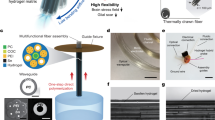
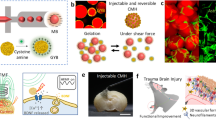
Abstract
Small materials with pliability and untethered mobility are particularly suitable for minimally invasive medical interventions inside the body. However, the capabilities and applicability of such soft ‘robots’ are restricted by foreign-body responses to them and by the need to get them cleared from the body after the intervention. Here we report the development of biodegradable magnetized biohybrid blood hydrogel fibres that evade immune recognition, and their applicability for targeted intracranial tumour therapy with real-time tracking through X-ray fluoroscopy. The gel fibres can be made of the patient’s own blood mixed with a small amount of magnetic particles and can be produced in about 15 min. We show that the locomotion of intracranially injected gel fibres through cerebrospinal fluid can be remotely controlled under a magnetic field and fluoroscopically tracked, and that a drug encapsulated in the gels can be released on demand under magnetic control, as we show for the delivery of doxorubicin to intracranial tumours in the minipigs. Biodegradable soft actuatable materials that avoid foreign-body responses may aid the development of personalized targeted interventions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results of this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Fomchenko, E. I. & Holland, E. C. Origins of brain tumors—a disease of stem cells? Nat. Clin. Pract. Neurol. 2, 288–289 (2006).
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 24, v1–v95 (2022).
Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 (2009).
Wen, P. Y. & Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 358, 492–507 (2008).
Li, J. et al. Micro/nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci. Robot. 2, eaam6431 (2017).
Chen, Y. et al. Bioinspired hydrogel actuator for soft robotics: opportunity and challenges. Nano Today 49, 101764 (2023).
Rus, D. & Tolley, M. T. Design, fabrication and control of soft robots. Nature 521, 467–475 (2015).
Horejs, C. Speedy soft robots. Nat. Rev. Mater. 5, 785–785 (2020).
Ebrahimi, N. et al. Magnetic actuation methods in bio/soft robotics. Adv. Funct. Mater. 31, 2005137 (2021).
Zhang, Y. et al. Submillimeter multifunctional ferromagnetic fiber robots for navigation, sensing, and modulation. Adv. Healthc. Mater. 12, 2300964 (2023).
Abdelaziz, M. E. M. K. et al. Fiberbots: robotic fibers for high-precision minimally invasive surgery. Sci. Adv. 10, adj1984 (2024).
Ye, Z. et al. Liquid-metal soft electronics coupled with multilegged robots for targeted delivery in the gastrointestinal tract. Device 2, 100181 (2024).
Leber, A. et al. Highly integrated multi-material fibers for soft robotics. Adv. Sci. 10, 2204016 (2023).
Barbot, A. et al. Floating magnetic microrobots for fiber functionalization. Sci. Robot. 4, eaax8336 (2019).
Xu, T. et al. Millimeter-scale flexible robots with programmable three-dimensional magnetization and motions. Sci. Robot. 4, eaav4494 (2019).
Guix, M. et al. Biohybrid soft robots with self-stimulating skeletons. Sci. Robot. 6, eabe7577 (2021).
Wang, B. et al. Endoscopy-assisted magnetic navigation of biohybrid soft microrobots with rapid endoluminal delivery and imaging. Sci. Robot. 6, eabd2813 (2021).
Mundaca-Uribe, R. et al. Towards multifunctional robotic pills. Nat. Biomed. Eng. 8, 1334–1346 (2024).
Kim, Y. et al. Ferromagnetic soft continuum robots. Sci. Robot. 4, eaax7329 (2019).
Liu, X. et al. Magnetic soft microfiberbots for robotic embolization. Sci. Robot. 9, eadh2479 (2024).
Xu, C. et al. Small-scale magnetic actuators with optimal six degrees-of-freedom. Adv. Mater. 33, 2100170 (2021).
Pilz da Cunha, M. et al. Bioinspired light-driven soft robots based on liquid crystal polymers. Chem. Soc. Rev. 49, 6568–6578 (2020).
