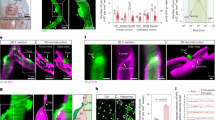
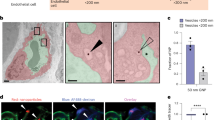
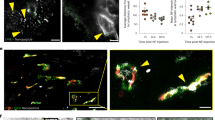
Abstract
The delivery of nanoparticles (NPs) into solid tumours is challenged by the tumour vascular basement membrane (BM), a critical barrier beneath the endothelium with robust mechanical properties resistant to conventional treatments. Here we propose an approach that uses nitric oxide (NO) to induce the opening of endothelial junctions, creating gaps between endothelial cells and enabling the navigation of NPs through these gaps. Subsequently, NO orchestrates a transient degradation of the BM encasing NP pools in a precise, localized action, allowing the enhanced passage of NPs into the tumour interstitial space through explosive eruptions. We have engineered a NO nanogenerator tailored for near-infrared laser-triggered on-demand NO release at tumour sites. Through breaching the BM barrier, this system results in an increase of clinical nanomedicines within the tumour, boosting the tumour suppression efficacy in both mouse and rabbit models. This approach delicately manages BM degradation, avoiding excessive degradation that might facilitate cancer metastasis. Our NO nanogenerator serves as a precise spatial catalytic degradation strategy for breaching the tumour vascular BM barrier, holding promise for NP delivery into non-tumour diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results of this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Jain, R. K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Luan, N. M. N. et al. The mechanisms of nanoparticle delivery to solid tumours. Nat. Rev. Bioeng. 2, 201–213 (2024).
Glassman, P. M. et al. Targeting drug delivery in the vascular system: focus on endothelium. Adv. Drug Deliv. Rev. 157, 96–117 (2020).
Wettschureck, N., Strilic, B. & Offermanns, S. Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol. Rev. 99, 1467–1525 (2019).
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 63, 131–135 (2011).
Wang, Q. et al. Breaking through the basement membrane barrier to improve nanotherapeutic delivery to tumours. Nat. Nanotechnol. 19, 95–105 (2024).
Rowe, R. G. & Weiss, S. J. Breaching the basement membrane: who, when and how? Trends Cell Biol. 18, 560–574 (2008).
Kelley, L. C., Lohmer, L. L., Hagedorn, E. J. & Sherwood, D. R. Traversing the basement membrane in vivo: a diversity of strategies. J. Cell Biol. 204, 291–302 (2014).
Jayadev, R. & Sherwood, D. R. Basement membranes. Curr. Biol. 27, R207–R211 (2017).
Nikolova, G., Strilic, B. & Lammert, E. The vascular niche and its basement membrane. Trends Cell Biol. 17, 19–25 (2007).
Du, B. et al. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 12, 1096–1102 (2017).
Pozzi, A., Yurchenco, P. D. & Iozzo, R. V. The nature and biology of basement membranes. Matrix Biol. 57–58, 1–11 (2017).
Reuten, R. et al. Basement membrane stiffness determines metastases formation. Nat. Mater. 20, 892–903 (2021).
Chang, J. & Chaudhuri, O. Beyond proteases: basement membrane mechanics and cancer invasion. J. Cell Biol. 218, 2456–2469 (2019).
Sung, Y. C. et al. Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies. Nat. Nanotechnol. 14, 1160–1169 (2019).
Kim, T. et al. Deep brain stimulation by blood–brain-barrier-crossing piezoelectric nanoparticles generating current and nitric oxide under focused ultrasound. Nat. Biomed. Eng. 7, 149–163 (2023).
Park, J. et al. In situ electrochemical generation of nitric oxide for neuronal modulation. Nat. Nanotechnol. 15, 690–697 (2020).
Zhu, D. et al. Nitrate-functionalized patch confers cardioprotection and improves heart repair after myocardial infarction via local nitric oxide delivery. Nat. Commun. 12, 4501 (2021).
Chen, H. et al. A nitric-oxide driven chemotactic nanomotor for enhanced immunotherapy of glioblastoma. Nat. Commun. 14, 941 (2023).
Chen, Y. et al. A tough nitric oxide-eluting hydrogel coating suppresses neointimal hyperplasia on vascular stent. Nat. Commun. 12, 7079 (2021).
