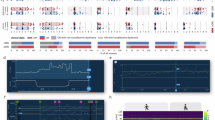
Abstract
Deep brain stimulation (DBS) has garnered widespread use as an effective treatment for advanced Parkinson’s disease. Conventional DBS (cDBS) provides electrical stimulation to the basal ganglia at fixed amplitude and frequency, yet patients’ therapeutic needs are often dynamic with residual symptom fluctuations or side effects. Adaptive DBS (aDBS) is an emerging technology that modulates stimulation with respect to real-time clinical, physiological or behavioural states, enabling therapy to dynamically align with patient-specific symptoms. Here we report an aDBS algorithm intended to mitigate movement slowness by delivering targeted stimulation increases during movement using decoded motor signals from the brain. Our approach demonstrated improvements in dominant hand movement speeds and study participant-reported therapeutic efficacy compared with an inverted control, as well as increased typing speed and reduced dyskinesia compared with cDBS. Furthermore, we demonstrate proof of principle of a machine learning pipeline capable of remotely optimizing aDBS parameters in a home setting. This work illustrates the potential of movement-responsive aDBS as a promising therapeutic approach and highlights how machine learning-assisted programming can simplify complex optimization to facilitate translational scalability.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All processed data supporting the analyses of this study or displayed in figures are available via the Dryad repository at https://doi.org/10.5061/dryad.4xgxd25hw (ref. 69). Any additional requests for raw data may be directed to the corresponding author and will be provided under reasonable request and with additional consent of the study participant.
Code availability
All machine learning and data analysis code is available via GitHub at https://github.com/Weill-Neurohub-OPTiMaL/dixon-2025-move-adbs (ref. 70). The rcssim package is available via GitHub at https://github.com/Weill-Neurohub-OPTiMaL/rcs-simulation (ref. 71).
References
Ashkan, K., Rogers, P., Bergman, H. & Ughratdar, I. Insights into the mechanisms of deep brain stimulation. Nat. Rev. Neurol. 13, 548–554 (2017).
McGregor, M. M. & Nelson, A. B. Circuit mechanisms of Parkinson’s disease. Neuron 101, 1042–1056 (2019).
Deuschl, G. et al. A randomized trial of deep brain stimulation for Parkinson’s disease. N. Engl. J. Med. 355, 896–908 (2006).
Follett, K. A. et al. Pallidal versus subthalamic deep brain stimulation for Parkinson’s disease. N. Engl. J. Med. 362, 2077–2091 (2010).
Odekerken, V. J. J. et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 12, 37–44 (2013).
Pozzi, N. G. & Isaias, I. U. in Handbook of Clinical Neurology 1st edn, Vol. 184 (eds Quartarone A., Ghilardi M.F. & Boller F.) 273–284 (Elsevier B.V., 2022).
Kühn, A. A., Kupsch, A., Schneider, G. H. & Brown, P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur. J. Neurosci. 23, 1956–1960 (2006).
Neumann, W. J. et al. Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease. Mov. Disord. 31, 1748–1751 (2016).
Lofredi, R. et al. Subthalamic beta bursts correlate with dopamine-dependent motor symptoms in 106 Parkinson’s patients. npj Parkinsons Dis. 9, 1–9 (2023).
Little, S. & Brown, P. Debugging adaptive deep brain stimulation for Parkinson’s disease. Mov. Disord. 35, 555–561 (2020).
Little, S. et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 74, 449–457 (2013).
Little, S. et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 87, 717–721 (2016).
Tinkhauser, G. et al. The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain 140, 1053–1067 (2017).
He, S. et al. Beta-triggered adaptive deep brain stimulation during reaching movement in Parkinson’s disease. Brain 146, 5015–5030 (2023).
Rosa, M. et al. Adaptive deep brain stimulation controls levodopa-induced side effects in Parkinsonian patients. Mov. Disord. 32, 628–629 (2017).
Arlotti, M. et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology 90, e971–e976 (2018).
Velisar, A. et al. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul. 12, 868–876 (2019).
Gilron, R. et al. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson’s disease. Nat. Biotechnol. 39, 1078–1085 (2021).
