Abstract
Chromosomal instability (CIN) is common in solid tumours and fuels evolutionary adaptation and poor prognosis by increasing intratumour heterogeneity. Systematic characterization of driver events in the TRACERx non-small-cell lung cancer (NSCLC) cohort identified that genetic alterations in six genes, including FAT1, result in homologous recombination (HR) repair deficiencies and CIN. Using orthogonal genetic and experimental approaches, we demonstrate that FAT1 alterations are positively selected before genome doubling and associated with HR deficiency. FAT1 ablation causes persistent replication stress, an elevated mitotic failure rate, nuclear deformation and elevated structural CIN, including chromosome translocations and radial chromosomes. FAT1 loss contributes to whole-genome doubling (a form of numerical CIN) through the dysregulation of YAP1. Co-depletion of YAP1 partially rescues numerical CIN caused by FAT1 loss but does not relieve HR deficiencies, nor structural CIN. Importantly, overexpression of constitutively active YAP15SA is sufficient to induce numerical CIN. Taken together, we show that FAT1 loss in NSCLC attenuates HR and exacerbates CIN through two distinct downstream mechanisms, leading to increased tumour heterogeneity.
Similar content being viewed by others
Main
Chromosomal instability (CIN) is pervasive during cancer evolution, particularly in non-small-cell lung cancer (NSCLC), where it is associated with poor recurrence-free survival1,2,3,4. CIN results in loss of heterozygosity (LOH) events, the burden of which correlates with the frequency of whole-genome doubling (WGD) events in solid tumours5,6. WGD not only mitigates the effect of LOH resulting from CIN, but also fosters ongoing CIN7,8,9,10. By duplicating the complete set of chromosomes, WGD is a key event during cancer evolution and correlates with poor prognosis and targeted therapy resistance7,11,12,13. Despite the importance of CIN and WGD in accelerating cancer evolution by promoting intratumour heterogeneity7, genetic events responsible for the initiation and maintenance of CIN and WGD in NSCLC have not been systematically investigated.
Tracking Cancer Evolution Through Therapy/Rx (TRACERx) is a longitudinal cancer study that utilizes multiregional sampling and whole-exome sequencing (WES)3 to identify and time genetic alterations and their relationship with WGD and CIN3. Here, we identify mutations in cancer driver genes that co-occur with WGD and CIN and further characterize their respective involvement in the DNA damage response (DDR) and CIN. We demonstrate that genetic perturbation of six TRACERx driver genes identified in our screen (particularly FAT1) causes deficiencies in homologous recombination (HR) repair and elevated CIN. Using NSCLC cell line models, we further characterize the molecular mechanisms by which FAT1 alterations drive WGD and CIN—known mediators of drug resistance12,14,15,16. These findings highlight the importance of FAT1 gene alterations in lung cancer evolution.
Results
Identification of DDR and CIN drivers in lung TRACERx
To identify alterations that correlate with WGD and CIN in NSCLC, we analysed multiregional WES data from tumours obtained from the first 100 patients within the lung TRACERx study3. Here, we identified 795 driver events in 91 genes, excluded known oncogenes that could not be modelled appropriately by genetic depletion approaches and focused on 37 tumour suppressor gene mutations that co-occurred with WGD (Fig. 1a). Pathway analysis revealed cellular processes related to genome maintenance, such as the DDR, transcription and chromatin remodelling (Supplementary Fig. 1)17,18,19. To assess how these genes contribute to genome integrity, we performed a multi-parametric RNA interference (RNAi) screen using high-content imaging in four different lung cancer cell lines harbouring mutations in KRAS, EGFR and TP53 to reflect the mutational landscape of the TRACERx cohort (Fig. 1b (top) and Extended Data Fig. 1a). DNA double-strand breaks (DSBs) induced by ionizing radiation, as well as replication stress induced by hydroxyurea, were chosen to model genotoxic stress. In parallel, the impact on chromosome loss was investigated utilizing a human artificial chromosome (HAC) reporter system20 (Fig. 1b (bottom) and Extended Data Fig. 1b). These combined approaches enabled the identification of six tumour suppressor driver genes—namely BAP1, CREBBP, FAT1, NCOA6, RAD21 and UBR5—as regulators of the DDR and maintenance of chromosomal stability in NSCLC (Fig. 1c). In addition, we also confirmed previously reported roles of WRN, FANCM, DICER1, SMARCA4/BRG1, ARID1B, ARID2, KDM5C and ATRX in the DDR21,22,23,24,25,26 (Extended Data Fig. 1a).
a, Flow chart depicting candidate gene selection for the DDR and CIN screens. b, Schematic of the design of the DDR and CIN screens. c, Venn diagram showing the six driver genes contributing to DDR and CIN. d, Validation of the six candidate genes by DR-GFP homologous recombination reporter assay; BRCA2 serves as a positive control. HR efficiencies are normalized to those of control samples. Statistical significance was determined by two-sided, one-sample t-test. The data represent means ± s.d. (n = 3 biological replicates, except for BRCA2, for which n = 2). e, Validation of the six candidate genes by DIvA U2OS-AsiSI site-directed resection assay. Statistical significance was determined by two-sided paired t-test. The data represent means ± s.d. (n = 3 biological replicates). f, Box plots quantifying RAD51 foci formation in A549 cells following depletion of the six candidate genes, following 6 Gy ionizing irradiation and 1 h of recovery. The box edges represent interquartile ranges, the horizontal lines represent median values and the ranges of the whiskers denote 1.5× the interquartile range (n = 3 biological replicates; >150 cells quantified per biological replicate). Statistical significance was determined by Kruskal–Wallis test with Dunn’s multiple comparison test. g, Driver mutation distribution and mutational timing of the six candidate genes in the TRACERx 421 cohort. ATM, CHEK2, ATR, CHEK1 and members of the Fanconi anaemia (FA)/BRCA pathway are included for comparison. FAT1 is highlighted in red. CTRL, control; EGFR, epidermal growth factor receptor; FACS, fluorescence-activated cell sorting; HU, hydroxyurea; IR, ionizing radiation; mut, mutant; nt, nucleotides; WT, wild type.
The effect of these genes on the DDR was initially validated using two orthogonal approaches; the DR-GFP HR reporter assay27 and a site-specific DSB-generating endonuclease system (DIvA)28. Loss of BAP1, FAT1, NCOA6, RAD21 or UBR5, but not CREBBP, was associated with HR repair deficiency and impaired single-stranded DNA (ssDNA) resection—a step required for accurate HR repair (Fig. 1d,e and Supplementary Fig. 2). Next, we assessed early HR repair signalling 1 h after 6 Gy ionizing radiation in both A549 and H1944 cells. A marked decrease in the formation of RAD51 ionizing radiation-induced foci (IRIFs) was observed following depletion of BAP1, FAT1, NCOA6, RAD21 or UBR5 (Fig. 1f, Extended Data Fig. 2a,b and Supplementary Figs. 3 and 4). These alterations in HR efficiency could not be fully explained by cell cycle changes (Extended Data Fig. 2c), which dictate the selection of activating HR or non-homologous end-joining (NHEJ) repair, suggesting that these genes may be involved in HR directly. FAT1 was prioritized as a top candidate of clinical relevance, as inactivating mutations in FAT1 were highly recurrent (~10%) in the TRACERx 421 cohort (comprising 421 patients and 1,644 tumour regions), with a notable proportion of FAT1 mutations occurring early before WGD (Fig. 1g and Supplementary Fig. 5a–c). Notably, ~20% of the lung TRACERx cohort were found to harbour other inactivating mutations that impair HR efficiency (Fig. 1g and Extended Data Fig. 2d).
FAT1 alterations are positively selected in lung cancer
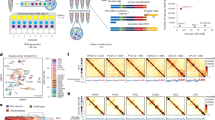
To quantify whether mutations in FAT1 were under positive selection, we measured the enrichment of FAT1 mutations before and after WGD using the ratio of the observed number of non-synonymous substitutions per non-synonymous site to the number of synonymous substitutions per synonymous site (dN/dS)29. Estimates of dN/dS above or below 1 suggest positive or negative selection, respectively, whereas estimates overlapping 1 imply that there is no evidence of selection. FAT1 mutations, which occurred more frequently in lung squamous cell carcinoma (LUSC) compared with lung adenocarcinoma (LUAD), were under greater positive selection before WGD occurrence in LUSC (Fig. 2a and Extended Data Fig. 3a). In the TRACERx 421 cohort, an enrichment of copy number deletion events was identified around the FAT1 genomic locus 4q35.2 only in patients with clonal WGD, indicating positive selection of 4q35.2 loss (Fig. 2b and Extended Data Fig. 3b–d). In LUSC, FAT1 promoter hypermethylation events reducing FAT1 expression levels and FAT1 copy number loss events were observed in the same tumours (Extended Data Fig. 3e–g). Furthermore, we detected a significant occurrence of mirrored subclonal allelic imbalance (MSAI) at the 4q35.2 locus, suggesting parallel evolution (Fig. 2c). Among genes encoded at the 4q35.2 locus, FAT1 has the lowest Genome Aggregation Database loss-of-function score in germline samples, implying that FAT1 loss is the least tolerated event within 4q35.2 (Fig. 2d). These results highlight the importance of FAT1 alterations.
a, Top, schematic of dN/dS ratio analysis. Bottom, results of dN/dS ratio analysis in the TRACERx 421 cohort, demonstrating that FAT1 truncation mutations are selected early in LUSC tumour evolution. The data points represent estimated dN/dS ratios and the error bars represent 95% confidence intervals calculated using the genesetdnds function from the R package dNdScv. The TRACERx 421 cohort comprised 233 males and 188 females (421 patients total), corresponding to a 55:45 male:female ratio. 93% of the cohort was from a White ethnic background and the mean age of the patients was 69 years, ranging between 34 and 92 years. Written informed consent was obtained. None of the patients was compensated for their involvement in the study. b, Genomic identification of significant targets In cancer (GISTIC) analysis of LUAD (141 patients) and LUSC tumours (80 patients) in TRACERx with clonal WGD only, demonstrating that SCNA loss at the FAT1 genomic locus (4q35.2; red text and highlighted) is positively selected in tumours with clonal WGD only. SCNA loci overlapping with common or rare chromosome fragile sites72,73 are annotated (in blue for common fragile sites and in green for rare fragile sites). c, MSAI analysis illustrating that the genomic region of chromosome 4 that harbours the FAT1 gene (arrows) is frequently lost in LUSC. Statistical significance was determined by Fisher’s exact test. In the schematic at the top, paternal and maternal chromosomes are indicated in blue and red, respectively. d, Top, schematic illustrating the location of the FAT1 gene on chromosome 4, together with other 4q35.2 genes within the frequently lost 4q35.2 genomic region. Bottom, selection pressures against losing genes. The data are from the Genome Aggregation Database (gnomAD) and demonstrate high selective pressure against deletion of the FAT1 genomic locus within 4q35.2. exp, expected; LOF, loss of function; obs, observed.