Kim, J. et al. Thermosensitive hydrogel releasing nitric oxide donor and anti-CTLA-4 micelles for anti-tumor immunotherapy. Nat. Commun. 13, 1479 (2022).
Li, J. L. et al. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat. Protoc. 7, 221–234 (2012).
Jiang, W. et al. Nitric oxide induces immunogenic cell death and potentiates cancer immunotherapy. ACS Nano 16, 3881–3894 (2022).
Miller, M. A. et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci. Transl. Med. 9, eaal0225 (2017).
Matsumoto, Y. et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 11, 533–538 (2016).
Otger, C., Ivar, N. & Alpha, S. Y. Adherens junctions as molecular regulators of emergent tissue mechanics. Nat. Rev. Mol. Cell Biol. 25, 252–269 (2024).
Giannotta, M., Trani, M. & Dejana, E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26, 441–454 (2013).
Sidibe, A. & Imhof, B. A. VE-cadherin phosphorylation decides: vascular permeability or diapedesis. Nat. Immunol. 15, 215–217 (2014).
Orsenigo, F. et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat. Commun. 3, 1208 (2012).
Kamaly, N. et al. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc. Natl Acad. Sci. USA 110, 6506–6511 (2013).
Setyawati, M. I. et al. Engineering tumoral vascular leakiness with gold nanoparticles. Nat. Commun. 14, 4269 (2023).
Quintero-Fabián, S. et al. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 9, 1370 (2019).
Kessenbrock, K., Plaks, V. & Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
Vandooren, J., Geurts, N., Martens, E., Van den Steen, P. E. & Opdenakker, G. Zymography methods for visualizing hydrolytic enzymes. Nat. Methods 10, 211–220 (2013).
Dong, X. et al. Enhanced drug delivery by nanoscale integration of a nitric oxide donor to induce tumor collagen depletion. Nano Lett. 19, 997–1008 (2019).
Ridnour, L. A. et al. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc. Natl Acad. Sci. USA 104, 16898–16903 (2007).
Underly, R. G. et al. Pericytes as inducers of rapid, matrix metalloproteinase-9-dependent capillary damage during ischemia. J. Neurosci. 37, 129–140 (2017).
Wang, X. et al. Susceptibility of rat steatotic liver to ischemia-reperfusion is treatable with liver-selective matrix metalloproteinase inhibition. Hepatology 72, 1771–1785 (2020).
Jiang, Y. et al. Near-infrared light-triggered NO release for spinal cord injury repair. Sci. Adv. 6, eabc3513 (2020).
Malhotra, K. et al. Unlocking long-term stability of upconversion nanoparticles with biocompatible phosphonate-based polymer coatings. Nano Lett. 22, 7285–7293 (2022).
Parisi, C., Seggio, M., Fraix, A. & Sortino, S. A high-performing metal-free photoactivatable nitric oxide donor with a green fluorescent reporter. ChemPhotoChem 4, 742–748 (2020).
Jiang, W. et al. Overcoming oxygen heterogeneity of tumor microenvironments to boost cancer immunotherapy by oxygen-switchable ROS/RNS nanogenerators. Nano Today 48, 101696 (2023).
Shen, Z. et al. Overcoming the oxygen dilemma in photoredox catalysis: near-infrared (NIR) light-triggered peroxynitrite generation for antibacterial applications. Angew. Chem. Int. Ed. 62, e202219153 (2023).
Sun, J. et al. Cascade reactions by nitric oxide and hydrogen radical for anti-hypoxia photodynamic therapy using an activatable photosensitizer. J. Am. Chem. Soc. 143, 868–878 (2021).
Kurz, A. R. M. et al. MST1-dependent vesicle trafficking regulates neutrophil transmigration through the vascular basement membrane. J. Clin. Invest. 126, 4125–4139 (2016).
Liu, C. et al. mRNA-based cancer therapeutics. Nat. Rev. Cancer 23, 526–543 (2023).
Lee, Y.-R. et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC–WWP1 inhibitory pathway. Science 364, eaau0159 (2019).
Lin, Y.-X. et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl. Med. 13, eaba9772 (2021).
