliative and include non-steroidal anti-inflammatory drugs (NSAIDS) or corticosteroids, approaches that neither counteract the root causes of disease nor prevent OA progression; corticosteroids may in fact worsen cartilage thinning5,6,7. Current treatments for RA include synthetic disease-modifying antirheumatic drugs (DMARDs) and biologic DMARDs such as tumour necrosis factor (TNF) inhibitors, interleukin (IL) inhibitors and B-cell inhibitors. These treatments are efficacious, although they can suppress the immune system, and their benefits often wane over the long term8.
There is extensive overlap between OA and RA in terms of the downstream molecular effectors that drive joint degeneration9. Both are associated with inflammation and upregulation of extracellular matrix (ECM)-degrading matrix metalloproteinases (MMPs)10. MMPs erode cartilage and the degradation byproducts have inflammatory signalling properties that further induce expression of inflammatory cytokines and MMPs, perpetuating a chronic catabolic cycle that leads to deep cartilage erosion, chondrocyte loss and subchondral bone exposure11,12,13.
Therapeutic MMP inhibition is a logical approach for OA and RA treatment. Development of MMP-selective antagonists is critical, as broad spectrum MMP inhibitors cause musculoskeletal toxicities in humans, probably due to universal disruption of normal tissue homeostasis14,15,16. Among MMPs, MMP13 is perhaps the most efficient at cleaving the key ECM components collagen II17 and fibronectin18, producing inflammatory fragments. Further, knockout mouse studies pinpoint MMP13 as a key proteolytic driver of cartilage loss in arthritis models19. Lastly, MMP13 expression is relatively confined to joint tissues and diseased or injured tissues characterized by fibrosis or inflammation20. This narrow expression pattern allows for a potentially wider therapeutic window with minimal toxicities. However, this hypothesis has yet to be tested fully, as clinical development of selective MMP inhibitors has been challenged by the extensive structural similarities between the catalytic sites of the various MMPs21.
RNA interference (RNAi) provides an ideal technique for gene-selective inhibition, as specificity is conferred by the genetic sequence. The translatability of small interfering (si)RNA therapeutics is also now well established based on the FDA approval of lipid nanoparticle and GalNAc–siRNA conjugate drugs, both of which have been successfully applied in humans for gene silencing in the liver22,23. Selective MMP13 gene knockdown using siRNA sequences would overcome the hurdle of structural similarity between MMPs, thus providing an opportunity to target MMP13 in the context of OA and RA. This notion is supported by recent PTOA model studies in which intra-articular delivery of MMP13 silencing siRNA nanoparticles (siNPs) protected against knee joint load-induced cartilage loss24,25.
To improve on these relatively complex nano- and microtechnologies for intra-articular MMP13 siRNA delivery, we sought an approach in which carrier-free siRNA conjugates delivered intravenously might preferentially accumulate within arthritic joints. A systemically delivered approach avoids the potential joint damage that can occur from repeated intra-articular injections and better enables treatment of MJOA and RA. Relative to siNPs, a molecularly defined conjugate is also simpler to manufacture and does not come with the added risk of carrier toxicities that often narrow the therapeutic window of cationic lipid or polymer siNP formulations.
Development of carrier-free siRNA conjugates for rheumatic diseases is an unchartered but high-impact endeavour26. Toward this goal, experimental siRNA–lipid conjugates that commandeer circulating plasma albumin for delivery to arthritic joints were engineered and tested in models of OA and RA. Albumin has unique promise as a therapeutic siRNA carrier, given its long circulation half-life (20 days)27 and natural tendency to accumulate in inflamed tissues, including arthritic joints28,29,30,31. Albumin has natural binding sites for long-chain fatty acids27,32,33,34,35,36 that can be co-opted by drugs or fatty acid-modified exogenous molecules, as exemplified by clinically approved albumin-based/binding formulations (for example, Abraxane, Semaglutide, Levemir, Optison and Tirzepatide)37,38,39. Binding to these sites yields the potential for increasing drug pharmacokinetic properties, as albumin accesses natural recycling and kidney reabsorption mechanisms that prevent its degradation and urinary clearance, thus prolonging its half-life27,40,41,42,43. Hitchhiking on albumin also can promote preferential accumulation in arthritic joints because their inflamed state causes leaky vasculature and permits local drug extravasation and sequestration (the ‘ELVIS’ effect44,45). Albumin is one of the most prominent proteins in synovial fluid (SF)46,47, and it also shows cartilage penetration, being 15-fold more concentrated in the deep layers of arthritic than healthy cartilage and having 2-fold higher penetration into human cartilage than IgG antibodies28,29. Recombinant fusion to albumin promotes protein accumulation in inflamed knees31,48, further motivating our approach.
Although covalent conjugation of siRNA to albumin has been explored to some extent49,50, lipid–siRNA conjugates that enable reversible in situ association with native circulating albumin has been proof-of-concept tested mostly in cancer studies32,51,52. We show here that systemic delivery of an albumin-hitchhiking siRNA–lipid conjugate targeting MMP13 achieves robust delivery, MMP13 silencing and therapeutic efficacy in joints afflicted by OA and RA.
Results and discussion
Circulating albumin robustly accumulates in PTOA joints
To determine the extent to which circulating albumin accumulates in arthritic joints, we intravenously (i.v.) delivered Cy5-labelled mouse serum albumin (MSA-Cy5) into mice with induced PTOA. In this model, the left knee undergoes repeated loading to create damage and inflammation, while the right knee remains unchallenged (Fig. 1a). At 24 h after delivery, abundant MSA-Cy5 was observed in the synovium and cartilage of PTOA knees but not in contralateral control knees (Fig. 1b and Supplementary Fig. 1). In contrast, Cy5-conjugated poly(ethylene glycol) (PEG-Cy5) did not accumulate preferentially in PTOA knees (Fig. 1c). Further, i.v. delivery of the albumin binding dye Evans blue resulted in preferential accumulation in synovia and cartilage of PTOA knees, but not contralateral healthy knees (Fig. 1d,e, and Supplementary Figs. 2 and 3). Interestingly, mRNAs of genes encoding the albumin delivery/transport factors caveolin-1 and secreted protein acidic and rich in cysteine (SPARC) were elevated in PTOA joint tissues (Supplementary Fig. 3d), consistent with previous reports of SPARC upregulation in arthritic joints53,54,55,56. Further, interstitial SPARC expression in tumours correlates with efficacy of albumin-based therapeutics in head and neck cancers57. These findings support the idea that hitchhiking on endogenous circulating albumin might be used to skew the biodistribution of siRNA conjugates to arthritic joints following i.v. delivery.
a–d, Left-knee mechanical loading was used to induce unilateral PTOA in mice, followed by i.v. delivery of 3 mg kg−1 MSA-Cy5 (N = 5), 3 mg kg−1 PEG-Cy5 (N = 3) and 200 µl of 2% w/v Evans blue (N = 4). Cy5 and Evans blue were measured by fluorescence imaging 24 h after delivery. Representative images are shown. a, Experimental timeline. b, MSA-Cy5 fluorescence in paired PTOA and healthy knees. c, Ratio of MSA-Cy5 and PEG-Cy5 fluorescence in PTOA and healthy knees for each treated mouse. d, Intravital Evans blue fluorescence in paired contralateral PTOA and contralateral non-loaded knees. e, PTOA and contralateral non-loaded knees were excised 24 h after Evans blue delivery. Evans blue was extracted and measured by densitometry (N = 3). Representative images of joints and resulting joint extracts are shown. f, Chemical modifications of siRNA ribose, backbone and terminus. g, Schematic of synthetic dicer-substrate siRNA and alternating 2′F and 2′OMe modified ‘zipper’ siRNA. h, siRNA stability in OA-derived synovial fluid: representative gel electrophoresis of dicer substrate and zipper siRNA sequences after incubation in synovial fluid collected from an untreated OA patient. i, Schematic representation of the si<(EGXL)2 series of zipper-modified siRNAs with divalent lipid end-modifications with variations in EG content. j, Left: FPLC chromatograph of elution of si<(EGXL)2 variants pre-incubated with human synovial fluid form normal joints. Right: percentage of total si<(EGXL)2 bound to albumin fractions. All error bars indicate s.d. of biological replicates. Knees labelled healthy indicate a non-loaded contralateral limb (right).
Enhanced stability, albumin binding and PTOA joint accumulation of optimized siRNA–lipid conjugates
Carrier-free siRNA conjugates must be chemically modified to protect against degradation by endo- and exonucleases. To stabilize the siRNAs, we engineered blunt-ended 19-mer RNA strands with alternating 2′-O-Me and 2′-F ribose modifications in a ‘zipper’ pattern and replaced phosphodiesters with phosphorothioate (PS) linkages for the last two bases of the 5′- and 3′-termini of the sense and antisense strands (Fig. 1f,g). Stability of the zipper-modified siRNA was assessed in synovial fluid from a treatment-naive OA patient, revealing siRNA stability through at least 24 h at 37 °C, whereas a more lightly modified dicer-substrate siRNA was >50% degraded within 1 h (Fig. 1h). Given the stability of the zipper-modified siRNA, all further siRNA modifications were done using this modification pattern.
The zipper siRNAs were end modified with bivalent C18 lipids intended to promote binding of the siRNA into the natural fatty acid (FA) binding pockets of albumin (Fig. 1i)32. A library of bivalent lipid structures was synthesized as recently reported51. Briefly, a splitter phosphoramidite (<) was added at the 5′ end of the sense strand, followed by addition of 0 (EG0), 1 (EG6), 3 (EG18) or 5 (EG30) hexa-ethylene glycol (EG6) phosphoramidite spacers to each branch. Both branches were terminally appended with a C18 lipid, thus generating siRNA<(EGxL)2, where X is the number of EG repeats linking the splitter to each terminal C18.
Binding of the different siRNA structures to albumin within human synovial fluid was assessed using fast protein liquid chromatography (FPLC). A proportion of each of the siRNA<(EGXL)2 conjugates was found in the albumin-containing synovial fluid fractions (Fig. 1j), whereas unconjugated, zipper-pattern modified siRNA was not. Notably, >80% of the total siRNA<(EG18L)2 detected by FPLC was found in the albumin-containing fraction, higher than any other siRNA–lipid conjugate tested. This observation was consistent with the previously measured high affinity binding of siRNA<(EG18L)2 to albumin (KD = 30 nM)51.
Cy5-labelled siRNA<(EGXL)2 constructs were next delivered i.v. (1 mg kg−1) to mice with PTOA induced in the left knee (Fig. 2a). While each accumulated to a greater extent in PTOA knees over healthy contralateral knees, siRNA<(EG18L)2 achieved the highest accumulation in PTOA joints (Fig. 2b and Supplementary Fig. 4). A benchmarking study was done to compare siRNA<(EG18L)2 to commonly used cholesterol-conjugated siRNA (siRNA-Chol). In contrast to the >4-fold enrichment of siRNA<(EG18L)2 in PTOA knees over healthy knees, parental siRNA and cholesterol-conjugated siRNA-Chol each showed only a 1.5- and 2-fold enrichment of delivery to PTOA vs healthy knees, respectively (Fig. 2c). Histological cryosections of PTOA knee synovium and cartilage illustrated holistic joint tissue penetration and cellular uptake of Cy5-labelled siRNA<(EG18L)2 (Supplementary Figs. 5–7), while siRNA-Chol was observed but at lower magnitude within PTOA joint compartments (Fig. 2d). Organ biodistribution studies performed by ex vivo Cy5 imaging showed that liver was the main off-target delivery site for both siRNA<(EG18L)2 and siRNA-Chol, while unconjugated siRNA accumulated primarily in kidneys (Supplementary Fig. 8), a major site of siRNA clearance.
a, Unilateral left knee mechanical loading, treatment (1 mg kg−1 i.v.) and endpoint protocol used in b–d. b, Representative ex-vivo IVIS images and quantitation of Cy5-labelled siRNA<(EGxL)2 accumulation in mouse knees 24 h after delivery. c, Left: representative intravital Cy5 fluorescence images taken 24 h after delivery of Cy5-labelled siRNA, siRNA-Chol or siRNA<(EG18L)2. Right: quantification of average Cy5 fluorescence in knees taken 24 h after mouse i.v. injection. Analysed using mixed effects analysis. d, DAPI-counterstained cryosections of knees from treated mice imaged and quantified for Cy5-labelled siRNA fluorescence in cartilage and synovial tissue. Dashed lines outline articular cartilage. e, In situ hybridization with an siMMP13 probe showing signal localization of siMMP13<(EG18L)2 in mice treated i.v. with 10 mg kg−1. Representative images show siRNA presence in the femoral cartilage (F), meniscus (M), tibial cartilage (T) and synovium. Serial tissue sections incubated with a negative control scrambled probe showed no signal. f, Flow cytometry assessment of cell type-specific uptake of Cy5-siRNA<(EG18L)2 in synovium. Mice were treated i.v. with 10 mg kg−1 following three bilateral mechanical loading sessions over a week. Cells isolated from synovia of 5 mice were pooled for analysis. Experimental and flow cytometry gating details are in Supplementary Fig. 11. All error bars indicate s.d. of biological replicates. Knees labelled healthy indicate a non-loaded contralateral limb (right).
