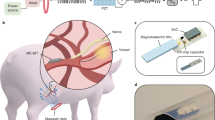
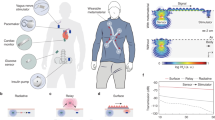
Abstract
Networks of miniature implants could enable simultaneous sensing and stimulation at different locations in the body, such as the heart and central or peripheral nervous system. This capability would support precise disease tracking and treatment or enable prosthetic technologies with many degrees of freedom. However, wireless power and data transfer are often inefficient through biological tissues, particularly as the number of implanted devices increases. Here we show that magnetoelectric wireless data and power transfer supports a network of millimetre-sized bioelectronic implants in which system efficiency improves with additional devices. We demonstrate wireless, battery-free networks ranging from one to six implants, where the total system efficiency increases from 0.2% to 1.3%, with each node receiving 2.2 mW at 1 cm distance. We show proof-of-concept networks of miniature spinal cord stimulators and cardiac pacing devices in large animals via efficient and robust wireless power transfer. These magnetoelectric implants provide a scalable network architecture of bioelectronic implants for next-generation electronic medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data supporting the results of this study are available within the paper and its Supplementary Information. Source data for Figs. 2a,c,d, 3b,d and 4f are available via figshare at https://doi.org/10.6084/m9.figshare.29431022, https://doi.org/10.6084/m9.figshare.29430995, https://doi.org/10.6084/m9.figshare.29431034, https://doi.org/10.6084/m9.figshare.29431040 and https://doi.org/10.6084/m9.figshare.29431061 (refs. 50,51,52,53,54).
Code availability
The custom MATLAB code used to preprocess and plot data for Figs. 2c, 3e and 4f are available via figshare at https://doi.org/10.6084/m9.figshare.29430995, https://doi.org/10.6084/m9.figshare.29431040 and https://doi.org/10.6084/m9.figshare.29431061 (refs. 51,53,54).
References
Lee, J. et al. Neural recording and stimulation using wireless networks of microimplants. Nat. Electron. 4, 604–614 (2021).
Tawakol, O. et al. In-vivo testing of a novel wireless intraspinal microstimulation interface for restoration of motor function following spinal cord injury. Artif. Organs 48, 263–273 (2024).
Becerra-Fajardo, L. et al. First-in-human demonstration of floating EMG sensors and stimulators wirelessly powered and operated by volume conduction. J. Neuroeng. Rehabil. 21, 4 (2024).
Becerra-Fajardo, L. et al. Floating EMG sensors and stimulators wirelessly powered and operated by volume conduction for networked neuroprosthetics. J. Neuroeng. Rehabil. 19, 57 (2022).
Benedict, B. C., Ghanbari, M. M. & Muller, R. Phased array beamforming methods for powering biomedical ultrasonic implants. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 69, 2756–2765 (2022).
Costello, J. T. et al. A low-power communication scheme for wireless, 1000 channel brain-machine interfaces. J. Neural. Eng. 19, 036037 (2022).
Dinis, H., Colmiais, I. & Mendes, P. M. Extending the limits of wireless power transfer to miniaturized implantable electronic devices. Micromachines 8, 359 (2017).
Lyu, H. et al. Synchronized biventricular heart pacing in a closed-chest porcine model based on wirelessly powered leadless pacemakers. Sci. Rep. 10, 2067 (2020).
Nguyen, N., Ha-Van, N. & Seo, C. Midfield wireless power transfer for deep-tissue biomedical implants. IEEE Antennas Wirel. Propag. Lett. 19, 2270–2274 (2020).
Lee, J. et al. A sub-mm3 wireless neural stimulator IC for visual cortical prosthesis with optical power harvesting and 7.5-kb/s data telemetry. IEEE J. Solid-State Circuits https://doi.org/10.1109/JSSC.2023.3349179 (2024).
Kim, W. et al. Magnetoelectrics enables large power delivery to mm-sized wireless bioelectronics. J. Appl. Phys. 134, 094103 (2023).
Chen, J. C. et al. A wireless millimetric magnetoelectric implant for the endovascular stimulation of peripheral nerves. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-022-00873-7 (2022).
Bichurin, M. I. et al. Resonance magnetoelectric effects in layered magnetostrictive-piezoelectric composites. Phys. Rev. B 68, 132408 (2003).
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2, 16093 (2017).
Bottomley, P. A. & Andrew, E. R. RF magnetic field penetration, phase shift and power dissipation in biological tissue: implications for NMR imaging. Phys. Med. Biol. 23, 630 (1978).
Alrashdan, F. T. et al. Wearable wireless power systems for ‘ME-BIT’ magnetoelectric-powered bio implants. J. Neural Eng. 18, 045011 (2021).
