Abstract
Accurate assessment of human epidermal growth factor receptor 2 (HER2) status is crucial for effective breast cancer treatment planning and improved patient outcomes. Traditional needle biopsies, limited in tissue sampling, often lead to inaccurate assessments due to intratumoural heterogeneity. Here, to address this, we introduce the deep-learning-based HER2 multimodal alignment and prediction (MAP) model, which leverages pretreatment multimodal breast cancer images for a more comprehensive reflection of tumour characteristics and provides more accurate HER2 status prediction. We develop patient response MAP models to demonstrate the HER2 prediction performance of our model compared with needle biopsies from patients receiving neoadjuvant therapy. A large-scale multimodal breast cancer dataset from 4 centres, consisting of 14,472 images from 6,991 cases, is adopted in this study, and the results consistently demonstrate the superiority of our HER2 MAP model in predicting patient response. These findings highlight the substantial advantages of our HER2 predictions. Our study provides physicians with a crucial tool for informed clinical decisions and treatment plans, aiming to improve outcomes in patients with breast cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
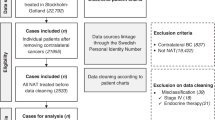
We possess multimodal datasets collected from four medical centres. The images and clinical information of centre C are available at https://wiki.cancerimagingarchive.net/pages/viewpage.action?pageId=70226903. Requests for academic use of in-house raw data can be addressed to the corresponding authors. All requests will be promptly reviewed to assess any intellectual property or patient confidentiality obligations, and will be processed in accordance with institutional and departmental guidelines, subject to a material transfer agreement. Source data are provided with this paper.
Code availability
Code for PyTorch implementation of MAP is available via GitHub at https://github.com/ZhangJD-ong/HER2-MAP-from-Multimodal-Breast-Data (ref. 46).
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Giaquinto, A. N. et al. Breast cancer statistics, 2022. CA Cancer J. Clin. 72, 524–541 (2022).
Zhang, J. et al. A robust and efficient AI assistant for breast tumor segmentation from DCE-MRI via a spatial–temporal framework. Patterns 4, 100826 (2023).
Park, Y. H. et al. Chemotherapy induces dynamic immune responses in breast cancers that impact treatment outcome. Nat. Commun. 11, 6175 (2020).
Bianchini, G., De Angelis, C., Licata, L. & Gianni, L. Treatment landscape of triple-negative breast cancer—expanded options, evolving needs. Nat. Rev. Clin. Oncol. 19, 91–113 (2022).
Asselain, B. et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 19, 27–39 (2018).
Agostinetto, E., Gligorov, J. & Piccart, M. Systemic therapy for early-stage breast cancer: learning from the past to build the future. Nat. Rev. Clin. Oncol. 19, 763–774 (2022).
McNamara, K. L. et al. Spatial proteomic characterization of HER2-positive breast tumors through neoadjuvant therapy predicts response. Nat. Cancer 2, 400–413 (2021).
Cardoso, F. et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30, 1194–1220 (2019).
Korde, L. A. et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J. Clin. Oncol. 39, 1485 (2021).
Wiklander, O. P. et al. Antibody-displaying extracellular vesicles for targeted cancer therapy. Nat. Biomed. Eng. 8, 1453–1468 (2024).
Von Minckwitz, G. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377, 122–131 (2017).
Gianni, L. et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 17, 791–800 (2016).
Rakha, E. A. & Ellis, I. O. Updated guideline recommendations for HER2 testing. Nat. Rev. Clin. Oncol. 11, 8–9 (2014).
Boba, M. et al. False-negative results of breast core needle biopsies—retrospective analysis of 988 biopsies. Pol. J. Radiol. 76, 25 (2011).
Filho, O. M. et al. Impact of HER2 heterogeneity on treatment response of early-stage HER2-positive breast cancer: phase II neoadjuvant clinical trial of T-DM1 combined with pertuzumab. Cancer Discov. 11, 2474–2487 (2021).