Interactions between albumin and siRNA<(EG18L)2 were further assessed by FPLC of synovial fluid from OA, RA or normal joints. Similar to what was observed using healthy patient-derived synovial fluid, Cy5-siRNA<(EG18L)2 eluted primarily in the albumin-containing fractions of OA patient and RA patient-derived synovial fluid samples, but with substantially greater intensity in arthritic samples (Supplementary Fig. 9a). In contrast, the parent Cy5-siRNA remained predominantly unbound. Western blotting confirmed the increased albumin content in OA- and RA-derived synovial fractions B12 and C1, the same fractions that siRNA<(EG18L)2 was predominantly associated with (Supplementary Fig. 9b). Complementary native gel electrophoresis analyses of synovial fluid fractions B12-C1 revealed co-localization of Cy5-siRNA<(EG18L)2 with a Coomassie-stained protein of 66 kDa, the known molecular weight of albumin (Supplementary Fig. 9c). Further, siRNA<(EG18L)2 showed stability in synovial fluid from OA joints for at least 96 h (Supplementary Fig. 9d). Taken together, these findings allowed us to assign siRNA<(EG18L)2 as our lead siRNA–lipid conjugate for albumin hitchhiking to arthritic joints, motivating a focus on this construct for subsequent therapeutic studies. We also found that siRNA<(EG18L)2 interacted with albumin across multiple species, including human, mouse and guinea pig, supporting its utility for all the OA and RA models used herein (Supplementary Fig. 9e).
PTOA joint accumulation and cell type-specific uptake of siRNA<(EG18L)2
RNA in situ hybridization (ISH) was utilized for label-free visualization of tissue localization and penetration of the siMMP13<(EG18L)2 delivered i.v. at a dose of 10 mg kg−1. Application of an ISH probe designed against siMMP13 to PTOA joint histological sections revealed the presence of siMMP13<(EG18L)2 within the meniscus and femoral/tibial cartilage, in addition to synovial localization (Fig. 2e). This label-free method illustrates that the therapeutically active siMMP13<(EG18L)2 compound achieves delivery and penetration into important joint tissues with known relevance to PTOA progression. To validate the reproducibility and specificity of the ISH approach, we also observed positive staining with a probe designed against the endogenous small nuclear RNA RNU6; furthermore, application of a negative control probe to the histological sections taken from PTOA joints of mice treated with siMMP13<(EG18L)2 or a saline vehicle control showed no visible staining, as did application of the siMMP13 probe to PTOA joint sections from saline-treated mice (Fig. 2e and Supplementary Fig. 10).
To characterize cell type-specific uptake of the selected siRNA<(EG18L)2 conjugate, Cy5-siRNA<(EG18L)2 (10 mg kg−1) or vehicle was delivered i.v. to mice with mechanical load-induced PTOA in both knees. Cell type-specific uptake by synovial fibroblasts, endothelial cells, macrophages, monocytes, dendritic cells and T cells was characterized 24 h after treatment using flow cytometry (Fig. 2f and Supplementary Fig. 11). A high percentage (83.1%) of the overall cells in the synovium were positive for uptake of Cy5-labelled siRNA<(EG18L)2, with fibroblasts, macrophages and endothelial cells exhibiting the highest proportion of Cy5-positive cells (>90%).
siRNA<(EG18L)2 accumulation in PTOA joints following subcutaneous and intra-articular delivery
Subcutaneous injection of siRNA<(EG18L)2 was tested as an alternative to the i.v. delivery route, given the utility of subcutaneous injections for patient self-administration of biologic drugs. Subcutaneous delivery of Cy5-siRNA<(EG18L)2 (2 mg kg−1) enabled preferential siRNA accumulation in PTOA knees over contralateral non-injured knees, with similar organ biodistribution profile as i.v. delivery (Extended Data Fig. 1a–c). However, subcutaneous delivery of siRNA<(EG18L)2 at 2 mg kg−1 did not achieve the absolute level of siRNA accumulation in PTOA knees seen with i.v. delivery at 1 mg kg−1 (Extended Data Fig. 1d). Notably, a large amount of the Cy5-siRNA<(EG18L)2 dose was retained at the injection site (data not shown), perhaps due to its rapid interaction with subcutaneous fat or cells at the injection site58. Intra-articular (i.a.) delivery, a common clinical route for steroid delivery, was also tested (0.25 mg kg−1), revealing greater Cy5-siRNA<(EG18L)2 retention in the PTOA over healthy knee and greater retention than Cy5-siRNA at 48 h post injection, achieving penetration into cartilage and synovia (Extended Data Fig. 1e–g and Supplementary Fig. 7b). While i.a.-delivered Cy5-siRNA<(EG18L)2 was retained primarily in the knee, Cy5-siRNA redistributed to kidneys, reflecting its relatively rapid removal from the joint by synovial drainage via vasculature and lymphatics, underscoring the importance of the lipid moieties for retention of siRNA<(EG18L)2 within the joint space.
Potent MMP13 knockdown in PTOA-afflicted joints by siRNA<(EG18L)2
siRNA sequences against mouse and guinea pig MMP13 (siMMP13) were screened in mouse ATDC5 chondrogenic cells and primary guinea pig chondrocytes, respectively, for MMP13 knockdown potency (Supplementary Fig. 12). Lead candidate sequences were then synthesized with the zipper modification pattern and MMP13 silencing potency reconfirmed upon transfection into TNF-stimulated ATDC5 cells (Supplementary Fig. 13). Albumin-binding zipper-pattern siMMP13 was then synthesized, thus generating siMMP13<(EG18L)2. Importantly, siMMP13<(EG18L)2, but not the parent zipper-pattern modified siMMP13, provided robust carrier-free (no lipofection reagent) Mmp13 knockdown (Supplementary Fig. 13). The siRNA<(EG18L)2 structure also showed efficient cellular uptake in ATDC5 cells; level of uptake was partially diminished in the presence of excess albumin but increased upon treatment with pro-inflammatory TNF stimulation, even in the presence of excess albumin (Supplementary Fig. 14).
MMP13 silencing by siMMP13<(EG18L)2 in PTOA-affected mouse knees was first assessed 5 days after i.v. treatment, finding >50% Mmp13 knockdown with a 5 mg kg−1 dose and >60% with a 10 mg kg−1 dose (Extended Data Fig. 2a). Neither unconjugated siMMP13 nor siMMP13-Chol (each at 10 mg kg−1 i.v.) significantly affected Mmp13 levels in PTOA knees. A single i.a. injection of siMMP13<(EG18L)2 at 1 mg kg−1 also decreased Mmp13 in PTOA knees (Extended Data Fig. 2b). While subcutaneous delivery of siMMP13<(EG18L)2 at a relatively high dose (50 mg kg−1) diminished Mmp13 in PTOA knees (Extended Data Fig. 2c), a lower dose (20 mg kg−1) reduced Mmp13 in PTOA knees only when co-delivered with an excess of mouse albumin (1:5 molar ratio). These results show promise for use of siMMP13<(EG18L)2 with multiple delivery routes. However, subcutaneous delivery may benefit from more optimization of excipients or penetration enhancers to improve absorption from the injection site into the systemic circulation.
Immunohistochemical (IHC) analysis of PTOA knees revealed abundant MMP13 in articular cartilage, synovium and meniscus compared with tissues in healthy knees. PTOA-induced MMP13 protein signal was markedly reduced in each PTOA knee compartment at 5 days after treatment with siMMP13<(EG18L)2 delivered at either 5 or 10 mg kg−1. However, neither siMMP13-Chol nor siMMP13 impacted MMP13 levels in PTOA knees (Extended Data Fig. 2d). MMP13 protein was undetectable in liver or kidneys by IHC (Extended Data Fig. 2e). RNA expression analyses similarly did not detect Mmp13 in liver, although low levels were found in kidney, albeit 6,000-fold lower than that detected in PTOA knees (Extended Data Fig. 2f). Regardless, kidney Mmp13 gene expression was unaffected in siMMP13<(EG18L)2-treated mice (Extended Data Fig. 2g), supporting the idea that siMMP13<(EG18L)2 might have a wide therapeutic window, with minimal risk of molecularly on-target side effects in two of the primary organs commonly associated with siRNA clearance, liver and kidney.
To further characterize cellular sources of MMP13 in PTOA synovium, we analysed single-cell RNA-sequencing from a murine anterior cruciate ligament (ACL) rupture-induced PTOA model59. Synovial fibroblasts, specifically Thy1+ Pdgfra+ Prg4− sublining fibroblasts were the primary Mmp13-expressing cell type in synovium, with some myeloid cells exhibiting a much lower degree of Mmp13 expression. While healthy synovium was essentially devoid of Mmp13-expressing cells, PTOA synovium exhibited a large increase in the number of Mmp13-positive synovial fibroblasts (Supplementary Fig. 15). Taken together with Cy5-siRNA<(EG18L)2 uptake results demonstrating a high degree of uptake by synovial fibroblasts (Fig. 2f and Supplementary Fig. 11), these results suggest that our siRNA<(EG18L)2 structure efficiently targets the primary Mmp13-expressing cells in synovium.
Sustained retention of siMMP13<(EG18L)2 in PTOA joints enables potent and durable MMP13 knockdown
Mice were subjected to mechanical left knee loading (3 times per week) over 5 weeks. They were i.v. treated with a single dose of Cy5-siRNA<(EG18L)2 at 10 mg kg−1 after the first week of loading, and longitudinal measurements of Cy5 retention in PTOA knees were performed at time points throughout the next 30 days (Extended Data Fig. 3a). At day 1, there was significant Cy5 signal in the cartilage/meniscus and synovial tissues (Extended Data Fig. 3b) that was retained for up to 30 days in PTOA knees treated systemically with Cy5-siRNA<(EG18L)2 (Extended Data Fig. 3c), and higher siRNA<(EG18L)2 levels were detected in PTOA knees over contralateral control knees (Extended Data Fig. 3d,e). Importantly, a single siMMP13<(EG18L)2 treatment maintained knockdown of MMP13 transcript and protein in PTOA joints through day 30 (Extended Data Fig. 3f,g).
Intra-articular dosing of siRNA<(EG18L)2 (1 mg kg−1) was similarly assessed, revealing preferential retention of Cy5-siRNA<(EG18L)2 in PTOA knees over contralateral healthy knees through 30 days post treatment (Extended Data Fig. 4). In a side-by-side comparison, an i.v. dose of 10 mg kg−1 and intra-articular dose of 1 mg kg−1 siRNA<(EG18L)2 had remarkably similar area under the curve (AUC) profiles, suggesting that ~10-fold higher dose should be used for the i.v. relative to the i.a. delivery route for siRNA<(EG18L)2 arthritic knee joint treatments. These trends were confirmed by a peptide nucleic acid (PNA) hybridization assay that enables absolute siRNA quantification in tissue. This measurement showed preferential accumulation of siRNA<(EG18L)2, but not free siRNA, in PTOA knees over contralateral healthy knees, and similar siRNA levels (measured as ng siRNA per mg tissue) when delivered at 10 mg kg−1 i.v. and at 1 mg kg−1 i.a. (Supplementary Fig. 16d). Broader organ biodistribution was also assessed at 30 days following administration of intravenous or intra-articular treatments. While signal remained in all organs, the siRNA<(EG18L)2 signal was highest within PTOA joints of all the tissues analysed (Supplementary Fig. 16).
siMMP13<(EG18L)2 diminishes molecular, histological and clinical manifestations of PTOA
Therapeutic efficacy of siMMP13<(EG18L)2 was tested in a mouse PTOA model after 5 weeks of mechanical knee loading (3 times per week). Mice were treated with siMMP13<(EG18L)2 or siControl<(EG18L)2 (10 mg kg−1) on day 7 and again on day 21 (Fig. 3a). The pressure-pain threshold in PTOA knees was measured by algometer, revealing a 45% reduction in threshold pressure tolerance in siControl<(EG18L)-treated mice, while siMMP13<(EG18L)2 lost only 25% (Fig. 3b). The siMMP13<(EG18L)2 cohort showed relief of joint sensitivity to a level similar to mice receiving intra-articular delivery of the FDA-approved sustained release corticosteroid Zilretta (8 mg kg−1) or 3× per week intraperitoneal treatment with the experimental MMP13-selective small-molecule inhibitor CL-82198 (ref. 19) (10 mg kg−1) (Fig. 3a,b). However, treatment with CL-82198 on the same schedule as siMMP13<(EG18L)2 (once every 2 weeks) produced a less-favourable pain tolerance in PTOA knees, as did treatment with the pan-MMP inhibitor Marimastat (10 mg kg−1 i.p., 3× weekly).
a, Schematic timeline for knee loading and treatment. Bilateral knee loading (3× per week, 5 weeks) was used to induce severe PTOA for comparing therapeutic impact of treatment with i.v. siMMP13<(EG18L)2, i.a. Zilretta, i.p. CL-82198 and i.p. Marimastat. b, Left: mechanical hyperalgesia was measured via algometer through day 35. Data over time displayed as mean + s.e.m. Right: AUC of average Smalgo readings over time was assessed. N = 8. c, Left: total MMP activity in mouse knees was measured using MMPsense 750 Fast (N = 8). Middle: mAbCII binding in knee joints was measured by fluorescence imaging (N = 8). Right: C2C fragments in mouse serum was measured by ELISA (N = 3). d, Knee joints were stained with toluidine blue (top row, femoral condyles shown) and H&E (bottom row, synovium lining and meniscus shown). Asterisks: white, healthy articular surface; red, loss of articular surface; yellow, healthy synovial lining/meniscus; black, synovial thickening/meniscal expansion and calcification. e, Joint cartilage damage was quantitated with the OARSI osteoarthritis cartilage histopathology assessment system (top) and DJD score (bottom). NS, not significant. In all panels, error bars indicate s.d. of biological replicates, unless specified otherwise.