Oehrn, C. R. et al. Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson’s disease: a blinded randomized feasibility trial. Nat. Med. 30, 3345–3356 (2024).
Kühn, A. A. et al. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain 127, 735–746 (2004).
Eisinger, R. S. et al. Parkinsonian beta dynamics during rest and movement in the dorsal pallidum and subthalamic nucleus. J. Neurosci. 40, 2859–2867 (2020).
Johnson, L. A. et al. Closed-loop deep brain stimulation effects on Parkinsonian motor symptoms in a non-human primate—is beta enough? Brain Stimul. 9, 892–896 (2016).
Iturrate, I. et al. Beta-driven closed-loop deep brain stimulation can compromise human motor behavior in Parkinson’s disease. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/696385v1.abstract (2019).
Bologna, M., Paparella, G., Fasano, A., Hallett, M. & Berardelli, A. Evolving concepts on bradykinesia. Brain 143, 727–750 (2020).
Chevalier, G. & Deniau, J. M. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 13, 277–280 (1990).
Ivry, R. B. & Spencer, R. M. C. The neural representation of time. Curr. Opin. Neurobiol. 14, 225–232 (2004).
Klaus, A., Alves Da Silva, J. & Costa, R. M. What, if, and when to move: basal ganglia circuits and self-paced action initiation. Annu. Rev. Neurosci. 42, 459–483 (2019).
Wichmann, T. & DeLong, M. R. Deep brain stimulation for movement disorders of basal ganglia origin: restoring function or functionality? Neurotherapeutics 13, 264–283 (2016).
Turner, R. S. & Desmurget, M. Basal ganglia contributions to motor control: a vigorous tutor. Curr. Opin. Neurobiol. 20, 704–716 (2010).
Darbin, O. et al. Subthalamic nucleus deep brain stimulation driven by primary motor cortex γ2 activity in parkinsonian monkeys. Sci. Rep. 12, 1–16 (2022).
Ferleger, B. I. et al. Fully implanted adaptive deep brain stimulation in freely moving essential tremor patients. J. Neural Eng. 17, 056026 (2020).
Herron, J. A. et al. Chronic electrocorticography for sensing movement intention and closed-loop deep brain stimulation with wearable sensors in an essential tremor patient. J. Neurosurg. 127, 580–587 (2017).
Opri, E. et al. Chronic embedded cortico-thalamic closed-loop deep brain stimulation for the treatment of essential tremor. Sci. Transl. Med. 12, eaay7680 (2020).
He, S. et al. Closed-loop deep brain stimulation for essential tremor based on thalamic local field potentials. Mov. Disord. 36, 863–873 (2021).
Sellers, K. K. et al. Analysis-rcs-data: open-source toolbox for the ingestion, time-alignment, and visualization of sense and stimulation data from the Medtronic Summit RC+S system. Front. Hum. Neurosci. 15, 1–14 (2021).
Strandquist, G. et al. Bringing the clinic home: an at-home multi-modal data collection ecosystem to support adaptive deep brain stimulation. J. Vis. Exp. 197, e65305 (2023).
Buzsáki, G. & Draguhn, A. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004).
Maciel, R., Zúñiga-Ramírez, C., Munhoz, R. P., Zurowski, M. & Fasano, A. Functional dyskinesias following subthalamic nucleus deep brain stimulation in Parkinson’s disease: a report of three cases. Mov. Disord. Clin. Pract. 8, 114–117 (2021).
Baizabal-Carvallo, J. F. & Jankovic, J. Movement disorders induced by deep brain stimulation. Parkinsonism Relat. Disord. 25, 1–9 (2016).
Rodríguez-Molinero, A. et al. Estimating dyskinesia severity in Parkinson’s disease by using a waist-worn sensor: concurrent validity study. Sci. Rep. 9, 1–7 (2019).
Dai, H., Zhang, P. & Lueth, T. C. Quantitative assessment of Parkinsonian tremor based on an inertial measurement unit. Sensors 15, 25055–25071 (2015).
Holt, A. B., Wilson, D., Shinn, M., Moehlis, J. & Netoff, T. I. Phasic burst stimulation: a closed-loop approach to tuning deep brain stimulation parameters for Parkinson’s disease. PLoS Comput. Biol. 12, e1005011 (2016).
