Abstract
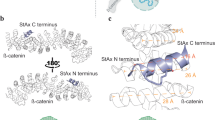
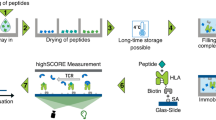
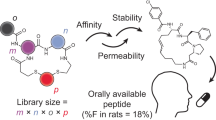
Peptides offer advantages for targeted therapy, including oral bioavailability, cellular permeability and high specificity, setting them apart from conventional small molecules and biologics. Here we develop an artificial intelligence algorithm, PepMimic, to transform a known receptor or an existing antibody of a target into a short peptide binder by mimicking the binding interfaces between targets and known binders. We apply PepMimic to drug targets PD-L1, CD38, BCMA, HER2 and CD4. Surface plasmon resonance imaging results show that 8% of the peptides exhibit dissociation constant (KD) values at the 10−8 M level, and 26 peptides achieving KD values as low as 10−9 M, substantially higher than random library screening conducted under identical conditions. We apply PepMimic to target proteins lacking available binders by first using existing algorithms to design protein binders, followed by designing peptide through simulating these artificial interfaces. We extensively validate the top-ranked peptides using tail vein injections in breast, myeloma and lung tumour mouse models. Experimental results demonstrate effective membrane binding and highlight their strong potential for clinical diagnostic imaging and targeted therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the training data, the test set, the unsupervised dataset and detailed splits are available via Zenodo at https://doi.org/10.5281/zenodo.13373108 (ref. 70). The complexes used for mimicry are extracted from PDB (https: //www.rcsb.org/), the PDB IDs of which are listed in Supplementary Tables 1–5. The synthesized and tested peptide mimicry generations, as well as their experimental KD values, are presented in Supplementary Data 1.
Code availability
Codes for running our peptide mimicry algorithm are available via GitHub at https://github.com/kxz18/PepMimic (ref. 71).
References
Muttenthaler, M., King, G. F., Adams, D. J. & Alewood, P. F. Trends in peptide-drug discovery. Nat. Rev. Drug Discov. 20, 309–325 (2021).
Wang, L. et al. Therapeutic peptides: current applications and future directions. Signal Transduct. Target. Ther. 7, 48 (2022).
Fosgerau, K. & Hoffmann, T. Peptide therapeutics: current status and future directions. Drug Discov. Today 20, 122–128 (2015).
Lee, A. C.-L., Harris, J. L., Khanna, K. K. & Hong, J.-H. A comprehensive review on current advances in peptide-drug development and design. Int. J. Mol. Sci. 20, 2383 (2019).
Vanhee, P. et al. Computational design of peptide ligands. Trends Biotechnol. 29, 231–239 (2011).
Gainza, P. et al. Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat. Methods 17, 184–192 (2020).
Watson, J. L. et al. De novo design of protein structure and function with RFdiffusion. Nature 620, 1089–1100 (2023).
Karoyan, P. et al. Human ACE2 peptide-mimics block SARS-CoV-2 pulmonary-cell infection. Commun. Biol. 4, 197 (2021).
S. E. Barrett, et al. Substrate interactions guide cyclase engineering and lasso peptide diversification. Nat. Chem. Biol. 21, 412–419 (2024).
Miljanich, G. P. Ziconotide: neuronal calcium-channel blocker for treating severe chronic pain. Curr. Med. Chem. 11, 3029–3040 (2004).
Stone, G. W. et al. Bivalirudin for patients with acute coronary syndromes. N. Engl. J. Med. 355, 2203–2216 (2006).
Wilson, A. C., Meethal, S. V., Bowen, R. L. & Atwood, C. S. Leuprolide acetate: a drug of diverse clinical applications. Expert Opin. Investigational Drugs 16, 1851–1863 (2007).
Burdick, D. et al. Assembly and aggregation properties of synthetic Alzheimer’s A4/β-amyloid peptide analogs. J. Biol. Chem. 267, 546–554 (1992).
Hruby, V. J. Designing peptide-receptor agonists and antagonists. Nat. Rev. Drug Discov. 1, 847–858 (2002).
Parisi, G. et al. Design of protein-binding peptides with controlled binding affinity: the case of SARS-CoV-2 receptor-binding domain and angiotensin-converting enzyme 2-derived peptides. Front. Mol. Biosci. 10, 1332359 (2024).
Hill, T. A., Shepherd, N. E., Diness, F. & Fairlie, D. P. Constraining cyclic peptides to mimic protein-structure motifs. Angew. Chem. Int. Ed. 53, 13020–13041 (2014).
