Abstract
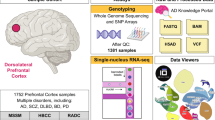
Recent work leveraging artificial intelligence has offered promise to dissect disease heterogeneity by identifying complex intermediate brain phenotypes, called dimensional neuroimaging endophenotypes (DNEs). We advance the argument that these DNEs capture the degree of expression of respective neuroanatomical patterns measured, offering a dimensional neuroanatomical representation for studying disease heterogeneity and similarities of neurologic and neuropsychiatric diseases. We investigate the presence of nine DNEs derived from independent yet harmonized studies on Alzheimer’s disease, autism spectrum disorder, late-life depression and schizophrenia in the UK Biobank study. Phenome-wide associations align with genome-wide associations, revealing 31 genomic loci (P < 5 × 10−8/9) associated with the nine DNEs. The nine DNEs, along with their polygenic risk scores, significantly enhanced the predictive accuracy for 14 systemic disease categories, particularly for conditions related to mental health and the central nervous system, as well as mortality outcomes. These findings underscore the potential of the nine DNEs to capture the expression of disease-related brain phenotypes in individuals of the general population and to relate such measures with genetics, lifestyle factors and chronic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The GWAS summary statistics and pre-trained AI models from this study are publicly accessible via the MEDICINE Knowledge Portal (https://labs-laboratory.com/medicine/) and Synapse (https://www.synapse.org/Synapse:syn64923248/wiki/630992)101. Genomic loci annotation used data from FUMA (https://fuma.ctglab.nl/). UKBB data can be requested at https://www.ukbiobank.ac.uk/. GWAS summary data from the PGC can be accessed at https://pgc.unc.edu/. MUSE atlas is generated via the pipeline at https://github.com/CBICA/MUSE. The raw data for the main figures and Supplementary Files 1–23 are publicly available via Zenodo at https://zenodo.org/records/15238099 (ref. 102).
Code availability
The software and resources used in this study are all publicly available via MLNI103 (HYDRA) at https://github.com/anbai106/mlni (DNEs for ASD1–3, LLD1–2, SCZ1–2) and Surreal-GAN104 at https://github.com/zhijian-yang/SurrealGAN (DNEs for AD1–2).
References
Hwang, G. et al. Assessment of neuroanatomical endophenotypes of autism spectrum disorder and association with characteristics of individuals with schizophrenia and the general population. JAMA Psychiatry 80, 498–507 (2023).
Wen, J. et al. Characterizing heterogeneity in neuroimaging, cognition, clinical symptoms, and genetics among patients with late-life depression. JAMA Psychiatry 79, 464–474 (2022).
Young, A. L. et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat. Commun. 9, 4273 (2018).
Yang, Z. et al. A deep learning framework identifies dimensional representations of Alzheimer’s disease from brain structure. Nat. Commun. 12, 7065 (2021).
Zhang, X. et al. Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 113, E6535–E6544 (2016).
Vogel, J. W. et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 27, 871–881 (2021).
Wen, J. et al. Multi-scale semi-supervised clustering of brain images: deriving disease subtypes. Med. Image Anal. 75, 102304 (2021).
Ferreira, D., Nordberg, A. & Westman, E. Biological subtypes of Alzheimer disease: a systematic review and meta-analysis. Neurology 94, 436–448 (2020).
Hodson, R. Precision medicine. Nature 537, S49–S49 (2016).
Davatzikos, C. et al. Precision diagnostics based on machine learning-derived imaging signatures. Magn. Reson. Imaging 64, 49–61 (2019).
Leonenko, G. et al. Identifying individuals with high risk of Alzheimer’s disease using polygenic risk scores. Nat. Commun. 12, 4506 (2021).
Wen, J. et al. Genetic and clinical correlates of two neuroanatomical AI dimensions in the Alzheimer’s disease continuum. Transl. Psychiatry 14, 1–14 (2024).
Chand, G. B. et al. Two distinct neuroanatomical subtypes of schizophrenia revealed using machine learning. Brain 143, 1027–1038 (2020).
Yang, Z., Wen, J. & Davatzikos, C. Surreal-GAN: semi-supervised representation learning via GAN for uncovering heterogeneous disease-related imaging patterns. International Conference on Learning Representations (2021).
Wingo, T. S. et al. Shared mechanisms across the major psychiatric and neurodegenerative diseases. Nat. Commun. 13, 4314 (2022).