Grado, L. L., Johnson, M. D. & Netoff, T. I. Bayesian adaptive dual control of deep brain stimulation in a computational model of Parkinson’s disease. PLoS Comput. Biol. 14, e1006606 (2018).
Popovych, O. V. & Tass, P. A. Adaptive delivery of continuous and delayed feedback deep brain stimulation—a computational study. Sci. Rep. 9, 10585 (2019).
Swann, N. C. et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J. Neural Eng. 15, e15–e16 (2018).
Gilron, R. et al. Sleep-aware adaptive deep brain stimulation control: chronic use at home with dual independent linear discriminate detectors. Front. Neurosci. 15, 732499 (2021).
Smyth, C. et al. Adaptive deep brain stimulation for sleep stage targeting in Parkinson’s disease. Brain Stimul. 16, 1292–1296 (2023).
Anjum, M. F. et al. Multi-night cortico-basal recordings reveal mechanisms of NREM slow-wave suppression and spontaneous awakenings in Parkinson’s disease. Nat. Commun. 15, 1793 (2024).
Kim, H. J., Jeon, B. S. & Paek, S. H. Non-motor symptoms and subthalamic deep brain stimulation in Parkinson’s disease. J. Mov. Disord. 8, 83–91 (2015).
Kurtis, M. M., Rajah, T., Delgado, L. F. & Dafsari, H. S. The effect of deep brain stimulation on the non-motor symptoms of Parkinson’s disease: a critical review of the current evidence. npj Parkinsons Dis. 3, 1–12 (2017).
Prange, S. et al. Limbic stimulation drives mania in STN-DBS in Parkinson disease: a prospective study. Ann. Neurol. 92, 411–417 (2022).
Hristova, A. et al. Effect and time course of deep brain stimulation of the globus pallidus and subthalamus on motor features of Parkinson’s disease. Clin. Neuropharmacol. 23, 208–211 (2000).
Koeglsperger, T., Palleis, C., Hell, F., Mehrkens, J. H. & Bötzel, K. Deep brain stimulation programming for movement disorders: current concepts and evidence-based strategies. Front. Neurol. 10, 1–20 (2019).
Merk, T. et al. Electrocorticography is superior to subthalamic local field potentials for movement decoding in Parkinson’s disease. eLife 11, e75126 (2022).
Ansó, J. et al. Concurrent stimulation and sensing in bi-directional brain interfaces: a multi-site translational experience. J. Neural Eng. 19, 026025 (2022).
Degenhart, A. D. et al. Histological evaluation of a chronically-implanted electrocorticographic electrode grid in a non-human primate. J. Neural Eng. 13, 139–148 (2016).
Volkova, K., Lebedev, M. A., Kaplan, A. & Ossadtchi, A. Decoding movement from electrocorticographic activity: a review. Front. Neuroinform. 13, 00074 (2019).
Blakely, T., Miller, K. J., Zanos, S. P., Rao, R. P. N. & Ojemann, J. G. Robust, long-term control of an electrocorticographic brain-computer interface with fixed parameters. Neurosurg. Focus 27, 4–8 (2009).
Pels, E. G. M. et al. Stability of a chronic implanted brain-computer interface in late-stage amyotrophic lateral sclerosis. Clin. Neurophysiol. 130, 1798–1803 (2019).
Asaad, W. F. & Sheth, S. A. What’s the n? On sample size vs. subject number for brain-behavior neurophysiology and neuromodulation. Neuron 112, 2086–2090 (2024).
Bundy, D. T., Pahwa, M., Szrama, N. & Leuthardt, E. C. Decoding three-dimensional reaching movements using electrocorticographic signals in humans. J. Neural Eng. 13, 026021 (2016).
Leuthardt, E. C., Schalk, G., Wolpaw, J. R., Ojemann, J. G. & Moran, D. W. A brain-computer interface using electrocorticographic signals in humans. J. Neural Eng. 1, 63–71 (2004).
Athalye, V. R., Carmena, J. M. & Costa, R. M. Neural reinforcement: re-entering and refining neural dynamics leading to desirable outcomes. Curr. Opin. Neurobiol. 60, 145–154 (2020).