Pan-MMP activity in PTOA joints was assessed in vivo on study day 30 using MMPSense, an MMP substrate that fluoresces upon proteolytic cleavage. Intravital imaging revealed significantly elevated MMP-driven fluorescence in PTOA knees of mice treated with siControl<(EG18L)2 compared with healthy knees (Fig. 3c). Decreased pan-MMP activity was seen in PTOA knees of mice treated with siMMP13<(EG18L)2, Zilretta and Marimastat, although the MMP13-selective small-molecule inhibitor CL-82198 did not significantly diminish pan-MMP activity in PTOA knees. A fluorescently labelled monoclonal antibody against aberrantly exposed CII collagen (mAbCII) was used to assess relative cartilage damage60,61,62, finding significantly elevated mAbCII localization to PTOA knees (Fig. 3c), thus confirming PTOA-induced cartilage damage in this model. However, mAbCII intensity in PTOA knees was diminished significantly in siMMP13<(EG18L)2-treated samples, approximating the mAbCII signal in healthy knees. Similar trends were observed for serum-based measurements of collagen C2C degradation fragments, a biomarker of cartilage degradation63. PTOA-induced mAbCII signal and serum C2C levels were also diminished by CL-82198 when dosed with higher frequency (3 times per week), underscoring the causative role of MMP13 in PTOA-induced collagen fragmentation and cartilage damage and supporting the hypothesis that MMP13 inhibitory strategies reduce structural OA manifestations. The long-acting steroid Zilretta had little impact on mAbCII binding in PTOA knees or on serum C2C levels, consistent with observations that steroids temporarily control arthritis pain but do not target underlying joint destruction and may even accelerate cartilage thinning64.
Histological analyses of PTOA joints collected on study day 30 revealed profound disruption of cartilage architecture, proteoglycan loss, synovial thickening, osteophyte formation and meniscal mineralization (Fig. 3d and Supplementary Fig. 20). In PTOA knees treated with siMMP13<(EG18L)2, cartilage morphology, proteoglycan content, synovial structure and meniscal anatomy were each relatively maintained, as quantitated using the Osteoarthritis Research Society International (OARSI) OA severity scoring system65,66 (Fig. 3e and Supplementary Table 1). Degenerative joint disease scoring (Supplementary Table 1) also indicated that there were significantly less structural changes in the mechanically loaded joints of mice treated with siMMP13<(EG18L)2 versus those treated with non-targeting siControl<(EG18L)2. Unlike what was seen with siMMP13<(EG18L)2, modest reductions in OA progression and joint degeneration were achieved in PTOA joints treated with CL-82198, although these trends did not reach statistical significance. OA progression and joint degeneration scores were not impacted by treatment with Zilretta or Marimastat.
Using microcomputed tomography (microCT) to quantify osteophytes and ectopic mineralization in PTOA mouse knees, the significant protection conferred by siMMP13<(EG18L)2 against meniscal mineralization and osteophyte formation was confirmed (Extended Data Fig. 5a–c). Importantly, pathologically elevated MMP13 expression in PTOA cartilage, meniscus and synovial compartments was reduced by treatment with siMMP13<(EG18L)2, correlating with decreased presence of degradation fragments (Extended Data Fig. 5d–g). These data show that MMP13 silencing in arthritic joints provides both molecular and clinically tangible benefits, reducing inflammation, clinical pain, articular cartilage loss and secondary manifestations of OA.
MMP13 knockdown in PTOA knees reprograms pro-inflammatory gene expression patterns
To understand the gene expression patterns that underlie the therapeutic outcomes associated with MMP13 silencing, we first measured the impact of PTOA on a small panel of pro-inflammatory genes with previously described roles in OA, including Ngf, Il1b, Tnf and Cdkn2a. These genes were each elevated in mouse PTOA knees compared with healthy knees (Extended Data Fig. 6a). We found that PTOA-induced levels of Ngf, Il1b, Tnf and Cdkn2a were significantly dampened as early as 5 days following i.v. delivery of siMMP13<(EG18L)2 at 10 mg kg−1, gene expression changes that were sustained through day 30. These findings motivated a broader and unbiased analysis of PTOA-induced gene expression changes in knee tissue, along with measuring the broader gene expression impact of MMP13 knockdown. Using a nanoString nCounter Inflammation 254 gene panel, unsupervised analysis of gene expression illustrated extensive PTOA-induced gene expression changes (Extended Data Fig. 6b). Notably, PTOA induction altered signalling along the pathway driven by p38 mitogen associated protein kinase (MAPK), a serine-threonine kinase that phosphorylates a network of substrates that respond to cell stress67. Relevant to OA, p38 MAPK signalling through its substrate MAPK-associated protein kinase 2 (MK2) regulates chondrocyte differentiation, cellular senescence, MMP upregulation and pro-inflammatory cytokine production68. MK2 signalling within human arthritic chondrocytes drives expression of prostaglandin E2, MMP3 and MMP13, leading to arthritic pain, inflammation and cartilage degradation69. Interestingly, we found that the PTOA-induced p38 MAPK gene cluster was the gene enrichment set most potently suppressed in siMMP13<(EG18L)2-treated samples (Extended Data Fig. 6c).
Treatment with siMMP13<(EG18L)2 also significantly suppressed IL-1, chemokine and TNF/NFκB pathway-associated gene clusters (Extended Data Fig. 6c). Notably, these clusters include several genes associated with OA pathogenesis, including those associated with chondrocyte proliferation (Mef2d), synovial fibroblast proliferation (Myc, Jun, Tgfb2, Pdgfa)70,71, angiogenesis (Pdgfba, Flt1, Hif1a), ECM degradation (Mmp3, Masp1), inflammatory signalling through nuclear factor-κB, (Nfκb1, Rela, Relb), cytokines (Il1b, Stat1), chemokines (Cxcl1, Cxcl10, Ccl3, Cxcr4) and toll-like receptors (TLR1, 2, 4, 5, 6 and 8).
Given that many inflammatory and stress response factors induce MMP13 expression and that proteolytic matrix degradation fragments further potentiate inflammation and expression of stress response factors72, these data suggest that siMMP13<(EG18L)2 uncouples a feed-forward inflammation-degradation cycle that drives arthritic joint pathology. These data support this hypothesis, given that selective MMP13 inhibition in arthritic joints suppresses expression of both pro-inflammatory factors and transcription factors that promote MMP13 expression.
K/BxN serum transfer arthritis (STA) multijoint rheumatoid/inflammatory arthritis mouse model
Rheumatoid arthritis is a systemic, multifactorial autoimmune disease that causes painful synovial inflammation and cartilage degradation in multiple joints simultaneously73. Increased MMP13 activity and consequent cartilage loss is a known feature of RA, and daily treatment with an experimental MMP13 small-molecule inhibitor can reduce RA pathogenesis4,74,75,76. Thus, we hypothesized that siMMP13<(EG18L)2 therapy would reduce cartilage loss, decrease induction of pro-inflammatory gene expression and suppress multiple features of RA. We used the transgenic K/BxN serum transfer model to test therapeutic efficacy of siMMP13<(EG18L)2 in an RA-like scenario. In this model, KRN, a T-cell receptor that binds to an endogenous glucose-6-phosphate isomerase (GPI) peptide presented by the IAg7 major histocompatibility complex class II (MHC-II) allele, drives production of anti-GPI autoantibodies and GPI-directed autoimmunity that localizes to articular cartilage/joint tissues. K/BxN mouse serum transiently confers RA to wild-type mouse serum recipients. MMP13 expression is increased in inflamed joints of K/BxN serum recipients, and MMP13-deficient mice are partially protected from K/BxN serum-induced RA76. Carrier-free siRNA delivery in RA has not yet been explored to our knowledge, but siMMP13<(EG18L)2 systemic administration for delivery to multiple afflicted joints is potentially advantageous. Interestingly, albumin-based delivery approaches have been shown to facilitate therapeutic outcomes in RA preclinical studies30,31,48,77,78,79,80,81,82,83,84. Importantly, i.v. delivery of albumin-binding Evans blue to mice resulted in concentrated Evans blue accumulation in arthritic forepaws and hindpaws of K/BxN serum recipients compared with unchallenged mice (Extended Data Fig. 7a,b). Likewise, i.v. delivery of Cy5-labelled MSA led to accumulation in arthritic forepaws and hindpaws of K/BxN serum recipients, but not in unchallenged wild-type mice (Extended Data Fig. 7c). Expression of caveolin-1 and SPARC was elevated in hindpaws of K/BxN recipient mice (Extended Data Fig. 7d). These data confirm increased albumin retention in RA-affected joints, supporting therapeutic testing of albumin-binding siRNA<(EG18L)2 in RA models.
Mice with STA showed accumulation of i.v.-injected Cy5-siRNA<(EG18L)2 within multiple inflamed joints (forepaw, wrist, hindpaw, ankle, knee) (Fig. 4a and Supplementary Fig. 17) at substantially higher levels than what was seen in healthy mice (Extended Data Fig. 7e–g) and exceeding that achieved by treatment with Cy5-siRNA or Cy5-siRNA-Chol (Fig. 4a). Fluorescence microscopy confirmed increased siRNA<(EG18L)2 in fore- and hindpaws of K/BxN recipients, with specific accumulation in soft tissue and cartilage (Fig. 4b). Longevity of siRNA retention was assessed in limbs of K/BXN serum recipients following a single-dose Cy5-siRNA<(EG18L)2. Intravital fluorescence was detected in limbs throughout the 30 days of mice monitoring (Supplementary Fig. 17c), supporting the notion of albumin-mediated delivery and retention of siRNA<(EG18L)2 in multiple afflicted joints in RA models.
a,b, In a mouse inflammatory arthritis (K/BxN serum transfer) model, mice were treated with Cy5-siRNA<(EG18L)2, Cy5-siRNA-Chol and Cy5-siRNA (1 mg kg−1 i.v.) 4 days after receiving K/BxN serum transfer. Joints of forepaw, hindpaw and knee were collected 24 h later and assessed by IVIS imaging (a) or fluorescence microscopy of hindpaw ankle joints (b). Representative images and quantitation of IVIS data are shown. N = 6. Dashed lines outline articular cartilage in fluorescence microscopy images of tissue cryosections. c, Relative Mmp13 mRNA in forepaws, knees and hindpaws of healthy untreated wild-type mice or in K/BxN serum recipients treated with siControl<(EG18L)2 or siMMP13<(EG18L)2 (10 mg kg−1 i.v.) was measured by RT–qPCR. N = 6. Error bars represent s.d. of biological replicates. d–f, K/BxN serum recipients were treated with siMMP13<(EG18L)2, methylprednisolone or CL-82198 (10 mg kg−1). d, Schematic timeline for K/BxN serum transfer and treatment. e, Photos of hindpaws were collected on treatment day 10, and representative images are shown. f, Ankle width change, clinical score, algometer ankle joint hyperalgesia and severity index were measured for 10 days after treatment. Error bars indicate s.e.m. (top) or s.d. (bottom) of biological replicates.
siMMP13<(EG18L)2 therapy reduces manifestations and symptoms of RA
Strong Mmp13 induction was seen in forepaws, knees and hindpaws of K/BxN serum recipients compared with healthy mice (Fig. 4c). At treatment day 8 following a single 10 mg kg−1 dose of siMMP13<(EG18L)2, mRNA and protein were decreased in forepaws, knees and hindpaws (Fig. 4c and Extended Data Fig. 8a). Similar to PTOA, MMP13 silencing in this RA model reduced expression of pro-inflammatory genes and cytokines encoding cyclooxygenase 2 (COX2), IL-6, TNF and IL-1β (Supplementary Fig. 18). Clinical parameters of RA were also reduced upon MMP13 knockdown, including ankle swelling, pressure-pain threshold, arthritis clinical score and disease severity index (Fig. 4d–f), and the number of afflicted joints per mouse was decreased (Supplementary Fig. 19). In parallel studies, K/BxN serum recipients were treated with a single dose of Cl-82198 (10 mg kg−1 i.p.) or methylprednisolone (10 mg kg−1 i.p.), neither of which improved ankle swelling (Fig. 4d–f). Although methylprednisolone, but not CL-82198, improved clinical score and pressure-pain tolerance, the effect size was not as pronounced as what was seen with siMMP13<(EG18L)2. Similarly, the disease severity score was somewhat improved upon treatment with CL-82198, but to a lesser extent than what was seen with siMMP13<(EG18L)2. Further, MMP13 silencing resulted in decreased mAbCII binding, indicative of cartilage preservation (Extended Data Fig. 8b) and decreased total MMP activity (Extended Data Fig. 8c).