Anttila, V. Analysis of shared heritability in common disorders of the brain. Science 360, eaap8757 (2018).
Wen, J. et al. The genetic architecture of biological age in nine human organ systems. Nat. Aging 4, 1290–1307 (2024).
Wen, J. Multiorgan biological age shows that no organ system is an island. Nat. Aging 4, 1182–1183 (2024).
Wen, J. et al. The genetic architecture of multimodal human brain age. Nat. Commun. 15, 2604 (2024).
Boquet-Pujadas, A. et al. MUTATE: a human genetic atlas of multiorgan artificial intelligence endophenotypes using genome-wide association summary statistics. Brief. Bioinform. 26, bbaf125 (2025).
Petersen, R. C. et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 74, 201–209 (2010).
Di Martino, A. et al. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci. Data 4, 170010 (2017).
Wen, J. et al. Subtyping brain diseases from imaging data. In Machine Learning for Brain Disorders (ed. Colliot, O.) 491–510 (Springer, 2023); https://doi.org/10.1007/978-1-0716-3195-9_16
Kendler, K. & Neale, M. Endophenotype: a conceptual analysis. Mol. Psychiatry 15, 789–797 (2010).
Cannon, T. D. & Keller, M. C. Endophenotypes in the genetic analyses of mental disorders. Annu. Rev. Clin. Psychol. 2, 267–290 (2006).
Gottesman, I. I. & Gould, T. D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160, 636–645 (2003).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Alfaro-Almagro, F. et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 166, 400–424 (2018).
Elliott, L. T. et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562, 210–216 (2018).
Wen, J. et al. Genomic loci influence patterns of structural covariance in the human brain. Proc. Natl Acad. Sci. USA 120, e2300842120 (2023).
Guan, H. & Liu, M. Domain adaptation for medical image analysis: a survey. IEEE Trans. Biomed. Eng. 69, 1173–1185 (2022).
Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61, 1000–1016 (2012).
Joseph, C., Wang, L., Wu, R., Manning, K. J. & Steffens, D. C. Structural brain changes and neuroticism in late-life depression: a neural basis for depression subtypes. Int. Psychogeriatr. 33, 515–520 (2021).
Tian, Y. E. et al. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat. Med. 29, 1221–1231 (2023).
McCracken, C. et al. Multi-organ imaging demonstrates the heart-brain-liver axis in UK Biobank participants. Nat. Commun. 13, 7839 (2022).
Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet 88, 76–82 (2011).
Jiang, L., Zheng, Z., Fang, H. & Yang, J. A generalized linear mixed model association tool for biobank-scale data. Nat. Genet. 53, 1616–1621 (2021).
Jiang, L. et al. A resource-efficient tool for mixed model association analysis of large-scale data. Nat. Genet. 51, 1749–1755 (2019).
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53, 1276–1282 (2021).
Demontis, D. et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat. Genet. 55, 198–208 (2023).
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444 (2019).
Mullins, N. et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 53, 817–829 (2021).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS) Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol. Psychiatry 23, 1181–1188 (2018).
O’Donovan, M. C. What have we learned from the Psychiatric Genomics Consortium. World Psychiatry 14, 291–293 (2015).
Cheverud, J. M. A comparison of genetic and pehnotypic correlations. Evolution 42, 958–968 (1988).
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11, e1004219 (2015).
McCutcheon, R. A., Krystal, J. H. & Howes, O. D. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry 19, 15–33 (2020).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Hordyjewska-Kowalczyk, E. et al. Functional analysis of novel RUNX2 mutations identified in patients with cleidocranial dysplasia. Clin. Genet. 96, 429–438 (2019).
Wamsley, B. et al. Molecular cascades and cell type–specific signatures in ASD revealed by single-cell genomics. Science 384, eadh2602 (2024).
Ayalew, M. et al. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol. Psychiatry 17, 887–905 (2012).
Siokas, V. et al. Myelin-associated oligodendrocyte basic protein rs616147 polymorphism as a risk factor for Parkinson’s disease. Acta Neurol. Scand. 145, 223–228 (2022).
Arnold, S. E. et al. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch. Gen. Psychiatry 58, 829–835 (2001).
Turetsky, B. I., Moberg, P. J., Arnold, S. E., Doty, R. L. & Gur, R. E. Low olfactory bulb volume in first-degree relatives of patients with schizophrenia. Am. J. Psychiatry 160, 703–708 (2003).