Sdrulla, A., Guan, Y. & Raja, S. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain. Pract. 18, 1048–1067 (2018).
Gill, M. L. et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 24, 1677–1682 (2018).
Angeli, C. A. et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 379, 1244–1250 (2018).
Rowald, A. et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat. Med. 28, 260–271 (2022).
Sayenko, D. G., Angeli, C., Harkema, S. J., Edgerton, V. R. & Gerasimenko, Y. P. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J. Neurophysiol. 111, 1088–1099 (2014).
Greiner, N. et al. Recruitment of upper-limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat. Commun. 12, 435 (2021).
Powell, M. P. et al. Epidural stimulation of the cervical spinal cord for post-stroke upper-limb paresis. Nat. Med. 29, 689–699 (2023).
North, R. B. & Prager, J. P. In Neuromodulation 2nd edn (eds. Krames, E. S., Peckham, P. H. & Rezai, A. R.) 587–596 (Academic, 2018).
Woods, J. E. et al. Miniature battery-free epidural cortical stimulators. Sci. Adv. 10, eadn0858 (2024).
Alò, K. et al. Factors affecting impedance of percutaneous leads in spinal cord stimulation. Neuromodulation Technol. Neural Interface 9, 128–135 (2006).
Toossi, A. et al. Comparative neuroanatomy of the lumbosacral spinal cord of the rat, cat, pig, monkey, and human. Sci. Rep. 11, 1955 (2021).
Steele, A. G. et al. Mapping lumbar efferent and afferent spinal circuitries via paddle array in a porcine model. J. Neurosci. Methods 405, 110104 (2024).
Zander, H. J., Graham, R. D., Anaya, C. J. & Lempka, S. F. Anatomical and technical factors affecting the neural response to epidural spinal cord stimulation. J. Neural Eng. 17, 036019 (2020).
Abraham, W. T. & Hayes, D. L. Cardiac resynchronization therapy for heart failure. Circulation 108, 2596–2603 (2003).
Brabham, W. W. & Gold, M. R. The role of AV and VV optimization for CRT. J. Arrhythmia 29, 153–161 (2013).
Kotsakou, M. et al. Pacemaker insertion. Ann. Transl. Med. 3, 42–42 (2015).
Vouliotis, A. I. et al. Leadless pacemakers: current achievements and future perspectives. Eur. Cardiol. 18, e49 (2023).
Furman, S., Hurzeler, P. & Mehra, R. Cardiac pacing and pacemakers IV. Threshold of cardiac stimulation. Am. Heart J. 94, 115–124 (1977).
Abbott. AveirTM Leadless Pacemaker IFU. Product Instructions for Use (2021); https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150035D.pdf
Medtronic. MicraTM AV MC1AVR1 Manual (2020); https://wwwp.medtronic.com/crs-upload/letters/401/401_Micra_AV_Implant_Manual_with_Medical_Procedure_and_EMI_Precautions.pdf
Zangooei, H., Mirbozorgi, S. A. & Mirbozorgi, S. Thermal analysis of heat transfer from catheters and implantable devices to the blood flow. Micromachines 12, 230 (2021).
Roujol, S., Tan, A. Y., Anter, E., Josephson, M. E. & Nezafat, R. Towards cardiac and respiratory motion characterization from electrophysiology data for improved real time MR-integration. J. Cardiovasc. Magn. Reson. 15, P68 (2013).
Werner, R., Ehrhardt, J., Schmidt, R. & Handels, H. Patient-specific finite element modeling of respiratory lung motion using 4D CT image data. Med. Phys. 36, 1500–1511 (2009).
Mukherjee, D. & Mallick, D. Magnetoelectric wireless power transfer system for biomedical implants. In 2021 IEEE International Midwest Symposium on Circuits and Systems (MWSCAS) https://doi.org/10.1109/MWSCAS47672.2021.9531861 (IEEE, 2021).
Rupp, T., Truong, B. D., Williams, S. & Roundy, S. Magnetoelectric transducer designs for use as wireless power receivers in wearable and implantable applications. Materials 12, 512 (2019).
García-Moreno, A. et al. Wireless networks of injectable microelectronic stimulators based on rectification of volume conducted high frequency currents. J. Neural Eng. 19, 056015 (2022).
Nair, V. et al. Miniature battery-free bioelectronics. Science 382, eabn4732 (2023).
Liu, Y., Zhang, J., Zhu, C. & Chan, C. C. A study on the safety analysis of an inductive power transfer system for kitchen appliances. Energies 15, 5218 (2022).
Singer, A. & Robinson, J. T. Wireless power delivery techniques for miniature implantable bioelectronics. Adv. Healthc. Mater. 10, 2100664 (2021).