Histologic examination of toluidine blue-stained cartilage collected on treatment day 8 in K/BxN recipient mice revealed substantial cartilage destruction in hindpaws, knees and forepaws, a phenotype that was diminished by siMMP13<(EG18L)2 treatment (Fig. 5a,b and Extended Data Fig. 9a,b; lower magnification images in Supplementary Figs. 20b and 21–23). Inflammation scoring illustrated similar trends (Fig. 5c and Extended Data Fig. 9c,d). MicroCT studies of bone structure in untreated K/BxN serum recipients revealed erosive bone disease characterized by increased bone surface to volume ratios, decreased bone mineral density (BMD) and decreased bone volume/total volume (BV/TV) ratio (Fig. 5d–g and Supplementary Fig. 23). Remarkably, treatment with siMMP13<(EG18L)2 protected K/BxN serum recipients from inflammation-induced changes in bone surface to volume, BMD and BV/TV measurements. Further, bone erosions characteristic of inflammatory arthritis were evident in forepaws, knees and hindpaws of untreated RA mice, but were minimal or absent in RA mice treated with siMMP13<(EG18L)2 (Extended Data Fig. 9e). In contrast, Cl-82198 and methylprednisolone did not diminish features of erosive bone disease detected by microCT. Serum C2C degradation fragments were significantly elevated in untreated, CL-82198-treated and methylprednisolone-treated K/BxN serum recipients (Extended Data Fig. 10a), while treatment with siMMP13<(EG18L)2 significantly reduced serum C2C fragments. These findings were confirmed using IHC for C1,2C in K/BxN serum recipients’ hindpaw/ankle and knee-joint tissues (Extended Data Fig. 10b).
K/BxN serum recipient mice were treated with siMMP13<(EG18L)2, methylprednisolone or CL-82198 (10 mg kg−1). a–c, Histological analysis of toluidine blue-stained ankle cartilage sections (a, top) and H&E-stained hindpaw sections (a, bottom) were used for cartilage destruction scoring (b) and inflammation scoring (c). Asterisks: yellow, articular surface; red, articular surface damage; black, healthy synovium; white, synovial inflammation. d–g, MicroCT-based 3D renderings of hindpaw scans (d, representative images) were used to measure bone surface:volume ratio (e), bone mineral density (f) and bone fraction (g). HA, hydroxyapatite, White arrows in d show bone erosions. All error bars indicate s.d. of biological replicates.
Hindpaw ankle articular cartilage and synovial RNA was collected from K/BxN serum transfer recipients on treatment day 8 and assessed using the nanoString nCounter mouse inflammation expression panel. These studies identified several gene clusters that were elevated in untreated K/BxN serum transfer recipients compared with healthy mice, many of which were downregulated in samples collected from K/BxN serum transfer recipients treated with siMMP13<(EG18L)2 (Extended Data Fig. 10c). Treated RA animals had gene expression patterns that mirrored the changes seen in PTOA knees upon MMP13 knockdown (for example, IL-1 signalling, NF-kB signalling and chemokine pathways were suppressed by treatment) (Extended Data Fig. 10d). In addition, the pro-inflammatory COX2-encoding gene Ptgs2, the chemokine genes Cxcl5, Ccl7 and Ccl19, and the RA-associated macrophage genes Tnfsf14 and Tr2 were significantly reduced in K/BxN hindpaws treated with siMMP13<(EG18L)2. Thus, the molecular markers of inflammation that characterize RA are dampened by MMP13 knockdown achieved by i.v. delivery of siMMP13<(EG18L)2.
Importantly, serum levels of toxicity markers, including blood urea nitrogen, alanine transaminase, aspartate amino transferase, creatine phosphokinase, lactate dehydrogenase and glucose, were detected within the normal ranges, lacking significant differences between healthy, PTOA or K/BxN serum recipient mice in any of the treatment groups (Supplementary Fig. 24). Liver, kidney, lung, heart and spleen of mice within all groups also showed a normal gross and histological structure (Supplementary Fig. 25).
MMP13 silencing in a Dunkin Hartley guinea pig ACL transection (ACLT) large animal model
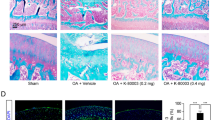
ACLT was done on the left knee of Dunkin Hartley guinea pigs (DHGPs) to establish proof of concept in PTOA in a larger species (Fig. 6a). Similar to humans, DHGPs develop OA with aging, with the medial knee compartment showing loss of proteoglycans, fibrillation, cloning of chondrocytes, osteophyte formation and subchondral bone sclerosis85. ACLT is used to mimic PTOA and accelerate the spontaneous OA phenotype86. MMP13 is strongly expressed and highly localized with OA cartilage lesions in DHGPs85. Cy5-siRNA<(EG18L)2 was delivered i.v. on post-surgical day 13 (1 mg kg−1 i.v.), and knees were imaged ex vivo to measure Cy5 fluorescence on day 14, revealing significantly increased Cy5-siRNA<(EG18L)2 accumulation in ACLT knees over unaltered knees (Fig. 6b), while free Cy5-siRNA did not exhibit preferential accumulation in ACLT-damaged knees. Ex vivo Cy5 imaging of organs showed abundant free siRNA in kidneys, while the albumin-binding siRNA<(EG18L)2 was found at highest levels in ACLT-damaged knees and liver, but not in healthy knees or in kidneys (Fig. 6c). Fluorescence microcopy confirmed delivery of Cy5-siRNA<(EG18L)2 but not unconjugated zipper-pattern siRNA to synovia of ACLT-damaged knees, with observable penetration into cartilage (Fig. 6d). Intravenous siMMP13<(EG18L)2 delivered on post-surgical day 7 at 10 mg kg−1 decreased Mmp13 expression in ACLT-damaged knees by >80% (Fig. 6e) and MMP13 protein expression by >70% on day 14 (Fig. 6f,g).
a, Study timeline for ACLT, treatment and sample collection in Dunkin Hartley guinea pigs. b–d, Cy5 fluorescence was measured ex vivo in healthy and ACLT knees (b) and organs (compared using multiple unpaired t-tests with no correction for multiple comparisons) (c) of guinea pigs by IVIS. Tissues were also assessed by fluorescence microscopy of sagittal ACLT knee cartilage/synovial cryosections (d) at 24 h after i.v. delivery of Cy5-siRNA or Cy5-siRNA<(EG18L)2 at 1 mg kg−1. Representative images are shown. In d: blue, DAPI; red, Cy5 siRNA. e, Guinea pig Mmp13 mRNA expression (ACLT/healthy knee) as determined by RT–qPCR. f,g, MMP13 immunofluorescence in cartilage/synovial cryosections of ACLT knee joints that were untreated (left) or treated with siMMP13<(EG18L)2 (10 mg kg−1 i.v.). Asterisks: white, synovium; magenta, femoral condyle cartilage. Representative images are shown with dashed lines outlining articular cartilage (f). MMP13 immunofluorescence intensity was quantitated using morphometric software (g). All error bars indicate s.d. of biological replicates. Knees labelled healthy indicate a contralateral limb (right) without ACL transection (non-surgical).
Outlook
A unique albumin-binding RNAi conjugate (siRNA<(EG18L)2) was engineered for MMP13-selective gene targeting in the context of arthritis. This optimized siRNA–lipid conjugate was capable of preferential accumulation and long-term retention within arthritic joints, robustly decreasing arthritis-induced MMP13 and interrupting the forward-feeding cycle of inflammatory joint damage to limit arthritis progression. Overall, this albumin-binding siRNA-L2 system shows promise as a systemic and/or local anti-MMP13-specific therapy. This siRNA delivery technology is readily amenable to modular engineering for targeting other arthritis disease-driving genes and/or gene combinations. Beyond the scope of arthritis, we believe this technology may be utilized to deliver to other diseased or injured tissues characterized by inflammation, including tumours, tissues undergoing ischaemic injury and bone fractures.
Methods
Materials
Phosphoramidites (2’-O-Me and 2’-F) and universal synthesis columns (MM1-2500-1) were purchased from Bioautomation, symmetrical branching CED phosphoramidite from ChemGenes (CLP-5215), and cyanine 5 phosphoramidite (10-5915), stearyl phosphoramidite (10-1979), hexaethyleneglycol phosphoramidite (10-1918), TEG cholesterol phosphoramidite (10-1976) and desalting columns (60-5010) were from Glen Research. Unless otherwise stated, materials and reagents were purchased from Fisher Scientific or Sigma-Aldrich. CL-82198 was purchased from MedChemExpress (HY-100359). Marimastat was purchased from APExBio (A4049). Clinical formulations of Zilretta and methylprednisolone were purchased through the Vanderbilt University Medical Center pharmacy. C2C ELISA kit and C1,2C antibody were from IBEX Pharmaceuticals.
siRNA synthesis, modification, annealing and storage
siRNA sequences were synthesized using modified (2’-F and 2’-O-Me) phosphoramidites with standard protecting groups on a MerMade 12 oligonucleotide synthesizer (Bioautomation). Amidites were dissolved at 0.1 M in anhydrous acetonitrile, except for 2’O-Me U-CE phosphoramidite (dissolved in 20% (v/v) anhydrous dimethylformamide) and stearyl phosphoramidite (dissolved in 3:1 (v/v) dichloromethane:acetonitrile). Coupling was performed under standard conditions. Strands were grown on controlled pore glass with a universal terminus (1 µmol scale, 1,000 Å pore), cleaved and deprotected using 1:1 methylamine:40% ammonium hydroxide, except Cy5 sequences which were deprotected in 30% ammonium hydroxide at room temperature for 20 h. Lipophilic RNAs were purified by reverse-phase (RP) high performance liquid chromatography (HPLC) using a Clarity Oligo-RP column (Phenomenex) under a linear gradient from 85% mobile phase A (50 mM triethylammonium acetate in water) to 100% mobile phase B (methanol), or 95% mobile phase A to 100% mobile phase B (acetonitrile). Oligonucleotide-containing fractions were dried, resuspended in nuclease-free water, sterile filtered and lyophilised. Conjugate molecular weight and purity was confirmed by liquid chromatography–mass spectrometry (LC–MS, ThermoFisher LTQ Orbitrap XL Linear Ion Trap mass spectrometer). Chromatography was performed using a Waters XBridge Oligonucleotide BEH C18 column under a linear gradient from 85% A (16.3 mM triethylamine, 400 mM hexafluoroisopropanol) to 100% B (methanol) at 45 °C. Mass spectrometry combined with liquid chromatography was utilized for product characterization (Supplementary Figs. 26–36). Purified oligonucleotide was resuspended in 0.9% sterile saline, annealed to the complementary strand by heating (95 °C) and stepwise cooling (15 °C per 9 min) to 25 °C and then stored at −80 °C.
MMP13 siRNA delivery to cultured cells
siRNA sequences used are listed in Supplementary Fig. 12. Seven candidate siRNA sequences (Dharmacon) targeting murine Mmp13 and 4 candidates (Dharmacon) targeting guinea pig Mmp13 were transfected (Lipofectamine 2000, ThermoFisher) into ATDC5 cells or primary guinea pig chondrocytes (respectively) in OptiMEM for 24 h. Stabilized ‘zipper’ siRNA sequences (50 nM and 100 nM, described below) were transfected into cells using Lipofectamine 2000 in OptiMEM for 24 h. For carrier-free delivery of siRNA and siRNA<(EG18L)2 to cells, siRNA (1,000 nM OptiMEM) was added to cells for 48 h. In all cases, complete medium supplemented with mouse or guinea pig tumour necrosis factor (TNF) (20 ng ml−1) replaced the siRNA containing media for an additional 24 h. RNA was collected, reverse transcribed and assessed by real-time polymerase chain reaction (PCR) using primers against mouse or guinea pig Mmp13. CellTiter-Glo (Promega) assay was used to assess cytotoxicity.
siRNA stability analysis
siRNA (1 nmol) was incubated at 37 °C for 0–24 h in 60% fetal bovine serum (FBS) in PBS, or in patient-derived arthritic joint synovial fluid (83-year-old male patient with untreated, active osteoarthritis). Samples were resolved on 2% agarose gels. Gels were stained with GelRed nucleic acid stain (Biotium) and imaged with UV transillumination.
siRNA<(EG18L)2 stability analysis (long-term)
siRNA<(EG18L)2 (2 nmol) was incubated at 37 °C for 0–96 h in 5 µl patient-derived arthritic joint synovial fluid with rocking. Samples were resolved using 4–20% Native PAGE (Mini-Protean) with 250 ng in each lane. Gels were stained with GelRed nucleic acid stain (Biotium) and imaged with UV transillumination.