Almandil, N. B. et al. Exome-wide analysis identify multiple variations in olfactory receptor genes (OR12D2 and OR5V1) associated with autism spectrum disorder in Saudi females. Front. Med. 10, 1051039 (2023).
Kuo, P.-H. et al. Genome-wide association study for autism spectrum disorder in Taiwanese Han population. PLoS ONE 10, e0138695 (2015).
Warnica, W. et al. Copy number variable microRNAs in schizophrenia and their neurodevelopmental gene targets. Biol. Psychiatry 77, 158–166 (2015).
Christen-Zaech, S. et al. Early olfactory involvement in Alzheimer’s disease. Can. J. Neurol. Sci. 30, 20–25 (2003).
Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 15, 11–24 (2019).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 7, e34408 (2018).
Sanderson, E. et al. Mendelian randomization. Nat. Rev. Methods Prim. 2, 1–21 (2022).
23andMe Research Team. et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 9, 1470 (2018).
Navari, S. & Dazzan, P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol. Med. 39, 1763–1777 (2009).
Chopra, S. et al. Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: a longitudinal, randomised, triple-blind, placebo-controlled MRI study. Neuropsychopharmacology 46, 1494–1501 (2021).
Zeldovich, L. Cold parenting? Childhood schizophrenia? How the diagnosis of autism has evolved over time. Science https://doi.org/10.1126/science.aau1206 (2018).
Moreau, C. A. et al. Dissecting autism and schizophrenia through neuroimaging genomics. Brain 144, 1943–1957 (2021).
Fu, Z. et al. Dynamic functional network reconfiguration underlying the pathophysiology of schizophrenia and autism spectrum disorder. Hum. Brain Mapp. 42, 80–94 (2021).
Bereczki, E. et al. Synaptic markers of cognitive decline in neurodegenerative diseases: a proteomic approach. Brain 141, 582–595 (2018).
Jiang, C.-C. et al. Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 7, 1–36 (2022).
Chi, S., Yu, J.-T., Tan, M.-S. & Tan, L. Depression in Alzheimer’s disease: epidemiology, mechanisms, and management. J. Alzheimers Dis. 42, 739–755 (2014).
Dafsari, F. S. & Jessen, F. Depression—an underrecognized target for prevention of dementia in Alzheimer’s disease. Transl. Psychiatry 10, 1–13 (2020).
Ly, M. et al. Late-life depression and increased risk of dementia: a longitudinal cohort study. Transl. Psychiatry 11, 1–10 (2021).
Lalousis, P. A. et al. Neurobiologically based stratification of recent-onset depression and psychosis: identification of two distinct transdiagnostic phenotypes. Biol. Psychiatry 92, 552–562 (2022).
Woo, M. Eyes hint at hidden mental-health conditions. Nature https://www.nature.com/articles/d41586-019-01114-9 (2019).
Tahsili-Fahadan, P. & Geocadin, R. G. Heart–brain axis. Circ. Res. 120, 559–572 (2017).
Leng, F. & Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17, 157–172 (2021).
Murphy, C. E., Walker, A. K. & Weickert, C. S. Neuroinflammation in schizophrenia: the role of nuclear factor kappa B. Transl. Psychiatry 11, 1–13 (2021).
Miller, A. H. & Raison, C. L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 (2016).
Tan, A. H., Lim, S. Y. & Lang, A. E. The microbiome–gut–brain axis in Parkinson disease — from basic research to the clinic. Nat. Rev. Neurol. 18, 476–495 (2022).
Morais, L. H., Schreiber, H. L. & Mazmanian, S. K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255 (2021).
Tost, H., Champagne, F. A. & Meyer-Lindenberg, A. Environmental influence in the brain, human welfare and mental health. Nat. Neurosci. 18, 1421–1431 (2015).
Ambitious Project Announced to Create the World’s Largest Longitudinal Imaging Dataset (UK Biobank, 2022); https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/news/ambitious-project-announced-to-create-the-world-s-largest-longitudinal-imaging-dataset
Wang, R., Chaudhari, P. & Davatzikos, C. Embracing the disharmony in medical imaging: a simple and effective framework for domain adaptation. Med. Image Anal. 76, 102309 (2022).