FAT1 ablation reduces HR efficiency
Considering the frequency of FAT1 alterations in NSCLC (Figs. 1g and 2, Extended Data Fig. 3 and Supplementary Fig. 5) and its potential role in genome maintenance (Fig. 1d–f), we further elucidated at which stage FAT1 acts in the DSB repair pathway by systematically investigating which mediators were affected by FAT1 depletion 1 h post-ionizing radiation. FAT1 knockdown did not impact IRIFs of early DSB repair mediators, including phosphorylated ATM, γH2A.X and 53BP1 oligomerization, which are associated with NHEJ22 (Fig. 3a and Supplementary Fig. 6a,b). However, IRIF formation of CtBP interacting protein (CtIP), which is responsible for initiating ssDNA resection22, was significantly impaired by FAT1 knockdown (Fig. 3a and Supplementary Fig. 6c). FAT1 depletion also reduced IRIF formation of the key HR mediator breast cancer type 1 susceptibility protein (BRCA1) in G2/M cells, using centromere protein F-positive staining as a marker (Fig. 3a and Supplementary Fig. 6d). CRISPR knockout of FAT1 in H1944 or A549 cells impaired RAD51 IRIF formation, but not γH2A.X (Extended Data Fig. 4a,b and Supplementary Fig. 6e). A time-course post-ionizing radiation demonstrated that FAT1 depletion was associated with persistent DNA damage, as manifested by the increased frequency of 53BP1 nuclear bodies (Extended Data Fig. 4c).
a, Box plots demonstrating the impact of FAT1 siRNA knockdown on early DNA damage signalling and 53BP1 binding in A549 cells. The boxes represent interquartile ranges, the black and red bars represent median and mean values, respectively, and the ranges of the whiskers denote 1.5× the interquartile range. Statistical significance was determined by two-sided Wilcoxon rank-sum test (n = 3 biological replicates). The total numbers of cells quantified per condition were as follows: n ≥ 370 (pATM), n ≥ 438 (γH2A.X), n ≥ 448 (53PB1), n ≥ 560 (CtIP) and n ≥ 218 (BRCA1). b, Schematic of the FAT1 functional domains. The full-length FAT1 protein is 4,588 amino acids. c, RAD51 IRIF formation following 6 Gy ionizing radiation and 1 h of recovery in FAT1 CRISPR knockout (sgFAT1) versus control A549 cells with overexpression of HA–FAT1ICD versus pcDNA3.1. The boxes represent interquartile ranges, the black and red bars represent median and mean values, respectively, and the ranges of the whiskers denote 1.5× the interquartile range. Statistical significance was determined by two-sided Kruskal–Wallis test followed by Dunn’s test with Bonferroni correction (n = 3 biological replicates). d–f, Top, cartoons depicting examples of HRD-related large-scale transition (LST; d), telomeric allelic imbalance (TAI; e) and LOH (f). Bottom left, Permutation analysis showing a correlation between FAT1 CNA and HRD-related genomic signatures based on TCGA LUAD data. Red lines indicates 90 and 95% confidence intervals, blue line indicates observed correlation value. Bottom right, FAT1 driver mutation scores for these respective genetic alterations, based on TRACERx LUAD data. For the TRACERx LUAD data, tumour numbers were as follows: n = 212 (WT) and n = 17 (mut). In the box and whisker plots, the boxes represent interquartile ranges, the lines represent median values and the ranges of the whiskers denote 1.5× the interquartile range. Statistical significance was determined by two-sided mixed-effects linear model with purity as a fixed covariate and tumour ID as a random variable. g, Top, cartoon showing the design of the EJ5–GFP distal end-joining reporter integrated in U2OS cells. Bottom, 53BP1 siRNA knockdown, but not FAT1 knockdown, affects the distal end-joining rate. The data represent means ± s.d. Statistical significance was determined by two-sided repeated measures one-way analysis of variance (ANOVA) with Holm–Šidák correction (n = 5 biological repeats). h, Top, cartoon showing the design of the EJ2–GFP alternative end-joining reporter integrated into U2OS cells. Bottom, FAT1 siRNA knockdown significantly reduces the alternative end-joining efficiency. The data represent means ± s.d. Statistical significance was determined by two-sided paired t-test (n = 4 biological repeats). EGF, epidermal growth factor-like domain; LAMG, laminin G-like domain; NLS, nuclear localization signal.
Although the size of full-length FAT1 (4,588 amino acids) limits its ectopic expression, the reduction in RAD51 foci formation could be partially rescued by overexpression of the FAT1 carboxy (C)-terminal intracellular domain30 (HA–FAT1ICD; amino acids 4,202–4,588), which exhibited nuclear localization, suggesting that nuclear FAT1 may promote efficient HR (Fig. 3b,c and Extended Data Fig. 4d,e). FAT1-knockout A549 cells were more sensitive to genotoxic stress induced by poly(ADP-ribose) polymerase inhibitors, cisplatin and hydroxyurea (Extended Data Fig. 5a,b). By analysing both the TRACERx and The Cancer Genome Atlas (TCGA) LUAD datasets, we observed a correlation between FAT1 loss and HR deficiency (HRD)-related genomic signatures, including elevated telomeric allelic imbalance (TAI)31, large-scale transitions (LST)32 and loss of heterozygosity (LOH)33 (Fig. 3d–f). Using established reporters for distal and alternative end-joining activities, respectively34, we confirmed that transient FAT1 siRNA depletion reduced the alternative end-joining efficiency without significantly reducing distal end joining (Fig. 3g,h). Utilizing WGS data from Genomics England, no significant difference was observed in ID6 and SBS3 mutational profiles (Extended Data Fig. 5c,d and Supplementary Fig. 7a,b), both of which are mutation signatures associated with NHEJ activity35.
FAT1 ablation leads to structural and numerical CIN
Indeed, FAT1 depletion resulted in an increased fork collapse rate and HAC loss (Fig. 4a and Extended Data Fig. 1b). Our analysis of the TCGA and Genomics England datasets revealed that FAT1 loss correlated with an increased weighted genome instability index score and total mutational burden, indicating elevated structural and numerical CIN (Fig. 4b,c). To validate this observation, we used U2OS and type 2 pneumocyte (T2P) cells to investigate the formation of micronuclei and 53BP1 nuclear bodies in G1 daughter cells, both established markers of unresolved replication stress and HRD36,37,38,39. FAT1 ablation at baseline and under replication stress induced by either low-dose aphidicolin or a short pulse of hydroxyurea exacerbated the formation of cyclin A-negative (G1-specific) 53BP1 nuclear bodies and micronuclei (Fig. 4d–h and Extended Data Fig. 6a). Notably, acentric micronucleus formation was significantly elevated following FAT1 depletion in A549 and U2OS cells following replication stress (Fig. 4f,g). Under these conditions, FAT1 ablation also resulted in an increased mitotic error rate at baseline and under replication stress, which manifested as an increased formation rate of chromatin bridges and lagging chromosomes (Fig. 5a and Extended Data Fig. 6b). Utilizing the DIvA site-directed DSB system28,40, we further demonstrated that FAT1 depletion resulted in increased illegitimate translocation of two DSBs induced on chromosome 17 (Fig. 5b). Concurrently, higher rates of structural chromosomal aberrations, including radial chromosomes and chromatid gaps, were observed upon FAT1 loss (Fig. 5c and Extended Data Fig. 6c). FAT1 silencing also reduced mitotic fidelity, as evidenced by deviations in the modal chromosome number (Fig. 5d–f and Extended Data Fig. 6d).
a, FAT1 knockout exacerbates replication fork stalling in A549 cells. Top, scheme of the nucleotide labeling used to measure replication fork stalling. Bottom (left) quantification; (right), representative image for the DNA fibre experiments. The data represent means ± s.d. Statistical significance was determined by two-sided paired t-test (n = 3 biological replicates; >600 forks counted in total). Scale bars, 20 µm. b, TCGA LUAD analysis showing that FAT1 copy number loss is significantly correlated with weighted genome instability index measurements. The blue lines indicate FAT1 loss and the red dotted lines indicate the 90 and 95% confidence intervals. Confidence intervals were generated using computational permutation analyses. c, Box plot comparing the numbers of indels in FAT1 WT versus mutated tumours in the Genomics England LUAD and LUSC cohorts. The boxes represent interquartile ranges, the lines represent median values and the ranges of the whiskers denote 1.5× the interquartile range. Statistical significance was determined by two-sided Wilcoxon rank-sum test. n = 818 (WT) and n = 16 (mut). d, Transient FAT1 siRNA knockdown induces the formation of 53BP1 bodies in cyclin A-negative U2OS cells following 4 mM hydroxyurea for 5 h and recovery for 24 h. Statistical significance was determined by two-sided Wilcoxon rank-sum test. Scale bars, 10 μm. The red bars in the graph to the left represent mean values (n = 5 biological replicates). e,f, Transient FAT1 siRNA knockdown in U2OS cells induces the formation of total micronuclei with or without replication stress induced by 5 h of 4 mM hydroxyurea followed by 24 h recovery (e), as well as the formation of both acentric and centromeric micronuclei following the hydroxyurea treatment (f). The data represent means ± s.d. Statistical significance was determined by one-way ANOVA with Bonferroni correction. Biological repeats: n = 8 (e) and n = 4 (f). g,h, FAT1 loss elevates the rate of micronuclei formation in response to replication stress induced by 0.2 µM aphidicolin treatment (24 h) following FAT1 CRISPR knockout in A549 cells (g) or transient siRNA knockdown in T2P cells (h). The data represent means ± s.d. Statistical significance was determined by two-sided Student’s t-test. Biological repeats: n = 4 (total micronuclei in g), n = 3 (centromeric and acentric micronuclei in g) and n = 8 (h).
a, Transient FAT1 knockdown significantly increases the mitotic error rate (lagging chromosomes plus DAPI bridges; left; data represent means ± s.d.) and the occurrence of nucleoplasmid bridges (middle; red bars represent mean values) in U2OS cells after 5 h treatment with 4 mM hydroxyurea and 24 h recovery. Statistical significance was determined by one-way ANOVA with Bonferroni correction (left) or Dunn’s test (middle). Right, selected maximum projection images following FAT1 knockdown, showing DAPI-stained mitotic U2OS cells following treatment with 4 mM hydroxyurea and 24 h recovery. Scale bars, 5 μm. Over 100 mitotic cells were scored across three biological replicates. b, Representative PCR-based semi-quantitative DIvA U2OS-AsiSI translocation assay. Transient FAT1 siRNA knockdown increases illegitimate repair products. PCR products generated from the uncut region and the legitimate repair product were used as the loading control. n = 3 biological replicates. c, Histogram (left) and representative images (right) showing that A549 cells with FAT1 loss exhibit a significantly increased number of chromosomal aberrations upon challenge with replication stress induced by 0.2 µM aphidicolin (APH) treatment. Scale bar, 5 μm. The data represent means ± s.d. Statistical significance was determined by one-way ANOVA with Holm–Šidák correction. A total of 60 metaphases were scored across three biological replicates per condition. Blue and red arrows indicate radial chromosomes and chromatid gaps, respectively. d–f, Transient FAT1 siRNA knockdown causes a significant numerical deviation in chromosome number in H1944 cells, as determined by multiple methodologies, including clonal fluorescence in situ hybridization (d), ImageStream high-throughput flow cytometry (e) and metaphase spreads (f). The histogram data represent means ± s.d. For the box plots, the boxes represent interquartile ranges, the black and red lines represent median and mean values, respectively, and the ranges of the whiskers denote 1.5× the interquartile range. Statistical significance was determined by two-sided Wilcoxon rank-sum test (d) or two-sided paired t-test (e). n = 3 biological replicates for all cases. bp, base pairs. NT, non-targeting.
Since FAT1 mutations are both common in lung cancer and evolutionarily selected before WGD (Fig. 2a–c), we experimentally validated the mitotic defect associated with FAT1 loss using the near-triploid (3N) LUAD cell line PC9 (which harbours an in-frame deletion at exon 19 of the epidermal growth factor receptor-encoding gene) and its isogenic WGD hexaploid (6N) clone12. Transient FAT1 depletion in PC9 cells significantly elevated the rate of stalled replication forks and mitotic errors, further confirming the involvement of FAT1 in genome maintenance (Extended Data Fig. 6e–g). Despite resulting in an elevated replication fork collapse rate, reduced formation of interphase and mitotic Fanconi anaemia complementation group D2 (FancD2) foci was also observed in FAT1-depleted WGD PC9 cells, suggesting a failure to recover from replication stress, leading to structural CIN (Extended Data Fig. 6e,g–j).