Cell culture
Immortalized mouse chondrogenic ATDC5 cells (ATCC) were cultured in DMEM/F-12, GlutaMAX medium with 10% FBS and 1% penicillin/streptomycin (P/S). Primary guinea pig knee-joint chondrocytes were cultured in DMEM with 10% FBS and 1% P/S. Cells were incubated at 37 °C in 5% CO2. All cells tested negative for Mycoplasma using MycoAlert Mycoplasma Detection kit (Lonza).
Quantitation of siRNA uptake in cultured cells
ATDC5 cells (15,000 per well) were seeded overnight in 8-well chamber slides, treated with Cy5-labelled siRNAs pre-complexed with mouse serum albumin (MSA, 10× molar excess) for 4 h in OptiMEM, washed with PBS, fixed and counterstained with DAPI. Cy5 imaging was performed by confocal microscopy on a Nikon Eclipse Ti-0E inverted microscopy base. Alternatively, ATDC5 cells (10,000 per well) in 96-well plates were seeded overnight, treated for 2 h with Cy5-labelled siRNA (100 nM) with or without 1 μM MSA or 10% serum, in OptiMEM. Cells were collected by trypsinization and assessed by flow cytometry on a Guava EasyCyte (Luminex), gating >500 cellular events.
Size exclusion chromatography (SEC)
Cy5-labelled siRNA molecules (1 μM) were incubated for 30 min with 100 μl patient-derived synovial fluid collected from joints of a non-arthritic donor (myocardial infarction death, no history of rheumatic disease), a donor with untreated osteoarthritis, or a donor with untreated rheumatoid arthritis. The siRNA-synovial fluid mixtures were filtered (0.22 µm) and injected into an AKTA Pure Chromatography System (Cytiva) for fractionation with three inline Superdex 200 Increase columns (10/300 GL) at 0.3 ml min−1 using 10 mM Tris-HCl, 0.15 M NaCl and 0.2% NaN3. Then, 1.5 ml fractions were collected (F9-C 96-well plate fraction collector, Cytiva). Cy5 fluorescence was measured in fractions (100 µl) in black-walled 96-well plates on a SynergyMx (Biotek). Albumin-associated fractions were determined empirically using known protein standards, examining A280 of eluent from each fraction. Fractions were resolved on 4–20% SDS–PAGE (Mini-Protean), transferred to nitrocellulose (iBlot2, Invitrogen) and stained with Coomassie blue or probed with anti-human albumin antibody (ab19180, Abcam) and IRDye 800CW donkey anti-goat IgG (Li-Cor).
Mouse models of arthritis
A mechanical overload model of post-traumatic osteoarthritis was used, using an ElectroForce 3100 test frame (TA Instruments). Knee joints of anaesthetized, 6-week-old C57BL/6 male mice were positioned in flexion at 140° with the tibia approximately vertical and placed directly under the loading point87. Repetitive knee loading was performed with 250 cycles of 9 N of compressive mechanical force as described in previous studies62,87. Loading was repeated three times weekly for up to 5 weeks in either the left knee only, or in both knees, as indicated. For modelling rheumatoid arthritis, serum (200 µl) collected from donor K/BxN transgenic mice was transferred by intraperitoneal delivery to 8-week-old male C57BL/6 mice recipients76.
Cy5 labelling
MSA (Sigma) and 40 kDa PEG (Sigma) were Cy5-labelled (Cy5 conjugation kit, Abcam) and purified, confirming Cy5 fluorescence using a plate fluorimeter (Tecan).
Pharmacokinetic and retention studies
For initial pharmacokinetic studies, Cy5-siRNA, Cy5-siChol, Cy5-siRNA<(EG0L)2, Cy5-siRNA<(EG6L)2, Cy5-siRNA<(EG18L)2 and Cy5-siRNA<(EG30L)2 molecules were delivered i.v. (1 mg kg−1), subcutaneously (2 mg kg−1) or intra-articularly (0.25 mg kg−1). Cy5-MSA (3 mg kg−1) and Cy5-PEG (3 mg kg−1) were delivered i.v. For longer-term retention studies, Cy5-siRNA<(EG18L)2 was delivered intravenously (10 mg kg−1), or by intra-articular injection (1 mg kg−1). Mouse hindlimbs were depilated before intravital Cy5 imaging (IVIS Lumina III, Caliper Life Sciences) at indicated time points. Ex vivo Cy5 imaging of organs and denuded limbs collected at necropsy was performed. Where indicated, knee joints were microdissected for Cy5 imaging of knee cartilage and synovial tissues. For Cy5 imaging in histological sections, hindlimbs were embedded into OCT freezing compound, serially sectioned at various depths along the joint at 20 µm, captured onto polyvinylidene chloride film coated with synthetic rubber cement (http://section-lab.jp/), placed on slides, formalin fixed and counterstained with DAPI (in some cases). Cy5 fluorescence was imaged using a Nikon Eclipse Ti inverted confocal microscope. Whole-joint imaging was performed by stitching 4 × 4 images at ×10 magnification.
Cellular uptake assessment via flow cytometry
Cy5-siRNA<(EG18L)2 (10 mg kg−1) or vehicle (0.9% NaCl) was delivered i.v. to mice that had undergone 1 week of bilateral loading as described above (Supplementary Fig. 11a). Mice were sacrificed 24 h after injection and synovial tissue was isolated from the anterior, medial and lateral compartments as previously described59. The posterior synovium was not collected. Two synovia were digested together in a volume of 1.5 ml digestion media (DMEM with 400 μg ml−1 collagenase IV, liberase and DNaseI). Synovia were digested for 40 min at 37 °C with intermittent vortexing at 0, 15, 30, 35 and 40 min, and then pelleted at 500 × g for 5 min at 4 °C. Each treatment group (vehicle and Cy5-siRNA<(EG18L)2) comprised 5 male C57/B6 mice, which were pooled and divided into controls and samples. Cold FACS buffer (PBS containing 1% FBS, 2 mM EDTA) was used for all antibody staining and wash steps. Non-specific binding was blocked using Mouse Seroblock FcR (Bio-Rad) for 5 min. CD45-PerCp/Cy5.5 (Biolegend), FAP-AF488 (R&D Systems) and CD31-PE (Invitrogen) were used to identify major synovial cell populations. CD3-SBV515 (Bio-Rad), CD11b-APC/Cy7 (Biolegend), F4/80-PE/Cy7 (Biolegend) and CD11c-BV605 (Biolegend) were used to identify specific immune populations. All cell populations were gated off fluorescence-minus-one (FMO) controls (Supplementary Fig. 11b). The Cy5 gate was established using the vehicle mouse sample and applied to each cell population (Supplementary Fig. 11c), then a %Cy5 positive proportion (Fig. 2f) and median fluorescence intensity (MFI) (Supplementary Fig. 11d) were calculated for all synovial cells and each cell type separately. Experiments included single-stained compensation controls to account for fluorescence bleed over. Acquisition was performed using a BD LSRFortessa cytometer with FACSDiva software (BD Biosciences), then data compensation and analysis was performed using FlowJo v.10 (TreeStar/BD Biosciences). Antibody details are listed in Supplementary Fig. 11e.
Peptide nucleic acid hybridization assay
Quantification of antisense strands in tissue was performed using a PNA hybridization assay as previously described88,89. In brief, knee joints were dissected into mixed cartilage/meniscus and synovial tissues (5–15 mg) and lysed in 300 μl QuantiGene homogenizing solution (Invitrogen) containing 0.2 mg ml−1 Proteinase K (Invitrogen). Sodium dodecyl sulfate (SDS) was precipitated from homogenates with 20 μl 3 M potassium chloride and centrifuged at 4,000 × g for 15 min. To anneal the PNA probe, 100 μl of hybridization buffer (50 mM Tris, 10% ACN, pH 8.8) and 5 pmol of Cy3-labelled probe (PNABio) were added to 150 μl of each homogenate. Samples were then incubated for 15 min at 90 °C and then at 50 °C before analysis by ion exchange on an iSeries LC equipped with an RF-20A fluorescence detector (Shimadzu) over a DNAPac PA100 anion-exchange column (ThermoFisher). Mobile phases consisted of buffer A (50% acetonitrile and 50% 25 mM Tris-HCl, pH 8.5; 1 mM ethylenediaminetetraacetate in water) and buffer B (800 mM sodium perchlorate in buffer A), and a gradient was obtained as follows: 10% buffer B for 4 min, 50% buffer B for 1 min and 50% to 100% buffer B within 5 min. The final mass of siRNA was calculated from a standard curve of known quantities of siRNA or EG18 spiked into untreated tissue homogenates.
Therapeutic treatment of arthritis models
Zipper-modified siRNA targeting mouse MMP13 (siMMP13) or non-targeting siRNA (siControl) was delivered to mice at either 5 mg kg−1 or 10 mg kg−1 i.v., 1 mg kg−1 intra-articular, or 20–50 mg kg−1 subcutaneous. Mice were treated in parallel studies with Marimastat (10 mg kg−1 i.p.), Cl-82198 (10 mg kg−1 i.p.), methylprednisolone (10 mg kg−1 i.p.) or Zilretta (8 mg kg−1)90.
In situ imaging of MMP activity and collagen fragments
Mice were injected with mAbCII-680 (50 μl of 1 mg ml−1, tail vein) or with MMPsense 750 fast (100 μl of resuspended formulation following instructions, tail vein, NEV10168, PerkinElmer). Hindlimbs were depilated for IVIS imaging of fluorescence intensity.
Gene expression analysis in animal tissues
At necropsy, hindlimbs were cleaned of excess muscle and placed in RNAlater solution (ThermoFisher). Anterior synovial tissue pads, menisci, and tibial and femoral articular surface cartilage specimens were collected by microdissection. Cartilage, meniscal tissue and synovial tissue were mixed in equal mass ratios and termed ‘whole joint’ and used for mRNA extraction (RNeasy Plus mini kit from Qiagen). RNA from whole joint, liver or kidney was reverse transcribed (iScript cDNA synthesis kit, Bio-Rad) and used for real-time PCR with the following: murine Taqman probes: Actb (Mm02619580_g1), Gapdh (Mm99999915_g1), Mmp13 (Mm00439491_m1), Il1b (Mm00434228_m1), Il6 (Mm00446190_m1), Cox2 (Mm03294838_g1), Tnf (Mm00443258_m1); Sparc: (Mm00486332_m1), Cav1 (Mm00483057_m1), Fcrn (Mm00438887_m1), Ngf (Mm00443039_m1), Cdkn1b (Mm00494449_m1); quinea pig Taqman probes: Actb (Cp03755210_g1), Gapdh (Cp03755743_g1), Mmp13 (APRWJ74). RNA was processed for nanoString analysis using the mouse nCounter Inflammation Panel by the Vanderbilt VANTAGE shared resource, hybridizing for 20 h following manufacturer directions (Nanostring Technologies). Data analysis was performed using nSolver software for comparison, unsupervised analysis and gene cluster analysis between groups. The source data file contains gene names and fold expression changes in knee RNA samples collected from healthy mice, untreated PTOA mice and PTOA mice treated with siMMP13<(EG18L)2, as well as hindpaw RNA samples collected from healthy mice, untreated K/BxN serum recipient mice and K/BxN serum recipients treated with siMMP13<(EG18L)2.
Mining of single-cell RNA-sequencing data for MMP13 expression in synovium
Single-cell RNA-sequencing (scRNAseq) data from ref. 59 (NIH GEO accession: GSE211584) were employed to identify Mmp13-expressing cells in murine PTOA synovium. Data import, quality control, dimensionality reduction and clustering were performed as described in the original publication to yield a cellular atlas of healthy and PTOA synovium comprising lining fibroblasts, sublining fibroblasts, myeloid cells, dendritic cells, T cells, pericytes, endothelial cells, lymphatic endothelial cells, Schwann cells, skeletal muscle and red blood cells. Healthy synovium represented cells from the ‘Sham’ condition (no injury loading, analgesia and anaesthesia only) and PTOA synovium represented merged cells from the ‘ACLR 7 d’ and ‘ACLR 28 d’ conditions, which underwent non-invasive anterior cruciate ligament rupture via mechanical loading. All conditions were made up of pooled male and female mice, with ~20,500 total cells in the analysis. Gene feature plots of normalized Mmp13 transcript expression were derived to demonstrate the cellular sources and PTOA-associated induction of Mmp13 in murine synovium.
Evans blue delivery and extraction
Evans blue (200 μl of 2% w/v in sterile saline) was delivered by intravenous injection. After 24 h, mice were perfused with PBS. Hindlimbs collected at necropsy were air dried overnight. Joint tissues were microdissected. Evans blue was extracted from dissected tissues in formamide at 55 °C for 24 h. Evans blue extracted from tissue was measured as absorbance at 610 nm.