Su, G.-H. et al. Radiogenomic-based multiomic analysis reveals imaging intratumor heterogeneity phenotypes and therapeutic targets. Sci. Adv. 9, eadf0837 (2023).
Cahill, R. A., Walsh, D., Landers, R. J. & Watson, R. G. Preoperative profiling of symptomatic breast cancer by diagnostic core biopsy. Ann. Surg. Oncol. 13, 45–51 (2006).
Ough, M., Velasco, J. & Hieken, T. J. A comparative analysis of core needle biopsy and final excision for breast cancer: histology and marker expression. Am. J. Surg. 201, 692–694 (2011).
Lorgis, V. et al. Discordance in early breast cancer for tumour grade, estrogen receptor, progesteron receptors and human epidermal receptor-2 status between core needle biopsy and surgical excisional primary tumour. Breast 20, 284–287 (2011).
Chen, J. et al. Comparison of core needle biopsy and excision specimens for the accurate evaluation of breast cancer molecular markers: a report of 1003 cases. Pathol. Oncol. Res. 23, 769–775 (2017).
Shah, V. I. et al. False-negative core needle biopsies of the breast: an analysis of clinical, radiologic, and pathologic findings in 27 consecutive cases of missed breast cancer. Cancer 97, 1824–1831 (2003).
Zhang, J., Wu, J., Zhou, X. S., Shi, F. & Shen, D. Recent advancements in artificial intelligence for breast cancer: image augmentation, segmentation, diagnosis, and prognosis approaches. Semin. Cancer Biol. 96, 11–25 (2023).
Nicosia, L. et al. Radiomic features applied to contrast enhancement spectral mammography: possibility to predict breast cancer molecular subtypes in a non-invasive manner. Int. J. Mol. Sci. 23, 15322 (2022).
Cui, H. et al. Radiogenomic analysis of prediction HER2 status in breast cancer by linking ultrasound radiomic feature module with biological functions. J. Transl. Med. 21, 44 (2023).
Ramtohul, T. et al. Multiparametric mri and radiomics for the prediction of HER2-zero, -low, and-positive breast cancers. Radiology 308, e222646 (2023).
Feng, L. et al. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a multicentre observational study. Lancet Digit. Health 4, e8–e17 (2022).
Zhou, H.-Y. et al. A transformer-based representation-learning model with unified processing of multimodal input for clinical diagnostics. Nat. Biomed. Eng. 7, 743–755 (2023).
Acosta, J. N., Falcone, G. J., Rajpurkar, P. & Topol, E. J. Multimodal biomedical AI. Nat. Med. 28, 1773–1784 (2022).
Qian, X. et al. Prospective assessment of breast cancer risk from multimodal multiview ultrasound images via clinically applicable deep learning. Nat. Biomed. Eng. 5, 522–532 (2021).
Boehm, K. M., Khosravi, P., Vanguri, R., Gao, J. & Shah, S. P. Harnessing multimodal data integration to advance precision oncology. Nat. Rev. Cancer 22, 114–126 (2022).
Li, Z. et al. Domain generalization for mammography detection via multi-style and multi-view contrastive learning. In Proc. 24th International Conference on Medical Image Computing and Computer-Assisted Intervention–MICCAI 2021 (eds de Bruijne, M. et al.) 98–108 (Springer, 2021).
Tian, Z., Shen, C., Chen, H. & He, T. FCOS: a simple and strong anchor-free object detector. IEEE Trans. Pattern Anal. Mach. Intell. 44, 1922–1933 (2020).
Zhou, B., Khosla, A., Lapedriza, A., Oliva, A. & Torralba, A. Learning deep features for discriminative localization. In Proc. IEEE Conference on Computer Vision and Pattern Recognition 2921–2929 (IEEE, 2016).
Lu, W. et al. Slidegraph+: whole slide image level graphs to predict HER2 status in breast cancer. Med. Image Anal. 80, 102486 (2022).
La Forgia, D. et al. Radiomic analysis in contrast-enhanced spectral mammography for predicting breast cancer histological outcome. Diagnostics 10, 708 (2020).