FAT1 depletion leads to mitotic errors and WGD
WGD events were highly prevalent in the lung TRACERx 421 cohort (84% of LUSC and 77% of LUAD) and FAT1 driver mutations were selected before WGD in LUSC tumours (Fig. 2a). However, FAT1 mutations and WGD did not significantly co-occur in the TRACERx 421 cohort (Fisher’s exact test; P = 0.179) (Extended Data Fig. 7a). This was probably due to the presence of other HR-related gene alterations (~20% of patients; Fig. 1g and Extended Data Fig. 2d), which also can contribute to WGD. To investigate whether FAT1 alterations drive WGD, we used the PC9 lung cancer model to quantify the proportion of actively replicating cells with >6N genome content (the basal ploidy of PC9 is 3N). Increased 5-ethynyl-2′-deoxyuridine (EdU) incorporation rates (Fig. 6a,b), as well as a significant increase in loading of the replicative helicase MCM7 beyond 6N, were observed in FAT1-knockout PC9 cells (Extended Data Fig. 7b), both indicating a second replication event post-6N and suggesting that FAT1 loss is associated with WGD. These observations were independent of p53 mutational status, as similar results were obtained in TP53 wild-type, near diploid, untransformed retinal pigment epithelial-1 (RPE-1) cells immortalized with the human telomerase reverse transcriptase subunit (hTERT) (Extended Data Fig. 7c).
To identify the cause of WGD following FAT1 depletion, we monitored cells using live-cell microscopy, tracking at single-cell resolution. Reported causes of WGD include cytokinesis defects, endoreplication, mitotic bypass and cyclin B1 dysregulation during G2 (refs. 41,42,43,44). No change in cyclin B1 level was observed upon FAT1 knockdown in G2, ruling out cyclin B1 dysregulation as the cause of WGD in the absence of FAT1 (Extended Data Fig. 7d,e). DNA synthesis, measured by EdU incorporation, was also unaltered in control versus FAT1-knockout WGD cells transiently blocked in mitosis using nocodazole (Extended Data Fig. 7f), thereby ruling out a role for FAT1 loss in driving WGD through endoreplication in a manner similar to that of cyclin E amplification reported recently44. To investigate mitotic bypass, we used the hTERT RPE-1 cell line expressing both H2B-mTurquoise and fluorescent, ubiquitination-based cell cycle indicator (FUCCI) (hereafter FUCCI–RPE-1 cells) for live-cell imaging (Supplementary Video 1). FAT1 depletion, irrespective of the induction of aphidicolin-induced replication stress, did not cause a significant increase in the rate of mitotic bypass (Fig. 6c and Supplementary Fig. 8). In contrast, FAT1-depleted cells demonstrated an elevated rate of cytokinesis failure, suggesting defects in this final step of cell division as the cause of the WGD associated with FAT1 deficiency (Fig. 6d, Extended Data Fig. 7g and Supplementary Video 2). FAT1 depletion was also associated with an increased rate of nuclear shape abnormalities in daughter cells after normal mitoses (Fig. 6e and Supplementary Video 3). Similarly, after FAT1 depletion, increases in multinucleation and nuclear morphology alterations were observed in fixed U2OS and RPE-1 cells (Extended Data Fig. 7h,i) and WGD PC9 cells, respectively, after replication stress exposure (Extended Data Fig. 8a).
a, Representative dot plots demonstrating FAT1 ablation in PC9 cells and assessment of EdU incorporation beyond the normal G2 phase, to visualize WGD. b, Top, representative western blot validating FAT1 knockout. Bottom, quantification of EdU incorporation beyond the normal G2 population showing that FAT1 knockout significantly increases the WGD population in PC9 cells. The data represent means ± s.d. Statistical significance was determined by one-way ANOVA with Bonferroni correction. Biological repeats: n = 7 (sgNT), n = 5 (sgFAT1 clone 1) and n = 4 (sgFAT1 clone 2). c, Schematic (left) and histogram (right) showing the impact of transient FAT1 knockdown in TERT RPE-1 cells on the promotion of WGD through mitotic bypass, as determined by live-cell imaging. The data represent means ± s.e.m. Statistical significance was determined by one-way ANOVA with Bonferroni correction. Biological repeats: n = 3 (with aphidicolin treatment) and n = 6 (without aphidicolin treatment). d,e, Schematics (left) and histograms (right) showing that transient FAT1 siRNA knockdown in TERT RPE-1 cells increases the rates of cytokinesis failure (d) and nuclear shape deformation (e), as determined by 30× magnification live-cell microscopy imaging at 20 min intervals. The data represent means ± s.e.m. Statistical significance was determined by two-sided paired t-test, At least 200 mitotic events were tracked per condition over five biological replicates. YFP, yellow fluorescent protein.
To delineate whether structural CIN precedes nuclear shape abnormalities, we performed live-cell spinning-disk confocal microscopy on FUCCI–RPE-1 cells in G2, allowing us to determine the timing and outcome of the pending mitosis and the fate of respective daughter cells at high resolution with low phototoxicity. We observed that FAT1 depletion not only increases chromatin bridge formation rates (Extended Data Fig. 8b, left) but reduces the maintenance of normal nuclear morphology following mitotic chromosomal bridge formation (Extended Data Fig. 8b (right) and Supplementary Videos 4 and 5). Next, we investigated the long-term outcome of daughter cells with deformed nuclear morphology, by observing the heritability and recurrence of nuclear deformities and mitotic errors in respective daughter cells following mitosis (Extended Data Fig. 8c). In addition, the number of normal second mitoses was significantly reduced following an initial nuclear deformation event (Extended Data Fig. 8c). Furthermore, FAT1-depleted cells had an accelerated rate of metaphase entry (Extended Data Fig. 8d), suggestive of a potential role for FAT1 in regulating mitotic timing. Taken together, these results demonstrate that FAT1 loss contributes to both an increased rate of structural CIN and elevated mitotic defects in daughter cells following CIN, contributing to WGD.
Since elevated CIN is known to synergize with WGD to generate increased intratumour heterogeneity3,45 and escape from targeted therapeutic pressure12,14,46,47, we also investigated whether FAT1 loss might enable WGD PC9 cells to accelerate cancer evolution and bypass targeted therapy treatment. PC9 cells were treated with a sensitizing concentration of the epidermal growth factor receptor inhibitor osimertinib (>90% inhibitory concentration) for 5 weeks (Extended Data Fig. 8e,f). An increase in clonal survival and clonal derivation rate was observed for FAT1-knockout cells over 3 months (Extended Data Fig. 8g,h). Osimertinib-resistant FAT1-knockout clones exhibited elevated ploidies compared with control clones (Extended Data Fig. 8i). Taken together, these results suggest that FAT1 loss might fuel WGD and elevated CIN, which in turn exacerbates cancer evolution and targeted therapy resistance, as previously demonstrated12,14,46,47.
FAT1 depletion leads to dysregulated Yes-associated protein 1 signalling
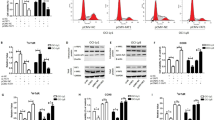
To determine the molecular mechanism by which FAT1 alterations lead to CIN, we investigated components of potential signalling pathways in which FAT1 has been implicated. FAT1 loss has been connected to dysregulation of the Hippo pathway, RAS–RAF–MEK–MAPK signalling and regulation of the level of Yes tyrosine kinase, which mediates hybrid–epithelial–mesenchymal transition48,49. Under our experimental conditions, no consistent FAT1 siRNA-mediated alterations of MEK–ERK phosphorylation or Yes tyrosine kinase levels (Fig. 7a) were observed. However, FAT1 depletion resulted in elevated nuclear localization of the key Hippo pathway activator YAP/TAZ (Fig. 7b and Extended Data Fig. 9a,b). Despite increased Yes-associated protein 1 (YAP1) nuclear localization, no significant alteration in YAP1 phosphorylation could be consistently observed following LATS1 or FAT1 depletion (Extended Data Fig. 9c) in T2P or FUCCI–RPE-1 cells.
a, Western blot demonstrating the impact of FAT1 knockdown on the Src–Mek–Erk signalling axis in hTERT RPE-1 and T2P cells. The results are representative of three repeats. b, Scatter plot showing the impact of transient siRNA depletion of FAT1 on nuclear YAP1 localization using the stringent PTEMF fixation buffer (Methods) in TERT RPE-1 cells. The red bars represent median values. Statistical significance was determined by two-sided Kruskal–Wallis test followed by Dunn’s multiple comparisons test. Over 170 cells were scored per condition over three biological replicates. c, Top, schematic illustrating the predicted domains of the FAT1 protein and respective regions cloned into a pCMV expression plasmid with an HA epitope tag. Bottom, TEAD activity in FAT1 knockout PC9 hexaploid WGD cells. The normalized TEAD activity was measured as an enrichment of the neonGreen signal over the untransfected background signal in each experiment. The HA signal was used to identify successful FAT1 rescue construct co-transfection at the single-cell level. TEAD activity was elevated in FAT1-knockout cells but could be further increased by overexpressing the constitutively active HA–YAP15SA mutant. Overexpression of the FAT1 wild-type construct repressed TEAD activity. However, overexpression of FAT1 mutants devoid of the MIB2 binding region (mScarlet–HA–FAT1MIB∆) and HA–FAT1ICD did not repress TEAD activity. The edges of the histograms represent mean values. Statistical significance was determined by two-sided Kruskal–Wallis test followed by Dunn’s multiple comparisons test. More than 95 cells were scored over three biological replicates. d, DSB resection assay, showing that transient knockdown of LATS1 and LATS2—both negative regulators of YAP1—represses ssDNA formation at DSB break sites (chr22:37194035, ssDNA measured 131 nucleotides from the DSB) in U2OS-AsiSI cells. The data represent means ± s.d. (n = 4 biological replicates). Statistical significance was determined by two-sided paired t-test. e, Both LATS1 and LATS2 siRNA knockdown in U2OS cells elevate rates of 53BP1 nuclear body formation when challenged with replication stress (5 h of 4 mM hydroxyurea followed by 24 h recovery). The boxes represent interquartile ranges, the black and red lines represent median and mean values, respectively, and the ranges of the whiskers denote 1.5× the interquartile range. Statistical significance was determined by two-sided Wilcoxon rank-sum test. More than 340 cells were scored over three biological replicates. f, Both LATS1 and LATS2 siRNA knockdown in U2OS cells induce centromeric and acentric micronuclei formation following challenge with replication stress (5 h of 4 mM hydroxyurea followed by 24 h recovery), suggestive of a mitotic segregation deficiency. Statistical significance was determined by repeated measures one-way ANOVA. The data represent means ± s.d. (n = 3 biological replicates). g, Transient siRNA knockdown of LATS1 in U2OS cells elevates the mitotic error rate. The data represent means ± s.d. Statistical significance was determined by repeated measures one-way ANOVA (n = 3 biological replicates). MIB2, MindBomb2-interacting domain; mS, mScarlet; TM, transmembrane domain.
Orthogonally, we designed a neonGreen reporter with 14xTEAD binding sites to elucidate how FAT1 regulates the ultimate output of the Hippo pathway, TEAD transcriptional activity. This is particularly important since YAP1 and TAZ/WWTR1 are highly analogous, functionally overlapping50 and both amplified in lung cancer3,51 (Extended Data Fig. 9d). By scoring HA-tagged construct and neonGreen reporter double-positive cells, we confirmed that FAT1 loss elevated TEAD transcriptional activity, despite the presence of multiple copies of YAP/TAZ in hexaploid WGD PC9 cells (Fig. 7c, condition 1 versus condition 2). Notably, overexpression of the constitutively active YAP1 mutant construct52 further stimulated TEAD activity in FAT1-knockout cells (HA-YAP15SA; Fig. 7c, condition 3).