Islam, M. A. et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2, 850–864 (2018).
He, H., Liu, L., Morin, E. E., Liu, M. & Schwendeman, A. Survey of clinical translation of cancer nanomedicines—lessons learned from successes and failures. Acc. Chem. Res. 52, 2445–2461 (2019).
Cheng, Y. H., He, C. L., Riviere, J. E., Monteiro-Riviere, N. A. & Lin, Z. M. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. ACS Nano 14, 3075–3095 (2020).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Poon, W., Kingston, B. R., Ouyang, B., Ngo, W. & Chan, W. C. W. A framework for designing delivery systems. Nat. Nanotechnol. 15, 819–829 (2020).
Horacio, C., Junjie, L., Kanjiro, M. & Kazunori, K. Controlling the biodistribution and clearance of nanomedicines. Nat. Rev. Bioeng. 2, 214–232 (2023).
Stater, E. P., Sonay, A. Y., Hart, C. & Grimm, J. The ancillary effects of nanoparticles and their implications for nanomedicine. Nat. Nanotechnol. 16, 1180–1194 (2021).
Chen, S. et al. Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion–drug conjugates with cell-membrane affinity. Nat. Biomed. Eng. 5, 1019–1037 (2021).
Wang, P. G. et al. Nitric oxide donors: chemical activities and biological applications. Chem. Rev. 102, 1091–1134 (2002).
SoRelle, R. Nobel prize awarded to scientists for nitric oxide discoveries. Circulation 98, 2365–2366 (1998).
Hou, J. et al. Targeted delivery of nitric oxide via a ‘bump-and-hole’-based enzyme–prodrug pair. Nat. Chem. Biol. 15, 151–160 (2019).
Acknowledgements
Y.W. discloses support for the research described in this study from the National Natural Science Foundation of China (52025036 and 52495010), National Key Research and Development Program of China (2020YFA0710700 and 2022YFC2303300) and Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0490000 and XDB0940000). W.J. discloses support for the research described in this study from the National Natural Science Foundation of China (52273156 and 52473154), National Key Research and Development Program of China (2022YFC2303700 and 2024YFA1212400), Young Elite Scientists Sponsorship Program by CAST (YESS20230281), Natural Science Foundation of Anhui Province (2308085Y01), Scientific Research Project of Anhui Provincial Department of Education (2023AH030113) and Fundamental Research Funds for the Central Universities (WK9100000088). This work was partially carried out at the USTC Center for Micro- and Nanoscale Research and Fabrication and Instruments Center for Physical Science, University of Science and Technology of China.
Author information
Authors and Affiliations
Contributions
W.J., Z.G. and Q.W. designed and performed the experiments and analysed the experimental data. Z.G. synthesized the mRNA. Z.C., W.D. and H.P. established the tumour models and provided transgenic reporter mice. Y.H., Q.L. and Z.C. helped with the animal experiments. W.J., Q.W. and H.L. wrote the original draft of the paper. Q.W. and Y.W. supervised the project. All authors reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Chao Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–55, Tables 1 and 2, methods and unprocessed blots and gels for Supplementary Figs. 12, 22, 35 and 37.
Supplementary Video 1
This video captures multiple eruptions at the same NP pool site in NO-treated tumours. Two consecutive on–off eruptions of varying intensity occur within 30 min, with the time indexed from the eruption’s initiation at 0 min. Imaging was performed every 0.5 min, with the normalized NP intensity shown in pseudocolour.
Supplementary Video 2
This video shows the process of NP extravasation in NO-treated 4T1 tumours. The initiation of NO injection is marked at 0 min. Imaging was performed every 0.5 min, with normalized the NP intensity shown in pseudocolour.
Supplementary Video 3
This video shows the formation process of NP pools in NO-treated 4T1 tumour vessels. This process is typically completed within 10 min. The video captures a representative field post-NO treatment, with NP pool formation defined as 0 min.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 5
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, W., Guo, Z., Wang, Q. et al. Enhanced nanoparticle delivery across vascular basement membranes of tumours using nitric oxide. Nat. Biomed. Eng 9, 1486–1501 (2025). https://doi.org/10.1038/s41551-025-01385-w
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01385-w