Immunofluorescence staining
Cryosections (20 µm) were fixed for 10 min with 4% paraformaldehyde, blocked in 5% donkey serum and probed with the following: anti-guinea pig MMP13 (1:100, ARP56350_P050, Aviva Systems Biology); goat anti‐rabbit Alexa Fluor 488 (1:500, ab150077, Abcam); Lycopersicon esculentum (tomato) lectin (LEL, TL) DyLight 488 (1:100, DL-1174-1). Slides were counterstained with DAPI, mounted with ProLong Gold Antifade and imaged on a Nikon Eclipse Ti inverted confocal microscope. Imaging settings remained constant across all treatment groups.
Staining of paraffin-embedded histological sections
Joints were formalin fixed, decalcified (20% tetrasodium EDTA) and paraffin embedded. Formalin-fixed paraffin-embedded coronal (knee) or sagittal (paw) sections (5 µm) were stained with haematoxylin and eosin (H&E), toluidine blue, or used for antigen retrieval in Epitope Retrieval 1 (Bond Rx) and immunohistochemistry. The following antibodies were used: anti-MMP13 (1:750, Abcam Ab39012), C1,2C (Col 2 3/4Cshort) polyclonal rabbit antibody (1:500, IBEX Pharmaceuticals, 50-1035). The Bond Refine Polymer detection system was used for visualization. Slides were imaged on the Leica SCN400 slide scanner (Leica Biosystems).
Clinical and histological scoring of arthritis
Knee-joint hyperalgesia was quantified using a hand-held algesiometer (Bioseb SMALGO, SMall animal ALGOmeter). For measurements, the pressure applicator tip was positioned on the medial aspect of the knee joint and force increased until eliciting a nociceptive response. Each limb was assessed in triplicate. The clinical score of arthritis was assessed as swelling/erythema on a scale of 0–3 (0, none; 1, slight; 2, moderate and in multiple digits; 3, pronounced and in entire paw)91. Ankle thickness was measured with calipers. Serum specimens were assessed for collagen degradation fragments using C2C enzyme-linked immunosorbent assay kit (IBEX Pharmaceuticals). Stained coronal knee-joint sections were scored from at least 2 mid-frontal coronal sections per joint66 using the OARSI and the DJD scales by a board-certified veterinary pathologist. OARSI scores (0–6 semiquantitative scale; Supplementary Table 1) were assigned from assessment of the medial and lateral plateaus of the tibia and femur65. DJD severity score (0–4 semiquantitative scale) was determined from H&E-stained sections using a semiquantitative index (Supplementary Table 1) based on cartilage erosion, subchondral osteosclerosis, synovial/meniscal metaplasia, subchondral osteosclerosis, inflammation, osteophytes and meniscal ectopic mineral deposits92. For the K/BxN STA study, hindpaws and forepaws were sectioned sagitally, with knee joints being sectioned coronally; they were also stained with H&E and toluidine blue for scoring purposes. Toluidine blue staining was verified by visualization of mouse skin mast cells and mouse ear cartilage. For the K/BxN serum transfer arthritis model, forepaw, hindpaw and knee-joint sections were also stained with H&E and toluidine blue and were scored using 3 scales adapted as previously described91,93 (Supplementary Table 2). Scoring was performed by a histopathologist blinded to the treatment.
Assessment of siRNA delivery to joint via RNA in situ hybridization
siMMP13<(EG18L)2 (10 mg kg−1) or vehicle (0.9% NaCl) was delivered i.v. to mice that had undergone 1 week of bilateral knee-joint loading as described above. Mice were killed 24 h after injection. Whole knee joints were dissected and fixed in 10% neutral buffered formalin at room temperature for 3 days. Joints were decalcified in 10% tetrasodium EDTA (pH 8) at room temperature under constant agitation for 4 days with a change to fresh EDTA solution after 2 days. Joints were embedded in paraffin, sectioned at 5 µm, mounted on SuperFrost Plus slides (12-550-15, Fisher Scientific) and baked at 60 °C overnight. Slides were deparaffinized through the following solvent series: xylene 10 min, xylene 10 min, 100% ethanol 5 min, 100% ethanol 5 min. Slides were then baked at 60 °C until dry, post fixed in 10% neutral buffered formalin overnight, washed in deionized (DI) water and baked at 60 °C until dry. Tissue sections were incubated with RNAscope hydrogen peroxide (322380, ACD) for 10 min at room temperature, washed in DI water, baked at 60 °C until dry, incubated with custom pretreatment reagent (provided by ACD for bone tissue specimens) for 30 min at 40 °C and washed in DI water. Tissue sections were then incubated as indicated with either positive control probe SR-RNU6-S1 (727871-S1, ACD), negative control probe SR-Scramble-S1 (727881-S1, ACD) or custom miRNAscope Singleplex Target Probe SR-siRNA-Mm-Mmp13 (ACD Sequence 155558, designed against the siMMP13 antisense sequence) for 2 h at 40 °C in a HybEZ II Hybridization System (321710, ACD). Amplification was performed with the miRNAscope HD Detection Kit-RED (324500, ACD) according to manufacturer instructions, with a reduced chromogen development time of 5 min. Slides were counterstained with filtered 50% Gill’s Hematoxylin I (HXGHE1LT, American Master Tech Scientific) for 2 min, washed under running DI water, dipped 5 times in 0.02% ammonia water and baked at 60 °C for 15 min. Slides were briefly dipped in xylene, mounted with Ecomount (EM897L, Biocare Medical) and coverslipped. Whole-slide imaging was performed in the Digital Histology Shared Resource at Vanderbilt University Medical Center, where slides were scanned at ×40 on a Leica SCN400 slide scanner.
MicroCT
Joints were fixed with formalin and submerged in 100% ethanol during microCT imaging with the ScanCo μCT-50 (Scanco Medical), with reconstructions completed in the ScanCo software (Scanco). Images were collected using 20-μm-thick slices, isotropic 12 μm voxel at 114 mA/70 kVp and 200 ms integration time. Initial contouring encompassed all mineralized joint components. Secondary contouring segmented out mineralization in the soft tissues surrounding cortical bone. Three-dimensional (3D) renderings (sigma: 1.5; support: 3; threshold: 388) were generated using Scanco software and are shown at a consistent density threshold (42.0% of maximum bone density, or 420 per mille). Imaging, contouring and sample measurements were acquired by a treatment-blinded user. Osteophyte length was measured on each sample in femur and tibia. For K/BxN serum recipients, contouring was performed over the calcaneus. Hydroxyapatite calibration phantoms were used to calibrate bone density values (g cm−3).
Guinea pig anterior cruciate ligament model
Dunkin Hartley guinea pigs (3-month-old male) (Charles River Laboratories) underwent left-knee ACLT surgery using procedures adapted from ref. 94. Surgery was performed under direct visualization in anaesthetized animals. A medial longitudinal parapatellar incision over the anterior knee exposed the patellar tendon. The patella was everted, the knee placed in flexion and the ACL incised. After confirming anterior joint laxity, the site was closed with the joint capsule continuously sutured. Ketoprofen was given immediately before and every 24 h post surgery for 3 days.
Blood chemistry
Whole blood was collected in EDTA-coated tubes or spun down at 1,000 × g for 10 min at 4 °C to isolate serum. Samples were then submitted to the Vanderbilt Translational Pathology Shared Resource for chemistry analyses.
Animal ethics statement
All animal experiments described herein were carried out according to protocols approved by Vanderbilt University’s Institutional Animal Care and Use Committee, and all studies followed the National Institutes of Health’s guidelines for the care and use of laboratory animals.
Statistical methods
Data are displayed as mean plus/minus standard deviation (unless stated otherwise). Statistical tests employed either one-way or two-way analysis of variance (ANOVA) with multiple comparisons test or two-tailed Student’s t-test between only two groups with α = 0.05 unless otherwise indicated. All statistical analyses were performed as described in figure captions using GraphPad Prism software, except for nanoString data analysis with nSolver v.3.0 (Nanostring Technologies).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Raw and normalized nanoString datasets are available at the Gene Expression Omnibus under accession identifier GSE233714. The remaining raw and analysed datasets from the study are available for research purposes from the corresponding author on reasonable request. Source data are provided with this paper.
References
Loeser, R. F. Osteoarthritis year in review 2013: biology. Osteoarthr. Cartil. 21, 1436–1442 (2013).
Gullo, T. R. et al. Defining multiple joint osteoarthritis, its frequency and impact in a community-based cohort. Semin. Arthritis Rheum. 48, 950–957 (2019).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Shiozawa, S., Tsumiyama, K., Yoshida, K. & Hashiramoto, A. Pathogenesis of joint destruction in rheumatoid arthritis. Arch. Immunol. Ther. Exp. 59, 89–95 (2011).
Bradley, J. D., Brandt, K. D., Katz, B. P., Kalasinski, L. A. & Ryan, S. I. Comparison of an antiinflammatory dose of ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee. N. Engl. J. Med. 325, 87–91 (1991).
Hochberg, M. C. et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 64, 465–474 (2012).
McAlindon, T. E. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 22, 363–388 (2014).
Benjamin, O., Goyal, A. & Lappin, S. L. Disease-Modifying Antirheumatic Drugs (DMARD) (StatPearls, 2022).
Bolduc, J. A., Collins, J. A. & Loeser, R. F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 132, 73–82 (2019).
Young, I. C. et al. A novel compressive stress-based osteoarthritis-like chondrocyte system. Exp. Biol. Med. 242, 1062–1071 (2017).
Jasin, H. E., Noyori, K., Takagi, T. & Taurog, J. D. Characteristics of anti-type II collagen antibody binding to articular cartilage. Arthritis Rheum. 36, 651–659 (1993).
Hollander, A. P. et al. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J. Clin. Invest. 96, 2859–2869 (1995).
Loeser, R. F. Integrins and chondrocyte–matrix interactions in articular cartilage. Matrix Biol. 39, 11–16 (2014).
Krzeski, P. et al. Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res. Ther. 9, R109 (2007).
Molina, J. R. et al. A phase I and pharmacokinetic study of the selective, non-peptidic inhibitor of matrix metalloproteinase BAY 12-9566 in combination with etoposide and carboplatin. Anticancer Drugs 16, 997–1002 (2005).
Clutterbuck, A. L., Asplin, K. E., Harris, P., Allaway, D. & Mobasheri, A. Targeting matrix metalloproteinases in inflammatory conditions. Curr. Drug Targets 10, 1245–1254 (2009).
Howes, J. M. et al. The recognition of collagen and triple-helical toolkit peptides by MMP-13: sequence specificity for binding and cleavage. J. Biol. Chem. 289, 24091–24101 (2014).
Zhang, X., Chen, C. T., Bhargava, M. & Torzilli, P. A. A comparative study of fibronectin cleavage by MMP-1, -3, -13, and -14. Cartilage 3, 267–277 (2012).
Wang, M. et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 15, R5 (2013).
Takaishi, H., Kimura, T., Dalal, S., Okada, Y. & D’Armiento, J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr. Pharm. Biotechnol. 9, 47–54 (2008).
Liu, J. & Khalil, R. A. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog. Mol. Biol. Transl. Sci. 148, 355–420 (2017).
Kristen, A. V. et al. Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis. Neurodegener. Dis. Manage. 9, 5–23 (2019).
Adams, D. et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid 30, 1–9 (2023).
Bedingfield, S. K. et al. Amelioration of post-traumatic osteoarthritis via nanoparticle depots delivering small interfering RNA to damaged cartilage. Nat. Biomed. Eng. 5, 1069–1083 (2021).
Bedingfield, S. K. et al. Top-down fabricated microplates for prolonged, intra-articular matrix metalloproteinase 13 siRNA nanocarrier delivery to reduce post-traumatic osteoarthritis. ACS Nano 15, 14475–14491 (2021).
DeJulius, C. R. et al. Engineering approaches for RNA-based and cell-based osteoarthritis therapies. Nat. Rev. Rheumatol. 20, 81–100 (2024).
Sleep, D., Cameron, J. & Evans, L. R. Albumin as a versatile platform for drug half-life extension. Biochim. Biophys. Acta 1830, 5526–5534 (2013).
Hsueh, M. F., Khabut, A., Kjellstrom, S., Onnerfjord, P. & Kraus, V. B. Cartilage permeability assessment based on proteomic analysis of plasma protein penetration. Osteoarthr. Cartil. 23, A132–A133 (2015).
Mannik, M. & Person, R. E. Deep penetration of antibodies into the articular cartilage of patients with rheumatoid arthritis. Rheumatol. Int. 14, 95–102 (1994).
Wunder, A. et al. Albumin-based drug delivery as novel therapeutic approach for rheumatoid arthritis. J. Immunol. 170, 4793–4801 (2003).