Next, we investigated which FAT1 domains are required to re-suppress TEAD transcriptional activity. In addition to the C-terminal HA–FAT1ICD fragment capable of rescuing RAD51 IRIF formation (Fig. 3b,c), a FAT1 construct encompassing the extracellular and transmembrane domain was generated (mScarlet–HA–FAT1WT in Fig. 7c), consisting of 3,735–4,588 amino acids of FAT1. Since FAT1 is known to cooperate with the E3 ligase MIB2 to regulate YAP/TAZ signalling53, we also generated a FAT1 mutant lacking the MIB2 interaction domain (mScarlet–HA–FAT1MIB∆ in Fig. 7c). Indeed, the mScarlet–HA–FAT1WT fragment successfully re-repressed TEAD transcription, whereas the FAT1 construct lacking the MIB2 interaction domain failed to do so (Fig. 7c (conditions 4 and 5) and Extended Data Fig. 9e), highlighting the role of FAT1–MIB2 interaction in the modulation of TEAD transcription. Notably, the HA–FAT1ICD construct, which lacks a transmembrane domain anchor compared with HA–FAT1WT (Supplementary Fig. 9), failed to re-repress TEAD transcription (Fig. 7c, condition 6), despite successfully rescuing RAD51 IRIF formation in A549 cells (Fig. 3b,c).
Next, we investigated whether depletion of LATS1/2—crucial modulators of YAP1 nuclear localization54—might lead to phenotypes resembling those of DDR and CIN observed following FAT1 depletion. Indeed, a marked reduction of the DNA end-resection rate, 53BP1 nuclear body formation and an increase in micronuclei formation were all observed following depletion of either LATS1 or LATS2 (Fig. 7d–f and Extended Data Fig. 10a). Using the site-directed endonuclease DIvA system, depletion of LATS2 caused illegitimate DSB repair, culminating in a translocation (Extended Data Fig. 10b). Notably, somatic copy number alteration (SCNA) loss of LATS2 genomic loci at 13q12.11 was positively selected for in LUSC (Extended Data Fig. 10c). Despite causing HRD, neither LATS1 nor LATS2 depletion disrupted the activation of early DDR signalling, such as phosphorylation of KAP1/TRIM28 or γH2A.X phosphorylation (Extended Data Fig. 10a). Similar to FAT1 depletion, LATS1 knockdown led to an elevated mitotic error rate (Fig. 7g).
FAT1 and YAP1 co-depletion reverses WGD but not HR defects
Given the involvement of FAT1 loss in YAP/TAZ activity (Fig. 7b,c), we systematically investigated whether WGD and numerical and structural CIN associated with FAT1 loss could be reversed by co-depleting YAP1. Despite FAT1/YAP1 co-depletion reversing the cell cycle arrest associated with YAP1 single knockdown (Extended Data Fig. 10d,e), co-depletion of FAT1 and YAP1 did not rescue HR activation defects after DSB formation (Fig. 8a–c). This result was orthogonally validated using the DR-GFP reporter assay, ssDNA formation after endonuclease-induced DSBs and RAD51 foci formation after ionizing irradiation (Fig. 8a–c). Next, since unresolved recombination intermediates due to HR repair deficiency can cause mitotic errors55, we quantified the rate of mitotic errors. YAP1/FAT1 co-depletion failed to rescue the elevated mitotic error and chromosomal bridges observed in FAT1-knockout A549 cells (Fig. 8d,e and Extended Data Fig. 10f).
a, Impact of FAT1/YAP1 siRNA co-depletion in U2OS cells, as determined by DR-GFP HR reporter assay. MRE11A siRNA served as a positive control. The HR efficiencies are normalized to those of the control samples. Statistical significance was determined by one-way ANOVA with Holm–Šidák correction. The data represent means ± s.d. Biological replicates: n = 5 (siCTRL and siFAT1), n = 3 (siYAP1) and n = 4 (siFAT1 + siYAP1 and siMRE11A). b, ssDNA resection rates for FAT1/YAP1 siRNA co-depletion, as determined by DIvA U2OS-AsiSI site-directed resection assay with the DSB site located at chr22:37194035, ssDNA measured 131 nucleotides from the DSB. Statistical significance was determined by one-way ANOVA with Holm–Šidák correction. The data represent means ± s.d. (n = 3 biological replicates). c, Box plots quantifying RAD51 foci formation in A549 cells following the loss of FAT1, or the combined loss of both FAT1 and YAP1, after 6 Gy ionizing irradiation and 1 h recovery. The boxes represent interquartile ranges, the black and red lines represents median and mean values, respectively, and the ranges of the whiskers denote 1.5× the interquartile range. Over 380 cells were scored across three biological replicates. Statistical significance was determined by uncorrected Dunn’s test. d,e, Plots (d) and representative images (e) illustrating the quantification of mitotic error rates in A549 cells after 24 h of aphidicolin treatment (0.2 µM). FAT1 wild-type or knockout cells were transiently depleted of YAP1 using RNAi. For the mitotic error analysis, statistical significance was determined by one-way ANOVA with Holm–Šidák multiple correction and the data represent means ± s.d. (biological repeats: n = 3 (FAT1 WT) and n = 4 (FAT1 knockout)). For the DAPI bridge and Fanconi anaemia complementation group D2 (FANCD2)-flanked DAPI bridge analyses, the red lines represent mean values, the boxes represent interquartile ranges and the ranges of the whiskers denote 1.5× the interquartile range, and statistical significance was determined by Dunn’s test with Bonferroni correction. Over 100 mitotic cells were scored across three biological replicates. Scale bars, 5 µM. f, Results of live-cell imaging analysis, showing that FAT1/YAP1 double siRNA knockdown in TERT–RPE-1 cells fully rescued the failed cytokinesis and WGD introduced by FAT1 knockdown (left) but only partially ameliorated the nuclear shape deformation (right). Statistical significance was determined by one-way ANOVA. At least five biological replicates were quantified per condition. Biological repeats: n = 4 (siCTRL), n = 7 (siFAT1) and n = 5 (siFAT1 + siYAP1). The data represent means ± s.e.m. g, Histogram illustrating the WGD populations in TP53 wild-type versus knockout RPE-1 cells with or without transient mScarlet–YAP5SA transfection. The data represent means ± s.e.m. Statistical significance was determined by two-sided Mann–Whitney test (n = 4 biological repeats). h, Histograms illustrating the total (left) and normalized (right) EdU+ WGD populations in FAT1 wild-type versus knockout PC9 cells, with or without transient mScarlet–YAP5SA transfection. The data represent means ± s.e.m. (n = 5 biological repeats). Statistical significance was determined by repeated measures one-way ANOVA with Benjamini–Hochberg correction (left) or Friedman test (right). KO, knockout.
Next, we investigated whether FAT1/YAP1 co-depletion might rescue the CIN phenotypes associated with FAT1 loss. Live-cell imaging experiments demonstrated that co-depletion of FAT1 and YAP1 reversed the cytokinesis failure phenotypes (Fig. 8f (left) and Extended Data Fig. 10g). However, the nuclear shape deformation observed following FAT1 depletion was only partially rescued (Fig. 8f, right).
Taken together, our observations suggest that FAT1 possesses dual roles. One outcome of FAT1 loss is HRD leading to unresolved recombination intermediates, replication stress, structural CIN and nuclear deformation (Figs. 3–5 and 8a–d); these events appear to be YAP1 independent. In contrast, hyperactive YAP1 leads to increased cytokinesis failure and WGD (Fig. 8f). To visualize the impact of constitutively active mScarlet–YAP15SA overexpression on WGD, we performed an EdU incorporation assay. Complementary to the literature56, mScarlet–YAP15SA overexpression promoted WGD in RPE-1 cells and this was independent of TP53 status (Fig. 8g and Supplementary Fig. 10). Concurrently, we observed an emerging WGD population in FAT1 wild-type and FAT1-knockout PC9 cells (Fig. 8h). These results suggest that constitutively active YAP1 is sufficient to promote WGD. We postulate that FAT1 loss might drive genome instability through two different routes—one through WGD, dependent on YAP1, and the second through HRD, driving structural CIN—in a TEAD/YAP1-independent manner.
Discussion
We systematically analysed 37 NSCLC drivers previously reported in the lung TRACERx study3 that are associated with CIN and WGD. Particularly, we show that depletion of either BAP1, FAT1, NCOA6, RAD21 or UBR5 is associated with downregulation of multiple key steps required for HR repair and elevated loss of a HAC, thereby confirming previous reports of the roles of BAP1, RAD21 and UBR5 in genome maintenance57,58,59. In addition, our DDR screen confirms previous reports characterizing the role of WRN, FANCM, DICER1, SMARCA4/BRG1, ARID1B, ARID2, KDM5C and ATRX in the DDR21,22,23,24,25,26. Despite FAT1 being frequently altered in normal somatic skin cells60 and in multiple cancers61,62,63, as well as being associated with NSCLC mortality2, the mechanistic links between FAT1 alterations, DDR and both numerical and structural CIN have remained elusive.
Through our comprehensive analyses, we have demonstrated that FAT1 ablation impacts HR efficiency, but not NHEJ efficiency, nor the early signalling events of the DDR. FAT1 loss results in hallmarks of HRD, such as increased sensitivity to replication stress inducers, elevated chromosome translocation, elevated fork collapse rates and increased HRD-predictive genetic scars in NSCLC. Our data suggest that FAT1 loss leads to end-resection deficiency after the chromatin remodelling step to initiate HR. The inability to accurately resolve replication stress or clastogenic breaks may lead to chromosome-level alterations36, especially structural CIN.
FAT1 alterations have been detected in normal somatic tissue60 and we hypothesize that FAT1 inactivation occurs early in tumorigenesis. Using the multiregional sequencing of the TRACERx study to time the occurrence of FAT1 alterations, we observed evidence of positive selection for tumour subclones with FAT1 alterations before WGD. Indeed, FAT1 depletion attenuates HR and causes unresolved replication stress, both of which contribute to mitotic errors and micronucleus formation39. Using live-cell microscopy, we demonstrated that FAT1 depletion not only elevates structural CIN, such as mitotic bridges, but also increases subsequent nuclear shape deformation.
FAT1 has been proposed to be tumour suppressive and involved in various signalling pathways, including the CaMK2, WNT and Hippo signalling pathways48,49,50,62. Components of the Hippo pathway have been reported to impact various aspects of genome maintenance, such as mitotic control, stabilization of replication forks, and modulation of nuclear shape, which can affect three-dimensional genome organization56,64,65,66. We confirmed that depletion of LATS1 or LATS2 can significantly affect HR efficiency and contribute to CIN64. We discovered that FAT1—at least in part through its intracellular C-terminal domain—is involved in the DDR and represses CIN through the modulation of YAP1, whereas its interaction with YAP1 is dependent on the interaction between FAT1 and the E3 ligase MIB2 (ref. 53). Notably, despite being able to reconstitute HR-related functions, overexpression of the HA–FAT1ICD construct, devoid of the transmembrane domain, failed to re-repress TEAD transcriptional activity in FAT1-knockout cells. In line with previous reports that HRD results in mitotic defects and structural CIN39,55, FAT1/YAP1 co-depletion failed to rescue both the HRD-related phenotypes and the formation of chromosomal bridges, suggesting that FAT1 loss contributes to HRD and structural CIN in a YAP1-independent manner. It has been reported that FAT1 can interact with MST1 and LATS1 (ref. 49), which can influence HR efficiency through their interaction with ATR and RASSF1A to control BRCA2 recruitment at DNA damage sites64,67.