Webster, J. G. Design of Cardiac Pacemakers (IEEE, 1995).
Baumgartner, C. et al. IT’IS Database for thermal and electromagnetic parameters of biological tissues. Version 4.2. IT’IS Foundation https://doi.org/10.13099/VIP21000-04-0 (2024).
Yu, Z. et al. MagNI: a magnetoelectrically powered and controlled wireless neurostimulating implant. IEEE Trans. Biomed. Circuits Syst. 14, 1241–1252 (2020).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Woods, J. E. et al. Dataset for distributed battery-free bioelectronic implants with improved network power transfer efficiency via magnetoelectrics Fig. 2a and Fig. 2d. figshare https://doi.org/10.6084/m9.figshare.29431022 (2025).
Woods, J. E. et al. Dataset for distributed battery-free bioelectronic implants with improved network power transfer efficiency via magnetoelectrics Fig. 2c. figshare https://doi.org/10.6084/m9.figshare.29430995 (2025).
Woods, J. E. et al. Dataset for distributed battery-free bioelectronic implants with improved network power transfer efficiency via magnetoelectrics Fig. 3b. figshare https://doi.org/10.6084/m9.figshare.29431034 (2025).
Woods, J. E. et al. Dataset for distributed battery-free bioelectronic implants with improved network power transfer efficiency via magnetoelectrics Fig. 3e. figshare https://doi.org/10.6084/m9.figshare.29431040 (2025).
Woods, J. E. et al. Dataset for distributed battery-free bioelectronic implants with improved network power transfer efficiency via magnetoelectrics Fig. 4f. figshare https://doi.org/10.6084/m9.figshare.29431061 (2025).
Acknowledgements
This work was supported in part by the National Heart Lung and Blood Institute of the National Institutes of Health under award no. R01HL144683 (to M.R.), the National Institutes of Health under award no. 1R01NS119587-01A (to D.G.S.), the Walter Neurological Restoration Initiative sponsored by The Paula and J. C. ‘Rusty’ Walter III and the Walter Oil and Gas Corporation, the Wings for Life Foundation (268) (to D.G.S.) and the National Science Foundation GRFP (to J.E.W. and E.C.). We acknowledge the pigs that took part in this study, without whom this would not be possible. We would also like to thank the Texas Heart Institute Veterinary Staff and the Houston Methodist Veterinary Staff for their care of the pigs used in this study.
Author information
Authors and Affiliations
Contributions
J.E.W. and E.C.C. designed and tested the PCBs. J.E.W., F.A. and W.T. assembled the ME devices. J.E.W. performed the testing, characterization, and simulation of the ME network. J.E.W., F.A., A.G.S., S.M.B., P.J.H., A.H.F. and D.G.S. designed and performed the porcine SCS experiments. J.E.W. and A.G.S. analysed the porcine SCS experiment data. J.E.W., F.A., W.T., E.C.C., M.J., D.B., A.P., A.M.-R., A.E. and M.R. designed and performed the porcine cardiac pacing experiments. J.E.W. and M.J. analysed the cardiac pacing experiment data. J.T.R., J.E.W. and F.A. contributed to the design of experiments. J.W. and J.T.R. prepared the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
J.T.R., F.A. and J.E.W. receive monetary and/or equity compensation from Motif Neurotech. A.F. is a consultant for Medtronic, Inc., Boston Scientific, Inc. and Abbott, Inc. M.R. receives monetary and/or equity compensation from Maxwell Biomedical, Rhythio and XNHealth, consults for Ziopatch and is the medical director for IRhythm. The terms of these arrangements have been reviewed and approved by Rice University, the Houston Methodist Research Institute, Baylor College of Medicine and The Texas Heart Institute in accordance with their policies on conflict of interest in research. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Peilong Feng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3, Figs. 1–10 and Descriptions of Movies 1 and 2.
Movie 1
A network of 12 wirelessly powered and programmed ME devices. The video shows a programmed sequence of blinking devices, all simultaneously powered and programmed by a single transmitter. The devices can be moved while still receiving power and data, as shown in the first segment by moving one of the devices. Arbitrary sequences can be programmed rapidly as shown in the second segment.
Movie 2
Heart movement simulator testing. The video shows the benchtop testing system used to characterize the cardiac pacing devices. Devices are placed on a moving platform while powered and controlled by a single external TX coil. Then, 2.5 cm of ground beef is placed between the TX coil and devices, and the beef and devices are submerged in 0.9% NaCl.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woods, J.E., Alrashdan, F., Chen, E.C. et al. Distributed battery-free bioelectronic implants with improved network power transfer efficiency via magnetoelectrics. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01489-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-025-01489-3