Liu, M. et al. Selective delivery of interleukine-1 receptor antagonist to inflamed joint by albumin fusion. BMC Biotechnol. 12, 68 (2012).
Hoogenboezem, E. N. & Duvall, C. L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 130, 73–89 (2018).
Kragh-Hansen, U. Molecular aspects of ligand binding to serum albumin. Pharmacol. Rev. 33, 17–53 (1981).
Curry, S., Mandelkow, H., Brick, P. & Franks, N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat. Struct. Biol. 5, 827–835 (1998).
Neumann, E. et al. Native albumin for targeted drug delivery. Expert Opin. Drug Deliv. 7, 915–925 (2010).
Carter, D. C. & Ho, J. X. Structure of serum albumin. Adv. Protein Chem. 45, 153–203 (1994).
Kratz, F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 132, 171–183 (2008).
Cohen, J. L. et al. Improved left ventricular endocardial border delineation and opacification with OPTISON (FS069), a new echocardiographic contrast agent. J. Am. Coll. Cardiol. 32, 746–752 (1998).
Frias, J. P. et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 385, 503–515 (2021).
Birn, H. et al. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J. Clin. Invest. 105, 1353–1361 (2000).
Birn, H. & Christensen, E. I. Renal albumin absorption in physiology and pathology. Kidney Int. 69, 440–449 (2006).
Amsellem, S. et al. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J. Am. Soc. Nephrol. 21, 1859–1867 (2010).
Sand, K. M. K. et al. Unraveling the interaction between FcRn and albumin: opportunities for design of albumin-based therapeutics. Front. Immunol. 5, 682–682 (2015).
Crielaard, B. J. et al. Glucocorticoid-loaded core-cross-linked polymeric micelles with tailorable release kinetics for targeted therapy of rheumatoid arthritis. Angew. Chem. Int. Ed. 51, 7254–7258 (2012).
Quan, L. D. et al. Development of a macromolecular prodrug for the treatment of inflammatory arthritis: mechanisms involved in arthrotropism and sustained therapeutic efficacy. Arthritis Res. Ther. 12, R170 (2010).
Bennike, T. et al. A normative study of the synovial fluid proteome from healthy porcine knee joints. J. Proteome Res. 13, 4377–4387 (2014).
King, J. D. et al. Joint fluid proteome after anterior cruciate ligament rupture reflects an acute posttraumatic inflammatory and chondrodegenerative state. Cartilage 11, 329–337 (2020).
Byeon, H. J. et al. Human serum albumin-TRAIL conjugate for the treatment of rheumatoid arthritis. Bioconjug. Chem. 25, 2212–2221 (2014).
Lau, S. et al. Enhanced extravasation, stability and in vivo cardiac gene silencing via in situ siRNA-albumin conjugation. Mol. Pharm. 9, 71–80 (2012).
Bienk, K. et al. An albumin-mediated cholesterol design-based strategy for tuning siRNA pharmacokinetics and gene silencing. J. Control. Release 232, 143–151 (2016).
Hoogenboezem, E.N. et al. Structural optimization of siRNA conjugates for albumin binding achieves effective MCL1-directed cancer therapy. Nat. Commun. 15, 1581 (2024).
Sarett, S. M. et al. Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc. Natl Acad. Sci. USA 114, E6490–E6497 (2017).
Sanchez, C. et al. Chondrocyte secretome: a source of novel insights and exploratory biomarkers of osteoarthritis. Osteoarthr. Cartil. 25, 1199–1209 (2017).
Sward, P., Frobell, R., Englund, M., Roos, H. & Struglics, A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)—a cross-sectional analysis. Osteoarthr. Cartil. 20, 1302–1308 (2012).
Nakamura, S. et al. Enhancement of SPARC (osteonectin) synthesis in arthritic cartilage. Increased levels in synovial fluids from patients with rheumatoid arthritis and regulation by growth factors and cytokines in chondrocyte cultures. Arthritis Rheum. 39, 539–551 (1996).
Nanba, Y. et al. Expression of osteonectin in articular cartilage of osteoarthritic knees. Acta Med. Okayama 51, 239–243 (1997).
Desai, N., Trieu, V., Damascelli, B. & Soon-Shiong, P. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl. Oncol. 2, 59–64 (2009).
Brown, K. M. et al. Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates. Nat. Biotechnol. 40, 1500–1508 (2022).
Knights, A. J. et al. Synovial fibroblasts assume distinct functional identities and secrete R-spondin 2 in osteoarthritis. Ann. Rheum. Dis. 82, 272–282 (2023).
Eichaker, L. R., Cho, H., Duvall, C. L., Werfel, T. A. & Hasty, K. A. Future nanomedicine for the diagnosis and treatment of osteoarthritis. Nanomed. Nanotechnol. Biol. Med. 9, 2203–2215 (2014).
O’Grady, K. P. et al. Drug-free ROS sponge polymeric microspheres reduce tissue damage from ischemic and mechanical injury. ACS Biomater. Sci. Eng. 4, 1251–1264 (2018).
Cho, H., Pinkhassik, E., David, V., Stuart, J. M. & Hasty, K. A. Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model. Nanomed. Nanotechnol. Biol. Med. 11, 939–946 (2015).
Ameye, L. G. et al. The chemical biomarkers C2C, Coll2-1, and Coll2-1NO2 provide complementary information on type II collagen catabolism in healthy and osteoarthritic mice. Arthritis Rheum. 56, 3336–3346 (2007).
McAlindon, T. E. et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA 317, 1967–1975 (2017).
Glasson, S. S., Chambers, M. G., Van Den Berg, W. B. & Little, C. B. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 18, S17–S23 (2010).
Bolon, B. et al. Rodent preclinical models for developing novel antiarthritic molecules: comparative biology and preferred methods for evaluating efficacy. J. Biomed. Biotechnol. 2011, 569068 (2011).
Coulthard, L. R., White, D. E., Jones, D. L., McDermott, M. F. & Burchill, S. A. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 15, 369–379 (2009).
Li, Z. et al. p38MAPK signaling pathway in osteoarthritis: pathological and therapeutic aspects. J. Inflamm. Res. 15, 723–734 (2022).
Jones, S. W. et al. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) modulates key biological pathways associated with OA disease pathology. Osteoarthr. Cartil. 17, 124–131 (2009).
Almasry, S. M., Soliman, H. M., El-Tarhouny, S. A., Algaidi, S. A. & Ragab, E. M. Platelet rich plasma enhances the immunohistochemical expression of platelet derived growth factor and vascular endothelial growth factor in the synovium of the meniscectomized rat models of osteoarthritis. Ann. Anat. 197, 38–49 (2015).
Solomon, L. A., Bérubé, N. G. & Beier, F. Transcriptional regulators of chondrocyte hypertrophy. Birth Defects Res. C 84, 123–130 (2008).
Adair-Kirk, T. L. & Senior, R. M. Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell Biol. 40, 1101–1110 (2008).
Guo, Q. et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 6, 15 (2018).
Jungel, A. et al. Effect of the oral application of a highly selective MMP-13 inhibitor in three different animal models of rheumatoid arthritis. Ann. Rheum. Dis. 69, 898–902 (2010).
Kazantseva, M. G., Hung, N. A., Highton, J. & Hessian, P. A. MMP expression in rheumatoid inflammation: the rs11568818 polymorphism is associated with MMP-7 expression at an extra-articular site. Genes Immun. 14, 162–169 (2013).
Singh, A. et al. Collagenase-3 (MMP-13) deficiency protects C57BL/6 mice from antibody-induced arthritis. Arthritis Res. Ther. 15, R222 (2013).
Granirer, L. W. A study of the albumin and globulin content in postpartum plasma and its use in rheumatoid arthritis. Science 111, 204 (1950).
Elewaut, D. Right on target: coupling methotrexate to albumin to treat rheumatoid arthritis. Rheumatology 43, 1067–1068 (2004).
Fiehn, C. et al. Albumin-coupled methotrexate (MTX-HSA) is a new anti-arthritic drug which acts synergistically to MTX. Rheumatology 43, 1097–1105 (2004).
Fiehn, C., Kratz, F., Sass, G., Muller-Ladner, U. & Neumann, E. Targeted drug delivery by in vivo coupling to endogenous albumin: an albumin-binding prodrug of methotrexate (MTX) is better than MTX in the treatment of murine collagen-induced arthritis. Ann. Rheum. Dis. 67, 1188–1191 (2008).
Bilthariya, U., Jain, N., Rajoriya, V. & Jain, A. K. Folate-conjugated albumin nanoparticles for rheumatoid arthritis-targeted delivery of etoricoxib. Drug Dev. Ind. Pharm. 41, 95–104 (2015).
Al-Rahim, A. M. et al. Folate-methotrexate loaded bovine serum albumin nanoparticles preparation: an in vitro drug targeting cytokines overwhelming expressed immune cells from rheumatoid arthritis patients. Anim. Biotechnol. 34, 166–182 (2021).
Lyu, J. et al. Treatment of rheumatoid arthritis by serum albumin nanoparticles coated with mannose to target neutrophils. ACS Appl. Mater. Interfaces 13, 266–276 (2021).
Liu, L. et al. Secreted protein acidic and rich in cysteine mediated biomimetic delivery of methotrexate by albumin-based nanomedicines for rheumatoid arthritis therapy. ACS Nano 13, 5036–5048 (2019).
Huebner, J. L., Otterness, I. G., Freund, E. M., Caterson, B. & Kraus, V. B. Collagenase 1 and collagenase 3 expression in a guinea pig model of osteoarthritis. Arthritis Rheum. 41, 877–890 (1998).
Thomas, N. P. et al. Synovial inflammation plays a greater role in post-traumatic osteoarthritis compared to idiopathic osteoarthritis in the Hartley guinea pig knee. BMC Musculoskelet. Disord. 18, 556 (2017).
Poulet, B., Hamilton, R. W., Shefelbine, S. & Pitsillides, A. A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 63, 137–147 (2011).
Roehl, I., Schuster, M. & Seiffert, S. Oligonucleotide detection method. Google Patents WO2010043512A1 WIPO (PCT) (2020).
Godinho, B. et al. Pharmacokinetic profiling of conjugated therapeutic oligonucleotides: a high-throughput method based upon serial blood microsampling coupled to peptide nucleic acid hybridization assay. Nucleic Acid Ther. 27, 323–334 (2017).
Kumar, A., Bendele, A. M., Blanks, R. C. & Bodick, N. Sustained efficacy of a single intra-articular dose of FX006 in a rat model of repeated localized knee arthritis. Osteoarthr. Cartil. 23, 151–160 (2015).
Zhou, H. F., Chan, H. W., Wickline, S. A., Lanza, G. M. & Pham, C. T. αvβ3-targeted nanotherapy suppresses inflammatory arthritis in mice. FASEB J. 23, 2978–2985 (2009).
Aigner, T. & Söder, S. Histopathologische Begutachtung der Gelenkdegeneration. Pathologe 27, 431–438 (2006).
Choi, Y. R. et al. A genome-engineered bioartificial implant for autoregulated anticytokine drug delivery. Sci. Adv. 7, eabj1414 (2021).
Jimenez, P. A., Harlan, P. M., Chavarria, A. E. & Haimes, H. B. Induction of osteoarthritis in guinea pigs by transection of the anterior cruciate ligament: radiographic and histopathological changes. Inflamm. Res. 44, S129–S130 (1995).