Conversely, FAT1/YAP1 co-depletion rescued both cytokinesis failure and WGD. Strikingly, overexpression of constitutively active mScarlet–YAP15SA was sufficient to drive WGD and increased numerical CIN. Taken together, our data reveal that FAT1 alterations may contribute to HRD, CIN and WGD through two distinct mechanisms (Extended Data Fig. 10h).
Multiple studies have suggested that WGD accelerates cancer evolution and promotes intratumour heterogeneity, which promotes targeted therapy resistance6,12,46,47. Here, using PC9 cells that are sensitive to osimertinib as an experimental model, we illustrate that FAT1 loss, through elevated genomic instability, may provide the evolutionary advantages that fuel targeted therapy resistance. Taken together, genomic observations and experimental results suggest a role for early positive selection of FAT1 alterations in LUSC tumorigenesis, potentially by driving cancer evolution and WGD through elevated CIN.
Recent studies have also identified that the YAP/TEAD signalling axis promotes therapy resistance68. In addition to FAT1, three other genes identified through our DDR and CIN screens—namely RAD21, BAP1 and NCOA6—have also been demonstrated to modulate the Hippo pathway69,70,71. Although YAP1 is not amplified in the TRACERx dataset, amplification of the WWTR1/TAZ genomic locus (3q25.1) and loss of the LATS2 genomic locus (13q12) are prevalent in LUSC3,51. Consistent with other studies61,68, our data highlight the importance of FAT1 and Hippo pathway dysregulation in genome instability, targeted therapy resistance and cancer evolution.
Methods
Ethical approval
The TRACERx study (NCT01888601; Clinicaltrials.gov) is sponsored by University College London (UCL/12/0279) and has been approved by an independent Research Ethics Committee (13/LO/1546). TRACERx is funded by Cancer Research UK (CRUK; C11496/A17786) and coordinated through the CRUK and University College London Cancer Trials Centre.
Cell line, cell culture, transfection and CRISPR knockout generation
H1944, H1650, H1792, HCC4006, A549 (CCL-185) and U2OS (HTB-96) cells were obtained from Cell Services at the Francis Crick Institute. The FUCCI–H2B-mTurquoise–RPE-1 cells have been described previously44 and were a kind gift from J. Diffley (Francis Crick Institute). The HA–ER–AsiSI–U2OS cells have been described previously28 and were a kind gift from G. Legube (Paul Sabatier University). The ER–KRAS–V12–T2P cells (referred to as T2P) have been described previously38 and were a kind gift from J. Downward (Francis Crick Institute). The TP53 wild-type and TP53-knockout RPE-1 cells have been described previously74. The HCT116 iRFP cell lines have been described previously75 and were a kind gift from K. Vousden (Francis Crick Institute). All of the cell lines were maintained in either McCoy’s 5A Medium, Dulbecco Modified Eagle′s Medium (DMEM) or RPMI 1640 Medium (all from Thermo Fisher Scientific) fortified with 10% foetal bovine serum in the presence of 1% penicillin–streptomycin (Thermo Fisher Scientific). Cells were grown at 37 °C under 5% CO2. All cell lines used were negative for Mycoplasma contamination and are frequently tested in-house at the Francis Crick Institute. DNA was transfected using GeneJuice (EMD Millipore) according to the manufacturer’s instructions.
CRISPR knockout cell lines were generated using a single-cell cloning approach. To minimize the off-target effect, CRISPR plasmids and guides were transiently transfected using GeneJuice (EMD Milipore) and underwent puromycin selection for 5 days before single-cell sorting to generate knockout clones. Single-cell clones were then cultured, validated and frozen. A549 clones were generated with two individual guides (sgFAT1#1 (AAACCCGGGAAGTCGAAGTCCTTGC) and sgFAT1#2 (AAACACGCTGGATGTGTAATGTAAC)), whereas PC9 clones were generated with both guides. The sgNT sequence as follows: GCGAGGTATTCGGCTCCGCG.
RNAi
A list of the siRNAs used is provided in Supplementary Table 1. Dharmacon ON-TARGETplus siRNA pools (Horizon Discovery) were used for high-content screening, HAC assay and RAD51 foci experiments in H1994 cells (Extended Data Figs. 1 and 2b,c and Supplementary Fig. 4). Ambion Silencer Select siRNA (Thermo Fisher Scientific) was used for all of the other RNAi experiments, except siRNA against MRE11A (L-009271-00-0005; Dharmacon; Fig. 8a). siRNA transfection was carried out using Lullaby reagent (OZ Biosciences) or Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific) according to the manufacturers’ instructions.
ssDNA resection and translocation assays
ssDNA resection assays were carried out as described previously28. Briefly, DIvA HA–ER–AsiSI–U2OS cells were plated overnight and siRNAs were transfected as described previously. Samples were subjected to a 4 h incubation in 300 nM 4-hydroxytamoxifen. Genomic DNA was extracted using the DNeasy Blood & Tissue kit (69504; Qiagen). For every 500 ng genomic DNA used, five units of RNase H1 (M0297; New England Biolabs) were added at 37 °C for 15 min. In vitro, restriction digestion with the BanI restriction enzyme was performed to assay for the presence of ssDNA around break sites. Then, 200 ng of samples were digested with 16 units of BanI at 37 °C for 12 h (New England Biolabs).
The following primers were used for the assay: 131-nucleotide forward (ACCATGAACGTGTTCCGAAT), 131-nucleotide reverse (GAGCTCCGCAAAGTTTCAA), 851-nucleotide forward (ACAGATCCAGAGCCACGAAA) and 851-nucleotide reverse (CCCACTCTCAGCCTTCTCAG),
The percentage of ssDNA generated by DNA resection was determined by quantitative PCR. ΔCT was defined as the difference in average cycles between a given digested sample and its undigested counterpart. To calculate the percentage of ssDNA, the following equation was used:
For the semi-quantitative DNA translocation assay40, 150 ng genomic DNA was used to perform the PCR reactions using the following primers: TRIM37 forward (AATTCGCAAACACCAACCGT), TRIM37 reverse (TCTGAAGTCTGCGCTTTCCA), MIS12 forward (GACTGGCATAAGCGTCTTCG), control chr1_82844750 forward (AGCACATGGGATTTTGCAGG) and control chr1_82844992 reverse (TTCCCTCCTTTGTGTCACCA).
Legitimate and illegitimate re-joining frequencies between MIS12 and TRIM37 (chr17_5390209 and chr17_57184285) were tested by PCR. The uncut site at chromosome 1 was used as a negative control.
Plasmid construction
A gene fragment containing the C-terminal intracellular domain of FAT1 transcript (NM_005245.4) was ordered from Integrated DNA Technologies. The gene fragment was subsequently subcloned into the pCMV-SPORT6 expression plasmid containing a His-HA tag at the amino (N) terminus of the open reading frame. To generate the mScarlet–HA–FAT1WT construct, gene fragments containing the mScarlet sequence and FAT1 sequences were ordered and ligated into the HA–FAT1ICD construct using NEBuilder HiFi DNA Assembly (New England Biolabs). Similarly, deletion of the MIB2 binding domain and generation of the HA–FAT1WT construct were achieved using NEBuilder HiFi DNA Assembly (New England Biolabs).
The TEAD transcriptional reporter comprises a multimerized TEAD-binding sequence (14xTBS), based on the sequence described by Schlegelmilch et al.76, upstream of a minimal promoter and a nuclear localization signal in frame with four copies of mNeonGreen followed by a Myc-tag. The sequence components were assembled by GeneArt (Thermo Fisher Scientific) in a pDONOR221 backbone. The final construct was obtained by cloning the insert into pLenti X1 Zeo DEST using Gateway technology (Thermo Fisher Scientific). The YAP5SA construct was obtained from Addgene (plasmid 27371)77 and subcloned into a pCMV-SPORT6 expression plasmid containing a His-HA tag at the N terminus of the open reading frame. The His-HA-eIF4A1 plasmid was a kind gift from M. Bushell (CRUK Scotland Institute) and was documented by Meijer et al.78.
All of the plasmids used in this study were then sequenced using Plasmidsaurus or Full Circle sequencing. The relevant sequences are included in Supplementary Information.
DNA fibre assay
DNA fibre assays were performed as described in ref. 55. A549 and PC9 cells were incubated with 5-chloro-2′-deoxyuridine for 20 min, challenged with replication stress induced by 2 mM hydroxyurea incubation for 2 h and then labelled with 5-iodo-2′-deoxyuridine for 20 min.
MCM7 loading assay
PC9 or RPE-1 cells were trypsinized and treated with modified CSK buffer (10 mM HEPES (pH 7.9), 100 mM NaCl, 3 mM MgCl2, 1 mM EGTA, 300 mM sucrose, 1% bovine serum albumin (BSA), 0.2% Triton X-100 and 1 mM dithiothreitol) on ice for 5 min to remove soluble proteins. Cells were washed with 1% BSA in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (Thermo Fisher Scientific) for 10 min. Cells were then washed, pelleted and permeabilized in 70% ethanol for 15 min. MCM7 staining was performed using a mouse anti-MCM7 antibody (sc-56324; Santa Cruz Biotechnology) followed by an Alexa Fluor 594 goat anti-mouse antibody (A11007; Invitrogen). DNA content was stained using 1 µg ml−1 4′,6-diamidino-2-phenylindole (DAPI) supplemented with 100 µg ml−1 RNase A. Cell doublets were excluded from all of the analyses.
Metaphase spreads
To assess numerical CIN, transfected cells were incubated with KaryoMAX Colcemid at 0.1 μg ml−1 for 1 h, collected and incubated for 7 min at 37 °C in pre-warmed hypotonic solution (75 mM KCl). Cells were pelleted and repeatedly resuspended in freshly prepared fixation buffer (3:1 methanol:glacial acetic acid) and spun three times, after which the cells were dropped onto glass slides. For hybridization, the slides were ethanol dehydrated and subsequently incubated overnight at 37 °C in a humidified chamber with All Human centromeric probes (KBI-20000G; Leica) according to the manufacturer’s instructions. Metaphase chromosome images were visualized using a 100× oil immersion objective on an Image Solutions DeltaVision microscope.
To assess chromosomal breakage and aberrations, metaphase spreads were prepared as previously described79. Briefly, 250,000 wild-type or FAT1-knockout cells were seeded in 6 cm dishes for one day before treatment with 0.2 µM aphidicolin for 24 h. Cells were treated with 0.2 µg ml−1 colcemid for 1 h at 37 °C before collection by trypsinization and incubation with 5 ml pre-warmed hypotonic swelling buffer (75 mM KCl) in 15 ml tubes at 37 °C for 10 min. 1 ml freshly prepared fixation buffer (3:1 methanol:acetic acid) was added to the 15 ml tube and mixed by inversion. Cells were pelleted by centrifugation at 300g for 4 min at room temperature. About 500 µl supernatant was left in the tubes and the cells were slowly resuspended before the addition of 5 ml fixation buffer, mixing and incubation for 10 min at room temperature. The fixation step was repeated by pelleting the cells, resuspension and the addition of fixation buffer, as above. To spread the metaphases, the fixed cells were dropped on glass slides at a 45° angle from a 30 cm height. Slides were left to air dry before staining with Giemsa (7% in 10 mM PIPES (pH 6.8)) for 10 min at room temperature, then mounted with DPX. Metaphase chromosome images were visualized using a 100× oil immersion objective on an upright Zeiss Axio Imager fluorescence microscope equipped with Volocity software.