Varol, E., Sotiras, A. & Davatzikos, C. HYDRA: revealing heterogeneity of imaging and genetic patterns through a multiple max-margin discriminative analysis framework. NeuroImage 145, 346–364 (2017).
Tustison, N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
Doshi, J. et al. MUSE: MUlti-atlas region segmentation utilizing ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage 127, 186–195 (2016).
Manichaikul, A. et al. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010).
Price, A. L., Zaitlen, N. A., Reich, D. & Patterson, N. New approaches to population stratification in genome-wide association studies. Nat. Rev. Genet. 11, 459–463 (2010).
Abraham, G., Qiu, Y. & Inouye, M. FlashPCA2: principal component analysis of Biobank-scale genotype datasets. Bioinformatics 33, 2776–2778 (2017).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet 81, 559–575 (2007).
Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826 (2017).
The GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Bowden, J. et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 36, 1783–1802 (2017).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Ge, T., Chen, C.-Y., Ni, Y., Feng, Y.-C. A. & Smoller, J. W. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat. Commun. 10, 1776 (2019).
Wen, J. et al. Convolutional neural networks for classification of Alzheimer’s disease: overview and reproducible evaluation. Med. Image Anal. 63, 101694 (2020).
Nadeau, C. & Bengio, Y. Inference for the generalization error. Mach. Learn. 52, 239–281 (2003).
Wen, J. MEDICINE: AI-derived GWAS summary statistics sharing portal. Preprint at https://doi.org/10.7303/syn64923248 (2025).
Wen, J. DNE_Figures_Dataset. Zenodo https://doi.org/10.5281/zenodo.15238098 (2025).
Wen, J. MLNI (version 0.1.4). GitHub https://github.com/anbai106/mlni (2025).
Yang, Z. Surreal-GAN (version 0.1.1). GitHub https://github.com/zhijian-yang/SurrealGAN (2025).
Acknowledgements
We express our sincere gratitude to the UK Biobank team (https://www.ukbiobank.ac.uk/) for their invaluable contribution to advancing clinical research in our field. We thank the PGC (https://pgc.unc.edu/) for generously sharing the GWAS summary statistics with the scientific community. This study used the UK Biobank resource under application numbers 35148 (C.D.) and 60698 (A.Z.). J.W. leads the MULTI consortium under UK Biobank Application number 647044; J.W. and the Laboratory of AI and Biomedical Science (LABS) are supported by the start-up funding from Columbia University. We also gratefully acknowledge the support of the imaging-based SysTem for AGing and NeurodeGenerative diseases (iSTAGING) consortium, funded by the National Institute on Aging through grant RF1 AG054409 at the University of Pennsylvania (C.D.). In addition, we acknowledge the funding program from the Rebecca L. Cooper Foundation at the University of Melbourne (A.Z.). Y.E.T. is supported by a National Health and Medical Research Council Investigator Grant (APP2026413).
Author information
Authors and Affiliations
Contributions
J.W. has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: J.W. and C.D. Acquisition, analysis or interpretation of data: all authors. Drafting of the paper: J.W. Critical revision of the paper for important intellectual content: J.W., I.S., Y.E.T., Z.Y., Y.C., G.E., G.H., E.V., A.B.-P., G.B.C., I.M.N., T.D.S., H.S, L.S., A.W.T., A.Z. and C.D. Statistical analysis: J.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Alexandra Badea, Luigi Lorenzini and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, Tables 1–11, Methods 1–3 and Text 1–6.
Supplementary Data 1
Supplementary Files 1–23.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, J., Skampardoni, I., Tian, Y.E. et al. Neuroimaging endophenotypes reveal underlying mechanisms and genetic factors contributing to progression and development of four brain disorders. Nat. Biomed. Eng 9, 1920–1937 (2025). https://doi.org/10.1038/s41551-025-01412-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01412-w
This article is cited by
-
Multi-organ AI endophenotypes chart the heterogeneity of brain, eye and heart pan-disease
Nature Mental Health (2026)
-
Pan-disease dimensions in the brain, eye and heart capture shared and specific heterogeneity
Nature Mental Health (2026)
-
MRI-based multi-organ clocks for healthy aging and disease assessment
Nature Medicine (2026)
-
Towards a multi-organ, multi-omics medical digital twin
Nature Biomedical Engineering (2025)
-
Brain–heart–eye axis revealed by multi-organ imaging genetics and proteomics
Nature Biomedical Engineering (2025)