Yuan, Y. et al. Accuracy of MRI in prediction of pathologic complete remission in breast cancer after preoperative therapy: a meta-analysis. Am. J. Roentgenol. 195, 260–268 (2010).
Baumgartner, A. et al. Ultrasound-based prediction of pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast 39, 19–23 (2018).
Weber, J. J. et al. MRI and prediction of pathologic complete response in the breast and axilla after neoadjuvant chemotherapy for breast cancer. J. Am. Coll. Surg. 225, 740–746 (2017).
Bitencourt, A. G. et al. MRI-based machine learning radiomics can predict HER2 expression level and pathologic response after neoadjuvant therapy in HER2 overexpressing breast cancer. eBioMedicine 61, 103042 (2020).
Turnbull, L. W. Dynamic contrast-enhanced MRI in the diagnosis and management of breast cancer. NMR Biomed. 22, 28–39 (2009).
Li, M. et al. Developing large pre-trained model for breast tumor segmentation from ultrasound images. In Proc. International Conference on Medical Image Computing and Computer-Assisted Intervention (eds Greenspan, H. et al.) 89–96 (Springer, 2023).
Mo, Y. et al. Hover-Trans: anatomy-aware Hover-Transformer for ROI-free breast cancer diagnosis in ultrasound images. IEEE Trans. Med. Imag. 42, 1696–1706 (2023).
Liu, Z. et al. Swin Transformer: hierarchical vision transformer using shifted windows. In Proc. IEEE/CVF International Conference on Computer Vision 10012–10022 (IEEE, 2021).
Zhang, J. HER2 MAP from multimodal breast data. GitHub https://github.com/ZhangJD-ong/HER2-MAP-from-Multimodal-Breast-Data (2025).
Acknowledgements
This work was supported in part by National Natural Science Foundation of China (grant numbers U23A20295 (D.S.), 82441023 (D.S.), 62131015 (D.S.), 82001986 (Zhenhui Li), 82360345 (Zhenhui Li), 82202143 (Zhenhui Li), 62250710165 (Zhenhui Li)), the China Ministry of Science and Technology (S20240085, STI2030-Major Projects-2022ZD0209000, STI2030-Major Projects-2022ZD0213100) (D.S.), Shanghai Municipal Central Guided Local Science and Technology Development Fund (grant number YDZX20233100001001) (D.S.), the Innovative Research Team of Yunnan Province (grant number 202505AS350013, Z.L.) and HPC Platform of ShanghaiTech University.
Author information
Authors and Affiliations
Contributions
In this study, J.Z., Zhenhui Li, Q.Z. and D.S. designed the method and drafted the paper. J.Z. and Y.L. wrote the code. J.Z., Y.L., P.L., J.L., Zheren Li and Zhen Li collected and processed the dataset. J.Z. and Y.L. provided statistical analysis and interpretation of the data. Z.C., K.W.Y.C., Zhenhui Li and D.S. coordinated and supervised the whole work. All authors were involved in critical revisions of the paper and have read and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
D.S. and Z.L. are consultant and employee of Shanghai United Imaging Intelligence Co., Ltd. The company had no role in designing or performing the surveillance, nor in analysing or interpreting the data. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes, Figs. 1 and 2, and Tables 1–4.
Source data
Source Data Figs. 3–5
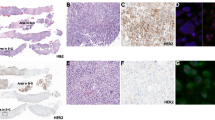
Figure 3a. HER2 status from needle biopsy, surgical biopsy and prediction. Figure 3b–f. Summary of HER2 prediction results. Figure 4a–d. HER2 status from needle biopsy and prediction. Figure 4e,f. Summary of therapy response prediction results. Figure 4k–r. AUROC of therapy response prediction results. Figure 5a. Percentage of contributions for each clinical feature. Figure 5b. Summary of therapy response prediction results of different combinations of modalities
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Li, Y., Li, Z. et al. Deep-learning-based HER2 status assessment from multimodal breast cancer data predicts neoadjuvant therapy response. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01495-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41551-025-01495-5