ImageStream fluorescence in situ hybridization
Transfected cells were processed for fluorescence in situ hybridization in suspension, as described elsewhere80. Briefly, following transfection, cells were harvested and fixed with freshly prepared 3:1 methanol:glacial acetic acid. Cells were subsequently hybridized with chromosome 15 satellite enumeration probe (LPE015G; CytoCell) performed in a thermocycler under the following conditions: 65 °C (2 h pre-annealing), 80 °C (5 min) and 37 °C (16 h) before analysis on an ImageStream X Mark II (Amnis).
Definition of FAT1 driver mutation in the TRACERx 421 cohort
FAT1 driver mutations in the TRACERx 421 cohort were defined as described in ref. 81. Briefly, FAT1 non-synonymous variants that were found to be deleterious (either stop–gain or predicted deleterious in two of the three computational approaches applied (that is, Sift82, Polyphen83 and MutationTaster81)) were classified as a driver mutation. Any tumour with any such mutations in FAT1 was considered to have a FAT1 loss.
Determination of WGD events in TRACERx 421
The WGD status for each tumour was estimated in two steps, as described in ref. 81. Briefly, if the genome-wide copy number of the major allele was ≥2 across at least 50% of the genome, this was assumed to reflect a WGD event13,84. A major allele copy number ≥3 across at least 50% genome was assumed to reflect two WGD events. Then, we leveraged additional information from the estimated copy number of mutations using a novel tool, ParallelGDDetect, available as an R package (https://github.com/amf71/ParallelGDDetect).
TRACERx 421 cohort
The TRACERx 421 cohort consisted of 233 males and 188 females (421 patients in total), corresponding to a 55:45 male:female ratio. 93% of the cohort was from a White ethnic background and the mean age of the patients was 69 years, ranging between 34 and 92 years. Written informed consent was obtained. None of the patients were compensated for their involvement in the study.
Mutation clonality in TRACERx 421
Reconstructed phylogenetic trees were used to classify mutations based on their inferred phylogenetic cancer cell fraction (phyloCCF)81. We classified as clonal in each tumour region every cluster whose 95% confidence interval of the phyloCCF of its mutations overlapped with the 95% confidence interval of the phyloCCF of the mutations in the mutation cluster within the trunk node of the tree (a minimum threshold of 0.9 was used for the left side of the 95% confidence interval on truncal mutations). Second, we defined as subclonal every mutation cluster in a tumour region whose mean phyloCCF across the corresponding mutations in that region was greater than 0 and not clonal (that is, the mutation cluster did not pass the previous tests). Lastly, any remaining mutation cluster was defined as absent in a tumour region otherwise. Furthermore, clonal mutations were defined as early or late depending on whether they occurred before or after WGD. A clonal mutation copy number consistent with the WGD ploidy was therefore considered early, or late, otherwise.
dN/dS analyses
The dN/dS point mutation estimate for FAT1 was calculated using the dndscv and geneci functions in the dNdScv29 R package. dndscv was run on different subsets of mutations (clonal early versus clonal late) from different subsets of samples (LUAD versus LUSC). The 95% confidence interval of the dN/dS estimate was obtained using the geneci function.
Enrichment analyses
All enrichment analyses were performed by counting the tumours in each category (for example, LUAD versus LUSC, WGD versus no WGD or early clonal versus late clonal) and performing a chi-squared test.
SCNA detection and MSAI
The identification of genome-wide allele-specific copy number states for multiregion WES is described in ref. 81. Briefly, logR data were calculated using VarScan 2, GC corrected using a wave-pattern GC correction method developed by Cheng and colleagues85 and processed using ASCAT (version 2.3)86. Sequenza (version 2.1.2)87 was utilized to offer supplementary assessments of tumour purity and ploidy for further examination. ASCAT was presented with the automatically selected models for ploidy and purity from either Sequenza or ASCAT, which were manually reviewed to generate SCNA profiles for each tumour area. Samples that had an estimated tumour purity below 10% were excluded. The data on ploidy, purity and copy number segmentation were fed into a multi-sample SCNA estimation approach51 to create a consistently minimal segmentation and genome-wide evaluation of LOH, as well as loss, gain and amplification copy number states in relation to sample ploidy.
The input SCNA profiles were used to identify allelic imbalance, which was subsequently employed to phase heterozygous single-nucleotide polymorphisms and re-estimate allele-specific copy numbers. Furthermore, MSAI, which occurs when SCNAs disrupt the same genomic region but affect different parental alleles within distinct tumour subclones, was detected as previously described57. We identified a subset of these MSAI events as parallel SCNA events that refer to the same class of event (gain/amplification or loss/LOH) in multiple samples from a given tumour but with major alleles from distinct haplotypes in the samples demonstrating the event. We then quantified the proportion of tumours where MSAI events occurred per cytoband, providing an empirical P value for cytoband 4q35.2.
EdU incorporation assay and cyclin B loading assay
The Click-iT Plus EdU Alexa Fluor 647 Flow Cytometry Assay kit (Thermo Fisher Scientific) was used for EdU incorporation assays. Cells were incubated with 10 μM EdU for 30 min before being fixed and processed per the manufacturer’s instructions. Cyclin B1 antibody was obtained from Abcam (ab32053; Abcam). DNA was stained in staining buffer (1 µg ml−1 DAPI and 100 µg ml−1 RNase A in PBS). Cell doublets were excluded from all of the analyses.
Microscopy and DNA damage foci studies
DDR foci and DNA fibre assays
The preparation of slides for DDR immunofluorescence foci studies was carried out as described in ref. 88. Briefly, cells were pre-seeded on glass coverslips, subjected to Cs-137 ionizing irradiation and allowed to recover. Cells were then washed once with PBS and pre-extracted with CSK buffer (100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 10 mM PIPES (pH 6.8), 10 mM β-glycerol phosphate, 50 mM NaF, 1 mM EDTA, 1 mM EGTA, 5 mM Na3VO4 and 0.5% Triton X-100). Cells were then washed once with CSK buffer and fixed with 4% paraformaldehyde on ice for 20 min. Samples were then washed three times with 0.1% Tris-buffered saline (TBS)-Tween, blocked with 10% goat serum for 1 h, washed twice with 0.1% TBS-Tween and incubated with primary antibody overnight at 4 °C (see Supplementary Table 1). The samples were subsequently washed and incubated with secondary antibodies (Alexa Fluor 488/594/647; Invitrogen) with DAPI in 1% goat serum for 1 h at room temperature. Samples were then washed with 0.1% TBS-Tween and mounted with Vectashield (H1200; Vector Laboratories). The samples were then double-blinded for quantification.
Images were acquired using a Zeiss Axio Imager M1 microscope with a 40×/1.3 NA Plan-Neofluar or 63×/1.4 NA Plan-Apochromat objective, equipped with an ORCA-spark CMOS camera (Hamamatsu) and pE-300white LED light source (CoolLED) and controlled by Micro-Manager 2.0 software89. Images were processed and counted in an unbiased way using the FindFoci ImageJ plugin90.
Segregation errors
For segregation errors, cells were fixed and permeablized in PTEMF buffer (4% paraformaldehyde, 0.2% Triton X-100, 20 mM PIPES and 2 mM MgCl2) for 15 min at room temperature. The samples were washed three times in PBS, blocked for 1 h with 3% BSA with 0.2% Triton X-100 and incubated overnight with the relevant primary antibodies. The samples were subsequently washed and incubated with secondary antibodies (Alexa Fluor 488/594/647; Invitrogen) with DAPI in 1% goat serum for 1 h at room temperature. Samples were then washed with 0.1% TBS-Tween and mounted with Vectashield (H1200; Vector Laboratories). Images were acquired using a Nikon Ti2 microscope with a 60×/1.45 NA Plan Apo TIRF objective and 1.5× intermediate magnification, equipped with a Prime BSI sCMOS camera (Teledyne Photometrics), motorized XY stage with piezo Z axis (Applied Scientific Instrumentation) and Spectra X LED light engine (Lumencor) and controlled with Micro-Manager 2.0 software89. Z stacks were acquired (31 μm × 0.2 μm z steps) and deconvolved using Microvolution software with ten iterations.
Live-cell widefield microscopy
Live-cell widefield microscopy was performed using a Nikon Ti2 inverted microscope with a Perfect Focus System using 1.5× intermediate magnification, equipped with a Prime BSI sCMOS camera (Teledyne Photometrics), motorized XY stage with piezo Z axis (ASI) and Spectra X LED light engine (Lumencor) and controlled with Micro-Manager 2.0 software89. Environmental conditions were maintained at 37 °C under 5% CO2 using a chamber and CO2 mixer (Okolab).
For live-cell imaging of the mitotic failure rate, the FUCCI–H2B-mTurquoise–RPE-1 cells were grown and transfected for 72 h on glass-bottomed plates (IBL) in DMEM culture media, as described above. Before imaging, the growth media was replaced with FluoroBrite DMEM (Thermo Fisher Scientific) with 10% foetal bovine serum to improve the image quality. To acclimatize to potential temperature fluctuations, the cells were allowed to settle for 1 h before imaging. Phase-contrast and fluorescence images were acquired with 20×/0.75 NA Plan Apo Ph2 objective every 20 min for over 72 h. The images were analysed using Fiji and the cells were tracked manually.
For the mitotic timing experiments, FUCCI–H2B-mTurquoise–RPE-1 cells were set up as above but imaged with a 40×/0.95 NA Plan Apo objective. Locations with high-level mVenus–geminin-expressing G2 cells were identified and recorded. Z stacks (25 μm × 0.5 μm z steps) of H2B-mTurquoise were taken at 5 min intervals over 12 h. Images were deconvolved over ten iterations using Microvolution software.
Live-cell confocal microscopy
Live-cell confocal microscopy was performed using either: (1) a Yokogawa CSU-W1 spinning-disk confocal scanhead on a Nikon Ti2 inverted microscope with a 40×/1.15 NA Apo objective and Prime 95B sCMOS camera (Teledyne Photometrics), controlled with NIS-Elements, at 37 °C under 5% CO2 with an environmental chamber and gas mixer (Okolab); or (2) an NL5 slit-scanning confocal unit on a Nikon Eclipse Ti inverted microscope with a 40×/1.25 NA Apo objective and Quest qCMOS camera (Hamamatsu), controlled with Micro-Manager 2.0 software89 at 37 °C under 5% CO2 using a stage top incubator and gas mixer (Tokai Hit). Locations with high-level mVenus–geminin-expressing G2 cells were first identified and recorded. Z stacks (25 μm × 0.5 μm z steps) of H2B-mTurquoise were taken at 5 min intervals over 12 h.
For Extended Data Fig. 7h,i and Supplementary Videos 4 and 5, a higher resolution was required to delineate whether mitotic error precedes nuclear deformation. A mixture of widefield deconvolution microscopy (n = 28 cells (siCTRL) and n = 27 cells (siFAT1)) and confocal microscopy (n = 50 cells (siCTRL) and n = 27 cells (siFAT1)) techniques were utilized to obtain sufficient images for statistical analysis.
Statistics and reproducibility
No statistical methods were used to predetermine the sample size. The statistical test types and biological n numbers used are detailed in the relevant figure captions. For immunofluorescence data, the numbers of cells sampled per biological repeat are documented accordingly. All of the data points shown are independent samples. For the experiments using statistical tests that assume normal distributions, we assumed that the data distribution was normal, but this was not formally tested.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA-seq, WES and reduced representation bisulfite sequencing (RRBS) data (in each case from the TRACERx study) used during this study have been deposited in the European Genome-phenome Archive, which is hosted by the European Bioinformatics Institute and Centre for Genomic Regulation, under the accession codes EGAS00001006517 (RNA-seq), EGAS00001006494 (WES) and EGAS00001006523 (RRBS). Access is controlled by the TRACERx data access committee. The Genomics England lung cohort is part of the 100,000 Genomes Project whose data are held in a secure research environment and are only available to registered users. For further information on how to obtain access, visit https://www.genomicsengland.co.uk/research/academic. Source data are provided with this paper.