Cavallo, A. & Neumann, W. J. Dopaminergic reinforcement in the motor system: implications for Parkinson’s disease and deep brain stimulation. Eur. J. Neurosci. 59, 457–472 (2024).
Yttri, E. A. & Dudman, J. T. Opponent and bidirectional control of movement velocity in the basal ganglia. Nature 533, 402–406 (2016).
Wu, M. C. K., David, S. V. & Gallant, J. L. Complete functional characterization of sensory neurons by system identification. Annu. Rev. Neurosci. 29, 477–505 (2006).
Jackson, A., Mavoori, J. & Fetz, E. E. Correlations between the same motor cortex cells and arm muscles during a trained task, free behavior, and natural sleep in the macaque monkey. J. Neurophysiol. 97, 360–374 (2007).
Wang, N. X. R., Olson, J. D., Ojemann, J. G., Rao, R. P. N. & Brunton, B. W. Unsupervised decoding of long-term, naturalistic human neural recordings with automated video and audio annotations. Front. Hum. Neurosci. 10, 00165 (2016).
Dixon, T. et al. Data from: Movement-responsive deep brain stimulation for Parkinson’s disease using a remotely optimized neural decoder. Dryad https://doi.org/10.5061/dryad.4xgxd25hw (2025).
Dixon, T. dixon-2025-move-adbs. GitHub https://github.com/Weill-Neurohub-OPTiMaL/dixon-2025-move-adbs (2025).
Dixon, T. rcssim. GitHub https://github.com/Weill-Neurohub-OPTiMaL/rcs-simulation (2025).
Acknowledgements
This work was supported by a Weill Neurohub Investigators Grant (J.L.G., J.A.H. and S.J.L.) and NIH UG3NS140730 award. The Weill Neurohub funding agency and NIH played no role in the study design, data collection, analysis, interpretation of the data or the writing of this paper. We would like to thank our participant, A. Perttula, for their involvement in the study and for supporting the research with their time for the experiments.
Author information
Authors and Affiliations
Contributions
T.C.D., J.A.H. and S.J.L. conceived the study and designed the research. T.C.D. and S.R. scheduled and oversaw the experiments. T.C.D. and D.L. designed and programmed the optimization pipeline and related tools. G.S., A.Z., T.F., R.B. and S.R. designed the data collection infrastructure. T.C.D., G.S., A.Z. and R.B. analysed the data. P.A.S. performed the implantation procedures. T.C.D. wrote the paper, and all other authors performed reviews. J.L.G., J.A.H. and S.J.L. acquired funding and supervised the study.
Corresponding author
Ethics declarations
Competing interests
T.C.D. is a current employee of iota Biosciences, Inc., but was not an employee when the study was designed and initiated. S.R. is a current employee of Medtronic, Inc., but was not an employee when the study was designed and initiated. S.J.L. is a consultant for iota Biosciences, Inc. P.A.S. received fellowship programme financial support from Medtronic, Inc., and Boston Scientific, Inc., is a consultant for InBrain Neuroelectronics, Inc., and serves on the DSMB for Neuralink, Inc. All other authors declare no competing interests. No commercial entity played any role in experimental design or analysis/interpretation of study results, provided financial support for this study, or has any obvious means of gaining or losing financially through this publication. A patent application has been submitted for the movement-responsive aDBS and optimization approach (applicant, UCSF; application number, 65/531,506; inventors, T.C.D., S.J.L., J.A.H., D.L. and P.A.S.).
Peer review
Peer review information
Nature Biomedical Engineering thanks Dominique Durand, Wolf-Julian Neumann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Consort checklist.
Supplementary Video 1
Real-time movement-responsive aDBS.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dixon, T.C., Strandquist, G., Zeng, A. et al. Movement-responsive deep brain stimulation for Parkinson’s disease using a remotely optimized neural decoder. Nat. Biomed. Eng 10, 110–124 (2026). https://doi.org/10.1038/s41551-025-01438-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01438-0
This article is cited by
-
From adaptive deep brain stimulation to adaptive circuit targeting
Nature Reviews Neurology (2025)