Bryan, C. M. et al. Computational design of a synthetic PD-1 agonist. Proc. Natl Acad. Sci. USA 118, e2102164118 (2021).
Ingraham, J. B. et al. Illuminating protein space with a programmable generative model. Nature 623, 1070–1078 (2023).
Lee, J. S., Kim, J. & Kim, P. M. Score-based generative modeling for de novo protein design. Nat. Comput. Sci. 3, 382–392 (2023).
Jin, W., Wohlwend, J., Barzilay, R. & Jaakkola, T. S. Iterative refinement graph neural network for antibody sequence–structure co-design. In International Conference on Learning Representations (2022); https://openreview.net/forum?id=LI2bhrE_2A
Kong, X., Huang, W. & Liu, Y. Conditional antibody design as 3-D equivariant graph translation. In The Eleventh International Conference on Learning Representations (2023); https://openreview.net/forum?id=LFHFQbjxIiP
Luo, S. et al. Antigen-specific antibody design and optimization with diffusion-based generative models for protein structures. Advances in Neural Information Processing Systems. 35, 9754–9767 (2022).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Jumper, J. et al. Highly accurate protein-structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Baek, M. et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021).
Krishna, R. et al. Generalized biomolecular modeling and design with RoseTTAFold All-Atom. Science 384, eadl2528 (2024).
Wang, F., Wang, Y., Feng, L., Zhang, C. & Lai, L. Target-specific de novo peptide-binder design with DiffPepBuilder. J. Chem. Inf. Model. 64, 9135–9149 (2024).
Li, J. et al. Full-atom peptide design based on multi-modal flow matching. In Proc. of the 41st International Conference on Machine Learning Vol. 235 (eds Salakhutdinov, R. et al.) 27615–27640 (PMLR, 2024).
Haitao Lin, O. et al. PPFLOW: target-aware peptide design with torsional flow matching. In Proc. 41st International Conference on Machine Learning (eds Salakhutdinov, R. et al.) vol. 235, 30510–30528 (PMLR, 2024).
Zhang, L., Rao, A. & Agrawala, M. Adding conditional control to text-to-image diffusion models. In Proc. IEEE/CVF International Conference on Computer Vision 3836–3847 (2023).
Rafailov, R. et al. Direct preference optimization: your language model is secretly a reward model. Adv. Neural Inf. Process. Syst. 36, 53728–53741 (2023).
You, J., Liu, B., Ying, Z., Pande, V. & Leskovec, J. Graph convolutional policy network for goal-directed molecular-graph generation. Advances in Neural Information Processing Systems 31 (2018).
Kong, X., Huang, W. & Liu, Y. End-to-end full-atom antibody design. In Proc. 40th International Conference on Machine Learning Vol. 202 (eds Krause, A. et al.) 17409–17429 (PMLR, 2023).
Jin, W., Barzilay, R. & Jaakkola, T. Antibody–antigen docking and design via hierarchical structure refinement. In Proc. of the 39th International Conference on Machine Learning Vol. 162 (eds Chaudhuri, K. et al.) 10217–10227 (PMLR, 2022).
Tsaban, T. et al. Harnessing protein-folding neural networks for peptide–protein docking. Nat. Commun. 13, 176 (2022).
Steinegger, M. & Söding, J. MMseqs2 enables sensitive protein-sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35, 1026–1028 (2017).
Alford, R. F. et al. The Rosetta all-atom energy function for macromolecular modeling and design. J. Chem. Theory Comput. 13, 3031–3048 (2017).
Kabsch, W. & Sander, C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 (1983).
van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Steinley, D. Properties of the Hubert–Arabie adjusted Rand index. Psychol. Methods 9, 386 (2004).
Henikoff, S. & Henikoff, J. G. Amino-acid substitution matrices from protein blocks. Proc. Natl Acad. Sci. USA 89, 10915–10919 (1992).
Lin, D. Y. et al. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T-cell receptors. Proc. Natl Acad. Sci. USA 105, 3011–3016 (2008).
Gainza, P. et al. De novo design of protein interactions with learned surface fingerprints. Nature 617, 176–184 (2023).
Kang-Pettinger, T. et al. Identification, binding, and structural characterization of single-domain anti-PD-L1 antibodies inhibitory of immune-regulatory proteins PD-1 and CD80. J. Biol. Chem. 299, 1 (2023).
Lee, H. T. et al. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci. Rep. 7, 5532 (2017).
Cheng, X. et al. Structure and interactions of the human programmed cell death 1 receptor. J. Biol. Chem. 288, 11771–11785 (2013).