Code availability
The code used to determine genome doubling is available at https://github.com/amf71/ParallelGDDetect. The code used to process data and generate figures is available at https://doi.org/10.5281/zenodo.13884283 (ref. 91).
References
Steele, C. D. et al. Signatures of copy number alterations in human cancer. Nature 606, 984–991 (2022).
Drews, R. M. et al. A pan-cancer compendium of chromosomal instability. Nature 606, 976–983 (2022).
Jamal-Hanjani, M. et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 376, 2109–2121 (2017).
Bakhoum, S. F. & Cantley, L. C. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell 174, 1347–1360 (2018).
Gemble, S. et al. Genetic instability from a single S phase after whole-genome duplication. Nature 604, 146–151 (2022).
Dewhurst, S. M. et al. Tolerance of whole-genome doubling propagates chromosomal instability and accelerates cancer genome evolution. Cancer Discov. 4, 175–185 (2014).
Lopez, S. et al. Interplay between whole-genome doubling and the accumulation of deleterious alterations in cancer evolution. Nat. Genet. 52, 283–293 (2020).
Watson, E. V. et al. Chromosome evolution screens recapitulate tissue-specific tumor aneuploidy patterns. Nat. Genet. 56, 900–912 (2024).
Bollen, Y. et al. Reconstructing single-cell karyotype alterations in colorectal cancer identifies punctuated and gradual diversification patterns. Nat. Genet. 53, 1187–1195 (2021).
Sottoriva, A. et al. A Big Bang model of human colorectal tumor growth. Nat. Genet. 47, 209–216 (2015).
Zack, T. I. et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140 (2013).
Hobor, S. et al. Mixed responses to targeted therapy driven by chromosomal instability through p53 dysfunction and genome doubling. Nat. Commun. 15, 4871 (2024).
Bielski, C. M. et al. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat. Genet. 50, 1189–1195 (2018).
Dharanipragada, P. et al. Blocking genomic instability prevents acquired resistance to MAPK inhibitor therapy in melanoma. Cancer Discov. 13, 880–909 (2023).
Isozaki, H. et al. Therapy-induced APOBEC3A drives evolution of persistent cancer cells. Nature 620, 393–401 (2023).
Ippolito, M. R. et al. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev. Cell 56, 2440–2454.e6 (2021).
Hawley, B. R., Lu, W. T., Wilczynska, A. & Bushell, M. The emerging role of RNAs in DNA damage repair. Cell Death Differ. 24, 580–587 (2017).
Brownlee, P. M., Meisenberg, C. & Downs, J. A. The SWI/SNF chromatin remodelling complex: its role in maintaining genome stability and preventing tumourigenesis. DNA Repair (Amst.) 32, 127–133 (2015).
Arnould, C. et al. Loop extrusion as a mechanism for formation of DNA damage repair foci. Nature 590, 660–665 (2021).
Lee, H. S. et al. A new assay for measuring chromosome instability (CIN) and identification of drugs that elevate CIN in cancer cells. BMC Cancer 13, 252 (2013).
Schiavoni, F. et al. Aneuploidy tolerance caused by BRG1 loss allows chromosome gains and recovery of fitness. Nat. Commun. 13, 1731 (2022).
Ciccia, A. & Elledge, S. J. The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204 (2010).
Aguilera, P. & Lopez-Contreras, A. J. ATRX, a guardian of chromatin. Trends Genet. 39, 505–519 (2023).
Francia, S. et al. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature 488, 231–235 (2012).
Davo-Martinez, C. et al. Different SWI/SNF complexes coordinately promote R-loop- and RAD52-dependent transcription-coupled homologous recombination. Nucleic Acids Res. 51, 9055–9074 (2023).
Rondinelli, B. et al. H3K4me3 demethylation by the histone demethylase KDM5C/JARID1C promotes DNA replication origin firing. Nucleic Acids Res. 43, 2560–2574 (2015).
Seluanov, A., Mao, Z. & Gorbunova, V. Analysis of DNA double-strand break (DSB) repair in mammalian cells. J. Vis. Exp. 2010, 2002 (2010).
Iacovoni, J. S. et al. High-resolution profiling of γH2AX around DNA double strand breaks in the mammalian genome. EMBO J. 29, 1446–1457 (2010).
Martincorena, I. et al. Universal patterns of selection in cancer and somatic tissues. Cell 171, 1029–1041.e21 (2017).
Sadeqzadeh, E. et al. Dual processing of FAT1 cadherin protein by human melanoma cells generates distinct protein products. J. Biol. Chem. 286, 28181–28191 (2011).
Birkbak, N. J. et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2, 366–375 (2012).
Popova, T. et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 72, 5454–5462 (2012).
Abkevich, V. et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br. J. Cancer 107, 1776–1782 (2012).
Bennardo, N., Cheng, A., Huang, N. & Stark, J. M. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 4, e1000110 (2008).
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Lukas, C. et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat. Cell Biol. 13, 243–253 (2011).
Feng, W. & Jasin, M. BRCA2 suppresses replication stress-induced mitotic and G1 abnormalities through homologous recombination. Nat. Commun. 8, 525 (2017).
Venkatesan, S. et al. Induction of APOBEC3 exacerbates DNA replication stress and chromosomal instability in early breast and lung cancer evolution. Cancer Discov. 11, 2456–2473 (2021).
Di Bona, M. & Bakhoum, S. F. Micronuclei and cancer. Cancer Discov. 14, 214–226 (2024).
Cohen, S. et al. Senataxin resolves RNA:DNA hybrids forming at DNA double-strand breaks to prevent translocations. Nat. Commun. 9, 533 (2018).
Sotillo, R. et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 11, 9–23 (2007).
Shi, Q. & King, R. W. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature 437, 1038–1042 (2005).
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864–870 (2005).
Zeng, J., Hills, S. A., Ozono, E. & Diffley, J. F. X. Cyclin E-induced replicative stress drives p53-dependent whole-genome duplication. Cell 186, 582–542.e14 (2023).
Misale, S., Di Nicolantonio, F., Sartore-Bianchi, A., Siena, S. & Bardelli, A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 4, 1269–1280 (2014).
Lukow, D. A. et al. Chromosomal instability accelerates the evolution of resistance to anti-cancer therapies. Dev. Cell 56, 2427–2439.e4 (2021).
Hata, A. N. et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat. Med. 22, 262–269 (2016).
Pastushenko, I. et al. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature 589, 448–455 (2021).
Martin, D. et al. Assembly and activation of the Hippo signalome by FAT1 tumor suppressor. Nat. Commun. 9, 2372 (2018).
Li, Z. et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell 34, 893–905.e8 (2018).
Watkins, T. B. K. et al. Pervasive chromosomal instability and karyotype order in tumour evolution. Nature 587, 126–132 (2020).
Zhao, B., Li, L., Tumaneng, K., Wang, C. Y. & Guan, K. L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFβ-TRCP. Genes Dev. 24, 72–85 (2010).
Li, R. et al. Endothelial FAT1 inhibits angiogenesis by controlling YAP/TAZ protein degradation via E3 ligase MIB2. Nat. Commun. 14, 1980 (2023).
Hao, Y., Chun, A., Cheung, K., Rashidi, B. & Yang, X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 283, 5496–5509 (2008).
Chan, Y. W., Fugger, K. & West, S. C. Unresolved recombination intermediates lead to ultra-fine anaphase bridges, chromosome breaks and aberrations. Nat. Cell Biol. 20, 92–103 (2018).
Ganem, N. J. et al. Cytokinesis failure triggers Hippo tumor suppressor pathway activation. Cell 158, 833–848 (2014).
Yu, H. et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc. Natl Acad. Sci. USA 111, 285–290 (2014).
Su, X. A. et al. RAD21 is a driver of chromosome 8 gain in Ewing sarcoma to mitigate replication stress. Genes Dev. 35, 556–572 (2021).
Zhang, T., Cronshaw, J., Kanu, N., Snijders, A. P. & Behrens, A. UBR5-mediated ubiquitination of ATMIN is required for ionizing radiation-induced ATM signaling and function. Proc. Natl Acad. Sci. USA 111, 12091–12096 (2014).
Martincorena, I. et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886 (2015).
Webster, A. L. H. et al. Genomic signature of Fanconi anaemia DNA repair pathway deficiency in cancer. Nature 612, 495–502 (2022).
Morris, L. G. et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat. Genet. 45, 253–261 (2013).
Martinez-Jimenez, F. et al. A compendium of mutational cancer driver genes. Nat. Rev. Cancer 20, 555–572 (2020).
Pefani, D. E. et al. RASSF1A–LATS1 signalling stabilizes replication forks by restricting CDK2-mediated phosphorylation of BRCA2. Nat. Cell Biol. 16, 962–971 (2014). 961–968.
Karoutas, A. & Akhtar, A. Functional mechanisms and abnormalities of the nuclear lamina. Nat. Cell Biol. 23, 116–126 (2021).
Nader, G. P. F. et al. Compromised nuclear envelope integrity drives TREX1-dependent DNA damage and tumor cell invasion. Cell 184, 5230–5246.e22 (2021).
Esashi, F. et al. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 434, 598–604 (2005).
Pfeifer, M. et al. Genome-wide CRISPR screens identify the YAP/TEAD axis as a driver of persister cells in EGFR mutant lung cancer. Commun. Biol. 7, 497 (2024).
Deng, P. et al. RAD21 amplification epigenetically suppresses interferon signaling to promote immune evasion in ovarian cancer. J. Clin. Invest. 132, e159628 (2022).
Lee, H. J. et al. The tumor suppressor BAP1 regulates the Hippo pathway in pancreatic ductal adenocarcinoma. Cancer Res. 80, 1656–1668 (2020).
Oh, H. et al. Yorkie promotes transcription by recruiting a histone methyltransferase complex. Cell Rep. 8, 449–459 (2014).
Durkin, S. G. & Glover, T. W. Chromosome fragile sites. Annu. Rev. Genet. 41, 169–192 (2007).
Kumar, R. et al. HumCFS: a database of fragile sites in human chromosomes. BMC Genomics 19, 985 (2019).
Sansregret, L. et al, APC/C Dysfunction limits excessive cancer chromosomal instability. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-16-0645 (2017).
Hock, A. K. et al. iRFP is a sensitive marker for cell number and tumor growth in high-throughput systems. Cell Cycle 13, 220–226 (2014).
Schlegelmilch, K. et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795 (2011).
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. https://doi.org/10.1101/gad.1602907 (2007).
Meijer, H. A. et al. Translational repression and eIF4A2 activity are critical for microRNA-mediated gene regulation. Science 340, 82–85 (2013).
Benitez, A. et al. GEN1 promotes common fragile site expression. Cell Rep. 42, 112062 (2023).
Maguire, O., Wallace, P. K. & Minderman, H. in Imaging Flow Cytometry. Methods in Molecular Biology (eds Barteneva, N. & Vorobjev, I.) Vol. 1389 (Humana Press, 2016).
Frankell, A. M. et al. The evolution of lung cancer and impact of subclonal selection in TRACERx. Nature 616, 525–533 (2023).
Kumar, P., Henikoff, S. & Ng, P. C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081 (2009).
Adzhubei, I., Jordan, D. M. & Sunyaev, S. R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet Chapter 7, Unit7.20 (2013).
Carter, S. L. et al. Absolute quantification of somatic DNA alterations in human cancer. Nat. Biotechnol. 30, 413–421 (2012).
Cheng, J. et al. Single-cell copy number variation detection. Genome Biol. 12, R80 (2011).