Shih, B. et al. Electronic skins and machine learning for intelligent soft robots. Sci. Robot. 5, eaaz9239 (2020).
Xu, T. et al. Multi-modal locomotion control of needle-like microrobots assembled by ferromagnetic nanoparticles. In Proc. IEEE/ASME Transactions on Mechatronics Vol. 27 4327–4338 (IEEE, 2022).
Mirvakili, S. M. et al. Actuation of untethered pneumatic artificial muscles and soft robots using magnetically induced liquid-to-gas phase transitions. Sci. Robot. 5, eaaz4239 (2020).
Yan, Y. et al. Soft magnetic skin for super-resolution tactile sensing with force self-decoupling. Sci. Robot. 6, eabc8801 (2021).
Yang, X. et al. An agglutinate magnetic spray transforms inanimate objects into millirobots for biomedical applications. Sci. Robot. 5, eabc8191 (2020).
Hu, X. et al. Magnetic soft micromachines made of linked microactuator networks. Sci. Adv. 7, eabe8436 (2021).
Wen, L. et al. Tensegrity metamaterials for soft robotics. Sci. Robot. 5, eabd9158 (2020).
Xu, T., Huang, C., Lai, Z. & Wu, X. Independent control strategy of multiple magnetic flexible millirobots for position control and path following. In Proc. IEEE Transactions on Robotics Vol. 38 2875–2887 (IEEE, 2022).
Alapan, Y. et al. Reprogrammable shape morphing of magnetic soft machines. Sci. Adv. 6, eabc6414 (2020).
Wang, Y. et al. Microrobots for targeted delivery and therapy in digestive system. ACS Nano 17, 27–50 (2023).
Wang, B. et al. Trends in micro-/nanorobotics: materials development, actuation, localization, and system integration for biomedical applications. Adv. Mater. 33, 2002047 (2021).
Drotman, D. et al. Electronics-free pneumatic circuits for controlling soft-legged robots. Sci. Robot. 6, eaay2627 (2021).
Le, X. et al. Recent progress in biomimetic anisotropic hydrogel actuators. Adv. Sci. 6, 1801584 (2019).
Feinberg, A. W. Biological soft robotics. Annu. Rev. Biomed. Eng. 17, 243–265 (2015).
Yang, C. & Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 3, 125–142 (2018).
Cianchetti, M. et al. Biomedical applications of soft robotics. Nat. Rev. Mater. 3, 143–153 (2018).
Huang, H.-W. et al. Soft micromachines with programmable motility and morphology. Nat. Commun. 7, 12263 (2016).
Ratner, B. D. Biomaterials: been there, done that, and evolving into the future. Annu. Rev. Biomed. Eng. 21, 171 (2019).
Anderson, J. M. et al. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86 (2008).
Schuerle, S. et al. Robotically controlled microprey to resolve initial attack modes preceding phagocytosis. Sci. Robot. 2, eaah6094 (2017).
Yasa, I. C. et al. Elucidating the interaction dynamics between microswimmer body and immune system for medical microrobots. Sci. Robot. 5, eaaz3867 (2020).
Cabanach, P. et al. Zwitterionic 3D-printed non-immunogenic stealth microrobots. Adv. Mater. 32, 2003013 (2020).
Zhang, H. et al. Dual-responsive biohybrid neutrobots for active target delivery. Sci. Robot. 6, eaaz9519 (2021).
Dai, Y. et al. Precise control of customized macrophage cell robot for targeted therapy of solid tumors with minimal invasion. Small 17, 2103986 (2021).
Fang-Yen, C. Biomechanical analysis of gait adaptation in the nematode Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 107, 20323–20328 (2010).
Yuan, J. et al. Gait synchronization in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 111, 6865–6870 (2014).
Deng, H. et al. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 44, 2782–2785 (2005).
Dong, X. et al. Toward a living soft microrobot through optogenetic locomotion control of Caenorhabditis elegans. Sci. Robot. 6, eabe3950 (2021).