Acknowledgements
We acknowledge the assistance of the Vanderbilt Translational Pathology Shared Resource (TPSR), which is supported by NCI/NIH Cancer Center Support Grant 2P30 CA068485-14 and the Shared Instrumentation Grant S10 OD023475 for the Leica Bond Rx. MicroCT analyses were supported in part by the NIH (S10RR027631-01). The histological technical assistance of C. Lowe and K. Thompson (Department of Pathology, Microbiology, and Immunology, Division of Comparative Medicine, Vanderbilt University Medical Center, Nashville, TN, USA) and M. Echanove González de Anleo (Biomedical Engineering Program, Centro de Investigación Médica Aplicada (CIMA), Pamplona, Spain), and the microCT technical assistance by S. Uppuganti (Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, USA) are acknowledged. We also appreciate the help of A. Knights (Department of Orthopaedic Surgery, University of Michigan, Ann Arbor, MI, USA) in planning and interpreting flow cytometry experiments. Flow Cytometry experiments were performed in the VMC Flow Cytometry Shared Resource, which is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). All LC-MS characterization was performed in the Mass Spectrometry Research Center at Vanderbilt University. We acknowledge funding from the DOD (DOD CDMRP OR130302), NIH (NIH R01 CA224241, NIH R01 EB019409, NIH R21 AR078636, NIH T32GM007347), the Canadian Natural Sciences and Engineering Research Council (NSERC), the Rheumatology Research Foundation (RRF), and the VA Merit Awards BX004151 and BX004472. The content in this report is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author information
Authors and Affiliations
Contributions
J.M.C. and C.L.D. conceptualized the study and experiments. J.M.C., E.N.H., J.H.L., A.G.S. and N.F. synthesized and characterized the siRNA conjugates and assisted with PNA hybridization. J.M.C. and M.C.K. performed in vitro siRNA studies. J.M.C., M.C.K., V.S., J.T.M. and C.R.D. performed in vivo flow experiments. J.M.C., M.C.K., V.S. and N.T.C. performed in situ hybridization experiments. J.M.C. and F.Y. performed in vivo pharmacokinetic studies. J.M.C. performed in vivo confocal microscopy imaging and analyses. D.L.M. and K.C.V. performed SEC synovial fluid analysis. J.M.C., F.Y. and V.S. performed in vivo therapeutic studies including MMPsense, RT–qPCR, microCT and histological tissue preparation. K.N.G.-C. performed pathologist-blinded OARSI, DJD and K/BxN STA scoring of histological sections. L.J.C. provided K/BxN serum and experimental insight for inflammatory arthritis studies. H.C. and K.A.H. provided mAbCII-680 reagent and experimental insight for in vivo cartilage damage studies. T.M. provided insight for in vivo flow cytometry experimental design and performed and contributed the single-cell RNA-seq analysis. R.C. and C.L.D. provided study mentorship and oversight. J.M.C., R.S.C. and C.L.D. co-wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Jean-Paul Desaulniers, Lei Wei, Huanghao Yang, Chuan Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterizing different routes of siRNA<(EG18L)2 delivery in PTOA mouse model.
a-c, Subcutaneous delivery of Cy5-siRNA<(EG18L)2 (2 mg/kg) was administered in a PTOA mouse model. Timeline for mechanical knee loading and treatment is shown (a). Intravital (b) and ex vivo (c) Cy5 fluorescence imaging was used to measure Cy5-siRNA<(EG18L)2 biodistribution to paired healthy and PTOA knees, along with organs, 24 hrs after subcutaneous treatment. N = 3. d, Quantification of subcutaneous vs. intravenous (i.v.) delivery route organ biodistribution 24 h after injection. Analyzed using multiple unpaired t-tests with no correction for multiple comparisons. e-g, Intra-articular delivery of Cy5-siRNA<(EG18L)2 (0.25 mg/kg) was characterized in a PTOA mouse model. Timeline for mechanical knee loading and treatment is shown (e). Ex vivo Cy5 fluorescence imaging was used to measure Cy5-siRNA<(EG18L)2 biodistribution to paired healthy and loaded knees, along with organs, 48 hrs after delivery (f). N = 3. Analyzed using multiple unpaired t-tests with no correction for multiple comparisons. (g) Cryohistology of the loaded knee joints 48 h after intra-articular injection with a focus on cartilage and synovial tissues. N = 3. Dashed lines outline articular cartilage. For all panels: Error bars indicate standard deviation of biological replicates. Radiant efficiency units are [photons/s] / [µW/cm²]. Knees labeled healthy indicate a non-loaded contralateral limb (right).
Extended Data Fig. 2 MMP13 knockdown in PTOA joints by i.v. delivery of siMMP13<(EG18L)2.
a, Mmp13 mRNA was measured by qRT-PCR in healthy and PTOA knees of mice 5 days after i.v. delivery of siMMP13<(EG18L)2, siMMP13-Chol, or free siMMP13 (5 and 10 mg/kg, where indicated). Timeline for mechanical knee loading and treatment is shown in Extended Data Fig 1. b, Mmp13 mRNA was measured by qRT-PCR in PTOA mouse knees 5 days after i.v., i.a., or subcutaneous siMMP13<(EG18L)2 delivery at the doses indicated. N = 5-6. c, Mmp13 mRNA was measured by qRT-PCR in PTOA mouse knees 5 days after subcutaneous siMMP13<(EG18L)2 delivery at the doses indicated. N = 5-6. d-e, MMP13 IHC in PTOA knees (d), liver (e, top), and kidneys (e, bottom) 5 days after i.v. delivery (10 mg/kg) of siMMP13<(EG18L)2, siMMP13-Chol, free siMMP13, or no treatment. f, Relative Mmp13 mRNA expression in liver and kidneys of untreated mice (relative to PTOA knee Mmp13 levels). N = 3. g, Relative Mmp13 mRNA in kidneys, 5 days post-injection with 10 mg/kg siMMP13<(EG18L)2 or free siMMP13, vs. untreated controls. N = 3. For all panels: Error bars indicate standard deviation of biological replicates.
Extended Data Fig. 3 Systemic (i.v.) siMMP13<(EG18L)2 (10 mg/kg) treatment achieves long-term retention and MMP13 silencing in PTOA knee joints.
a, Timeline for mouse left knee mechanical loading (3 times / week) and single treatment (day 0; 7 days after initiation of mechanical loading) with Cy5-siRNA<(EG18L)2 or siMMP13<(EG18L)2. b, Cryosection confocal microscopy imaging of Cy5-siRNA<(EG18L)2 in joint tissues taken down on day 1 post-treatment. c-e, Cy5-siRNA<(EG18L)2 was visualized on day 30 post-treatment by confocal microscopy of knee joint cryosections (c, representative images with dashed lines outlining articular cartilage), and at time points from 0-30 days post-treatment by longitudinal intravital Cy5 imaging (d). Semiquantitative analysis of the AUC (Cy5 fluorescence x days) was calculated (e) (N = 4). f-g, Knee joint Mmp13 was measured at the indicated time points by qRT-PCR (e) and IHC (f) in healthy or PTOA knee tissues collected at the indicated time points from mice treated with or without siMMP13<(EG18L)2. N = 5-6. For all panels: Error bars indicate standard deviation of biological replicates. Knees labeled healthy indicate a non-loaded contralateral limb (right).
Extended Data Fig. 4 Healthy and PTOA knee joint retention after intra-articular delivery over a 30-day time course.
a, Representative IVIS images of healthy and PTOA mouse knee joints over 30 days after a single 1 mg/kg intra-articular (i.a.) injection of Cy5-siRNA<(EG18L)2 or Cy5-siRNA. b, Semiquantitative analysis of time-course fluorescent radiant efficiency within healthy and OA mouse knee joints over 30 days (N = 3). AUC was calculated based on the fluorescence intensity profiles. Radiant efficiency over time plotted as mean + SEM. AUC plot error bars indicate standard deviation of biological replicates. c, Semiquantitative analysis of time-course fraction of retention within healthy and OA mouse knee joints over 30 days (N = 3). AUC was calculated based on the fraction of retention profiles. Radiant efficiency over time plotted as mean + SEM. AUC plot error bars indicate standard deviation of biological replicates. d, Representative confocal microscopy of knee joint cryosections at the 30-day endpoint showing i.a. Cy5-siRNA<(EG18L)2, and i.a. Cy5-siRNA in loaded, PTOA, knees with a specific focus on synovial and cartilage/meniscus tissues. Dashed lines outline articular cartilage. Knees labeled healthy indicate a non-loaded contralateral limb (right).
Extended Data Fig. 5 Intravenous albumin-hitchhiking siMMP13<(EG18L)2 treatment provides whole knee joint protection by reducing osteophyte formation and pathologic mineralization and is associated with a reduction in MMP13 protein and collagen degradation fragments.
a, MicroCT-based 3-dimensional (3D) renderings of meniscal/ectopic mineralization and osteophyte growth in healthy and PTOA mice treated with siMMP13<(EG18L)2 or siControl<(EG18L)2. Red asterisk = osteophyte outgrowth. b, Measurements of femoral and tibial osteophyte size at largest outgrowth from normal cortical bone structure. N = 8. c, Ectopic mineralization (measured as mg of hydroxyapatite) in menisci and in the form of osteophytes was quantified. N = 8. d-f, MMP13 IHC analyses of PTOA joint cartilage (d), meniscus (e), and synovium (f) show reduced MMP13 protein levels in animals treated with siMMP13<(EG18L)2. g, IHC on C1,2 C collagen 2 degradation fragments. Samples here are from the PTOA therapeutic study in Main Fig. 3. For panels b-c: Error bars indicate standard deviation of biological replicates.
Extended Data Fig. 6 Systemic siMMP13<(EG18L)2 delivery alters inflammatory gene expression profiles in mechanically-loaded PTOA joints.
a, qRT-PCR of IL-1B, TNFalpha, NGF, and P16INK4a (Cdkn2a) at days 5, 10, 20, and 30 after a single i.v. injection of 10 mg/kg siMMP13<(EG18L)2. Analyzed using a mixed effects analysis. N = 3-7. Error bars indicate standard deviation. b, Unsupervised sorting of treatment groups as quantified by nanoString at day 10 after treatment of PTOA knees with 10 mg/kg siMMP13<(EG18L)2. Gene expression is shown as high- (green) or low-expression (red) sorted vertically by differences between treatment groups. c, Gene cluster expression changes at day 10 post-treatment in PTOA knees.
Extended Data Fig. 7 Characterizing albumin-based drug delivery in the K/BxN serum transfer arthritis model.
a-c, Evans Blue or Cy5-MSA was delivered i.v. to wild type (healthy) and K/BxN serum recipient mice 4 days after serum transfer. Joints of forepaws and hindpaws were used for Evans Blue extraction (a, representative images), measuring Evans Blue absorbance in extracts (a, right). Evans Blue was also visualized by fluorescence microscopy in joint cryosections (b). MSA-Cy5 delivery to joints was visualized by intravital Cy5 fluorescence imaging (c). d, qRT-PCR was used to measure relative gene expression of albumin transport-associated genes (Sparc and Caveolin-1 [Cav1]) in healthy and K/BxN arthritis hind paws. N = 7 for Sparc; N = 6 for Cav1. e-g, Cy5-siRNA<(EG18L)2 (1 mg/kg, i.v.) was delivered to healthy and K/BxN serum recipient mice. Cy5 fluorescence in organs (e) and limb joints (f), analyzed using multiple unpaired t-tests with no correction for multiple comparisons, were measured ex vivo at 24 hrs. Cy5 fluorescence was assessed in knee and hindpaw cryosections by fluorescence microscopy (g). For panel e, N = 3 for heart, lungs, liver, and spleen; N = 6 for kidney, forepaw, knee, and hindpaw. For all panels: Error bars indicate standard deviation of biological replicates.
Extended Data Fig. 8 Intravenous siMMP13<(EG18L)2 delivery silences MMP13 and protects cartilage in the joints of mice given K/BxN serum transfer.
K/BxN STA mice were treated with or without siMMP13<(EG18L)2 (10 mg/kg, i.v.), then assessed at day 10. a, MMP13 IHC analysis of cartilage and synovium in multiple joints was used to assess silencing of MMP13 expression. b, Fluorescent mAbCII delivered to mice was detected by ex vivo fluorescence imaging to measure relative cartilage damage in healthy and K/BxN STA mice undergoing treatment (representative images shown). N = 14. c, Pan-MMP activity was measured via delivery of MMPsense 750 Fast to mice, followed by detection using ex vivo fluorescence imaging. N = 14. For all panels: Error bars indicate standard deviation of biological replicates.
Extended Data Fig. 9 Intravenous delivery of siMMP13<(EG18L)2 protects cartilage and bone while reducing inflammation in multiple joints of the K/BxN STA model.
a-b, Toluidine Blue stained histological knee and forepaw sections (a) were scored using the Cartilage Destruction Score (b). Hindpaw data is shown in Fig. 5. c-d, H&E-stained histological knee and forepaw sections (c) were scored using the Inflammation Score (d). Hindpaw data is shown in Fig. 5. e, Histological bone destruction score quantification of hindpaw, knee, and forepaw joint tissues. For panels b, d, and e: N = 7. Error bars indicate standard deviation of biological replicates.
Extended Data Fig. 10 Intravenous siMMP13<(EG18L)2 treatment reduces presence of cartilage degradation fragments in serum and diminishes the inflammatory gene expression profile in the joints of the K/BxN STA model.
a, Relative serum C2C collagen 2 degradation fragment levels in serum were measured on day 10 following treatment by ELISA. Error bars indicate standard deviation of biological replicates. N = 6-7. b, Presence and localization of C1,2C collagen 2 degradation fragments were assessed by IHC on day 10 post-treatment. c-d, RNA harvested from K/BxN recipient mouse joints was assessed by the nanoString nCounter Mouse Inflammatory 294 Gene Expression panel. Clustering of gene expression changes [high- (green) or low- (red)] sorted vertically by differences between treatment groups (c). Analysis of relevant gene clusters from tissues that were harvested on day 10 post-treatment (d). N = 4.
Supplementary information
Supplementary Information
Supplementary Figs. 1–36.
Supplementary Tables 1 and 2
Supplementary table
Source data
Source Data
Source data for main figures, extended data figures, nanostring data set with labelled tabs and unprocessed gel for Fig. 1h.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Colazo, J.M., Keech, M.C., Shah, V. et al. siRNA conjugate with high albumin affinity and degradation resistance for delivery and treatment of arthritis in mice and guinea pigs. Nat. Biomed. Eng 9, 1366–1383 (2025). https://doi.org/10.1038/s41551-025-01376-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01376-x