Tan, S. et al. Distinct PD-L1 binding characteristics of therapeutic monoclonal antibody durvalumab. Protein Cell 9, 135–139 (2018).
Zak, K. M. et al. Structural biology of the immune-checkpoint receptor PD-1 and its ligands PD-L1/PD-L2. Structure 25, 1163–1174 (2017).
Chevalier, A. et al. Massively parallel de novo protein design for targeted therapeutics. Nature 550, 74–79 (2017).
Davies, D. R. & Cohen, G. H. Interactions of protein antigens with antibodies. Proc. Natl Acad. Sci. USA 93, 7–12 (1996).
Muyldermans, S. Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem. 82, 775–797 (2013).
Schymkowitz, J. et al. The FoldX web server: an online force field. Nucleic Acids Res. 33, W382–W388 (2005).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv https://doi.org/10.1101/2021.10.04.463034 (2021).
Wei Yang, D. R. et al. Design of high-affinity binders to convex protein target sites. Preprint at bioRxiv https://doi.org/10.1101/2024.05.01.592114 (2024).
Liu, X. et al. Targeting Trop-2 in solid tumors: a look into structures and novel epitopes. Front. Immunol. 14, 1332489 (2023).
Ho, J. & Salimans, T. Classifier-free diffusion guidance. In NeurIPS 2021 Workshop on Deep Generative Models and Downstream Applications (2021).
Kyte, J. & Doolittle, R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 (1982).
Helen, M. & Berman, et al. Protein Data Bank. Nucleic Acids Res. 28, 235–242 (2000).
Tomer Tsaban, J. K. et al. Harnessing protein folding neural networks for peptide–protein docking. Nat. Commun. 13, 176 (2022).
Steinegger, M. & Söding, J. Mmseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35, 1026–1028 (2017).
Kunchur Guruprasad, B. V. B. R. & Madhusudan, W. P. Correlation between stability of a protein and its dipeptide composition: a novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. 4, 155–161 (1990).
Chothia, C. & Janin, J. Principles of protein–protein recognition. Nature 256, 705–708 (1975).
Basu, S. & Wallner, B. Dockq: a quality measure for protein–protein docking models. PLoS ONE 11, e0161879 (2016).
Mitternacht, S. Freesasa: an open source C library for solvent accessible surface area calculations. F1000Research 5, 189 (2016).
Kingma, D. P. & Welling, M. Auto-encoding variational Bayes. Preprint at https://arxiv.org/abs/1312.6114 (2013).
Golub, G. H. & Van Loan, C. F. Matrix Computations (JHU Press, 2013).
Jonathan Ho, A. J. & Pieter Abbeel, D. Diffusion probabilistic models. Adv. Neural Inf. Process. Syst. 33, 6840–6851 (2020).
Kong, X., Huang, W. & Liu, Y. End-to-end full-atom antibody design. In Proc. 40th International Conference on Machine Learning (eds Krause, A. et al.) vol. 202, 17409–17429 (PMLR, 2023).
John Jumper, R. E. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Kong, X., Jia, Y., Huang, W. & Liu, Y. PepBench: dataset for protein-binding peptide design. Zenodo https://doi.org/10.5281/zenodo.13373108 (2024).
Kong, X. kxz18/PepMimic: checkpoints. Zenodo https://doi.org/10.5281/zenodo.15699103 (2025).
Acknowledgements
This work is jointly supported by, National Key Plan for Scientific Research and Development of China (grant no. 2023YFC3043300, to J.M.), the Natural Science Foundation of Fujian Province (grant no. 2024J010027, to W.H.), the National Key R&D Program of China (grant no. 2022ZD0160502, to Y.L.) and the National Natural Science Foundation of China (grant no. 61925601, to Y.L.).
Author information
Authors and Affiliations
Contributions
X.K. and J.M. conceived and designed the generative model with contributions from R.J. X.K. and R.J. processed the data for training and inference, as well as conducted the computational experiments, with help from R.G. to construct the AlphaFold filtering pipeline. Z.W. conducted the wet-lab experiments. X.K., Z.W. and J.M. analysed the results. X.K., R.J., Z.W. and J.M. wrote the manuscript, with contributions from H.L. The study was supervised by W.-Y.M., W.H., Y.L. and J.M.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Leyi Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods 1–4, Figs. 1–12, Tables 1–7 and Algorithms 1–9.
Supplementary Data
All peptides tested by SPRi.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, X., Jiao, R., Lin, H. et al. Peptide design through binding interface mimicry with PepMimic. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01507-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-025-01507-4