Xia, N. et al. Decoupling and reprogramming the wiggling motion of midge larvae using a soft robotic platform. Adv. Mater. 34, 2109126 (2022).
Floyd, S. et al. Control methodologies for a heterogeneous group of untethered magnetic micro-robots. Int. J. Robot. Res. 30, 1553–1565 (2011).
Acknowledgements
We thank C. Zhu from Shenzhen Top Biotechnology Limited Company for their technical support and assistance with the animal experiments. We thank L. Zheng from Shenzhen University for their technical support in materials characterization. We thank the Instrument Analysis Center of Shenzhen University for assistance with the SEM analysis. T.X. discloses support for the publication of this study from the National Key Research and Development Project (2023YFB4705300). B.W. discloses support for the research described in this study from the National Natural Science Foundation of China (52475308), the Shenzhen Science and Technology Innovation Committee Projects (JCYJ20220531103409021) and the Shenzhen Medical Research Fund (SMRF A2303074). J.S. discloses support for the publication of this study from the National Natural Science Foundation of China (52471256) and the Shenzhen Science and Technology Innovation Committee Projects (SGDX20220530111405038, JCYJ20240813115822030). T.X. also discloses support for the publication of this study from the National Natural Science Foundation of China (62022087 and U22A2064), the Shenzhen Science and Technology Innovation Committee Projects (RCJC20231211085926038, RCBS20200714114920190 and JCYJ20220818101611025) and the Guangdong Basic and Applied Basic Research Foundation (2022B1515120010). Z.Y. discloses support for the publication of this study from the Guangdong University Students Science and Technology Innovation Cultivation Special Fund Project (pdjh2023b0450). L.Z. discloses support for the publication of this study from the Hong Kong Research Grants Council (RGC) with Research Impact Fund (R4015-21), the Research Fellow Scheme (RFS2122-4S03), the Strategic Topics Grant (STG1/E-401/23-N) and the Croucher Foundation Grant (CAS20403). X.W. discloses support for the research described in this study from the National Natural Science Foundation of China (62125307). We also disclose support from the SIAT-CUHK Joint Laboratory of Robotics and Intelligent Systems and the Multi-scale Medical Robotics Centre (MRC), InnoHK, at the Hong Kong Science Park.
Author information
Authors and Affiliations
Contributions
Conceptualization, B.W., L.Z. and T.X.; methodology, B.W., J.S. and C.H.; investigation, B.W., J.S., C.H., Z.Y. and J.H.; supervision, B.W., L.Z. and T.X.; writing—original draft, B.W. and C.H.; writing—review and editing, B.W., J.S., L.Z., X.W., Z.G. and T.X.; funding acquisition, B.W., J.S., Z.Y., L.Z. and T.X.
Corresponding authors
Ethics declarations
Competing interests
B.W. is an inventor with a Chinese patent (patent number ZL202111388615.2, filed on 22 November 2021) on the fabrication method of a magnetic biodegradable blood hydrogel. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Qiang He, Christopher Nguyen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes, figures, tables, references and video captions.
Supplementary Video 1
Multimodal locomotion of BBHFs.
Supplementary Video 2
Motion of BBHFs in confined environments.
Supplementary Video 3
Motion of BBHF in a brain phantom.
Supplementary Video 4
Motion through the porcine cerebral cortex with gullies.
Supplementary Video 5
Splitting and drug release process of a BBHF.
Source data
Source Data Figs. 2–5 and 7
Source data for Figs. 2–5 and 7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, B., Shen, J., Huang, C. et al. Magnetically driven biohybrid blood hydrogel fibres for personalized intracranial tumour therapy under fluoroscopic tracking. Nat. Biomed. Eng 9, 1471–1485 (2025). https://doi.org/10.1038/s41551-025-01382-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01382-z
This article is cited by
-
A movable long-term implantable soft microfibre for dynamic bioelectronics
Nature (2025)
-
Biocompatible low-voltage electrothermal actuators with biological operational temperature range
Communications Materials (2025)