Van Loo, P. et al. Allele-specific copy number analysis of tumors. Proc. Natl Acad. Sci. USA 107, 16910–16915 (2010).
Favero, F. et al. Sequenza: allele-specific copy number and mutation profiles from tumor sequencing data. Ann. Oncol. 26, 64–70 (2015).
Britton, S., Coates, J. & Jackson, S. P. A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J. Cell Biol. 202, 579–595 (2013).
Edelstein, A. D. et al. Advanced methods of microscope control using μManager software. J. Biol. Methods 1, e10 (2014).
Herbert, A. D., Carr, A. M. & Hoffmann, E. FindFoci: a focus detection algorithm with automated parameter training that closely matches human assignments, reduces human inconsistencies and increases speed of analysis. PLoS ONE 9, e114749 (2014).
Lu, W.-T. Data and code for “TRACERx analysis identifies a role for FAT1 in regulating chromsomal instability and whole-genome doubling via Hippo signaling”. Zenodo https://doi.org/10.5281/zenodo.13884283 (2024).
Acknowledgements
C.M.-R. is supported by the Rosetrees Trust (M630) and Wellcome Trust. K.L. is funded by the UK Medical Research Council (MR/V033077/1), Rosetrees Trust and Cotswold Trust (A2437), Royal Marsden Cancer Charity (thanks to the Ross Russell Family and Macfarlanes donations), Melanoma Research Alliance and CRUK (C69256/A30194). M.J.-H. received the CRUK Career Establishment Award, as well as funding from CRUK, the International Association for the Study of Lung Cancer International Lung Cancer Foundation, the Lung Cancer Research Foundation, the Rosetrees Trust, the UK and Ireland Neuroendocrine Tumour Society, the National Institute for Health Research (NIHR) and the NIHR University College London Hospitals Biomedical Research Centre. N.J.B. is supported by the Lundbeck Foundation (R272-2017-4040), Aarhus University Research Foundation (AUFF-E-2018-7-14) and Novo Nordisk Foundation (NNF21OC0071483 and NNF23OC0085954). L.-P.Z., T. A.W. and E.G. received support from the ERC Consolidator Grant THESEUS (grant agreement no. 617844). E.G. was further funded by the ERC Advanced Grant PROTEUS (grant agreement no. 835297). N.M. is funded by the Wellcome Trust and Royal Society (grant number 211179/Z/18/Z), and also receives funding from CRUK (DRCPFA-Nov23/100003), the Rosetrees Trust, the NIHR Biomedical Research Centre at University College London Hospitals and the CRUK-funded University College London Experimental Cancer Medicine Centre. W.-T.L. is grateful for support from the Kuok Foundation. N.K. is supported by the Breast Cancer Research Foundation (BCRF 23-157), Rosetrees Trust and CRUK. J.B. is funded by the Danish Cancer Society (R322-A17482) and Novo Nordisk Foundation (NNF20OC0060590) and is grateful for the support. C.S. is a Royal Society Napier Research Professor (RSRP\R\210001). His work is supported by the Francis Crick Institute, which receives core funding from CRUK (CC2041), the UK Medical Research Council (CC2041) and the Wellcome Trust (CC2041). For the purpose of open access, C.S. has applied a CC BY public copyright licence to any author accepted manuscript version arising from this submission. C.S. is funded by CRUK (TRACERx (C11496/A17786), PEACE (C416/A21999) and the CRUK Cancer Immunotherapy Catalyst Network), the CRUK Lung Cancer Centre of Excellence (C11496/A30025), the Rosetrees Trust, the Butterfield and Stoneygate Trusts, the Novo Nordisk Foundation (ID16584), the Royal Society Professorship Enhancement Award (RP/EA/180007 and RF\ERE\231118), the NIHR University College London Hospitals Biomedical Research Centre, the CRUK–University College London Centre, the Experimental Cancer Medicine Centre, the US Breast Cancer Research Foundation (BCRF-22-157), the CRUK Early Detection and Diagnosis Primer Award (grant EDDPMA-Nov21/100034), the Mark Foundation for Cancer Research ASPIRE Award (grant 21-029-ASP) and the ASPIRE Phase II award (grant 23-034-ASP). C.S. is in receipt of an ERC Advanced Grant (PROTEUS) from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement number 835297). The TRACERx study (NCT01888601; Clinicaltrials.gov) is sponsored by University College London (UCL/12/0279) and has been approved by an independent Research Ethics Committee (13/LO/1546). TRACERx is funded by CRUK (C11496/A17786) and coordinated through the CRUK and University College London Cancer Trials Centre, which has a core grant from CRUK (C444/A15953). We gratefully acknowledge the patients and relatives who participated in the TRACERx study. This research was made possible through access to data in the National Genomic Research Library, which is managed by Genomics England (a wholly owned company of the Department of Health and Social Care). The National Genomic Research Library holds data provided by patients and collected by the National Health Service (NHS) as part of their care, as well as data collected as part of their participation in research. The National Genomic Research Library is funded by the National Institute for Health Research and NHS England. The Wellcome Trust, CRUK and the Medical Research Council are involved in the research infrastructure of Genomic England. We thank G. Legube (Paul Sabatier University), J. Diffley (Francis Crick Institute), J. Downward (Francis Crick Institute) and K. Vousden (Francis Crick Institute) for providing materials. We thank J. Zeng, A. Bertolin, J. Diffley, T. Klockner, U. Baruchel (Francis Crick Institute), S.F. Bakhoum (Memorial Sloan Kettering Cancer Center) and the members of the C.S. laboratory for discussion and insights. We thank the scientific platforms in the Francis Crick Institute—particularly Advance Light Microscopy, Cell Services, Flow Cytometry and High Throughput Screening—for support. We are grateful for assistance from the Flow Cytometry unit of the CRUK City of London Centre at the Barts Cancer Insitute and University College London Cancer Institute. This work was supported by the Francis Crick Institute, which receives core funding from CRUK (CC2041), the UK Medical Research Council (CC2041) and the Wellcome Trust (CC2041). This work was also supported by the CRUK Lung Cancer Centre of Excellence and the CRUK City of London Centre Award (C7893/A26233).
Funding
Open Access funding provided by The Francis Crick Institute.
Author information
Authors and Affiliations
Consortia
Contributions
W.-T.L., N.K., L.-P.Z. and C.S. conceived of the project concept. L.-P.Z. and M.H. designed the high-throughput screening experiments. L.-P.Z., M.J. and M.H. executed the high-throughput screening experiments. L.-P.Z., H.X., R.E.S. and M.H. analysed the high-throughput screening experiments. C.B., J.R.M.B., C.M.-R., O.P., F.G.-V., I.U., K.T., Y.-H.L., D.B., T.K., M.A.B., D.S.-L., N.S., K.L., N.J.B. and N.M. performed the genomics and statistical analyses. A.M. and M.R. contributed to the imaging analysis and imaging method development. L.T. and S.E.M. developed the ImageStream fluorescence in situ hybridization methods. W.-T.L., L.-P.Z., T.A.W., E.I., B.A., S.V., G.S., K.F. and N.K. carried out the experiments and developed the methods. L.-P.Z., E.G., S.E.M., K.L., N.J.B., N.T., K.F. and N.M. provided feedback on the experimental design and data analyses. M.J.-H. designed the PEACE and TRACERx study protocols. L.-P.Z., E.G., M.J.-H., S.E.M., N.T., K.F. and N.M. provided feedback on the manuscript. W.-T.L. and N.K. jointly coordinated and designed the experiments, performed the experiment analyses and wrote the manuscript. J.B. and C.S. provided strategic oversight and helped to write the manuscript. L.-P.Z., T. A.W. and E.G. received support from the ERC Consolidator Grant THESEUS (grant agreement no. 617844). E.G. was further funded by the ERC Advanced Grant PROTEUS, (grant agreement no. 835297).
Corresponding authors
Ethics declarations
Competing interests
D.B. reports personal fees from NanoString and AstraZeneca and has a patent (PCT/GB2020/050221) issued on methods for cancer prognostication. M.A.B. has consulted for Achilles Therapeutics. M.J.-H. has received funding from CRUK, the National Institutes of Health National Cancer Institute, the International Association for the Study of Lung Cancer International Lung Cancer Foundation, the Lung Cancer Research Foundation, the Rosetrees Trust, the UK and Ireland Neuroendocrine Tumour Society and the NIHR. M.J.-H. has consulted for, and is a member of, the Achilles Therapeutics Scientific Advisory Board (SAB) and Steering Committee, has received speaker honoraria from Pfizer, Astex Pharmaceuticals, Oslo Cancer Cluster, Bristol Myers Squibb and Genentech. M.J.-H. is listed as a co-inventor on a European patent application relating to methods for the detection of lung cancer (PCT/US2017/028013). This patent has been licensed to commercial entities and, under the terms of their employment, M.J.-H. is due a share of any revenue generated from such license(s). M.J.-H. is also listed as a co-inventor on a GB priority patent application (GB2400424.4) with title ‘Treatment and prevention of lung cancer’. K.L. has a patent pending on indel burden and checkpoint inhibitor response, has received speaker fees from Roche Tissue Diagnostics and research funding from the CRUK Therapeutic Discovery Laboratories–Ono Pharmaceutical–LifeArc alliance and Genesis Therapeutics and has held consulting roles with Ellipses Pharma, Monopteros and Kynos Therapeutics. N.J.B. is listed as a co-inventor on a patent related to the identification of responders to cancer treatment (PCT/GB2018/051912), has submitted a patent application (PCT/GB2020/050221) on methods for cancer prognostication and has a patent on methods for predicting anti-cancer response (US14/466,208). N.M. has stock options in, and has consulted for, Achilles Therapeutics and holds a European patent in determining human leukocyte antigen (HLA) LOH (PCT/GB2018/052004), has a patent pending on determining HLA disruption (PCT/EP2023/059039) and is a co-inventor on a patent on the identification of responders to cancer treatment (PCT/GB2018/051912). N.K. acknowledges grants from AstraZeneca. C.S. acknowledges grants from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Pfizer, Roche VENTANA, Invitae (previously ArcherDX; a collaboration on minimal residual disease sequencing technologies), Ono Pharmaceutical and Personalis. He is Chief Investigator for the AZ MeRmaiD-1 and -2 clinical trials and is the Steering Committee Chair. He is also Co-Chief Investigator of the NHS Galleri Trial funded by GRAIL and a paid member of GRAIL’s SAB. He receives consultant fees from Achilles Therapeutics (and is also a SAB member), Bicycle Therapeutics (and is also a SAB member), Genentech, Medicxi, the China Innovation Centre of Roche (formerly the Roche Innovation Centre – Shanghai); Metabomed, until July 2022, Relay Therapeutics (and is also a SAB member), SAGA Diagnostics (and is also a SAB member) and the Sarah Cannon Research Institute. C.S. has received honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Illumina, MSD, Novartis, Pfizer and Roche VENTANA. C.S. has previously held stock options in ApoGen Biotechnologies and GRAIL and currently has stock options in EPIC Bioscience, Bicycle Therapeutics, Relay Therapeutics and Achilles Therapeutics. C.S. is also a co-founder of Achilles Therapeutics. C.S. declares patents and patent applications relating to methods for the detection of lung cancer (PCT/US2017/028013), neoantigen targeting (PCT/EP2016/059401), the identification of patent response to immune checkpoint blockade (PCT/EP2016/071471), the identification of patients who respond to cancer treatment (PCT/GB2018/051912), the determination of HLA LOH (PCT/GB2018/052004), the prediction of survival rates of patients with cancer (PCT/GB2020/050221) and methods and systems for tumour monitoring (PCT/EP2022/077987). C.S. is an inventor on a European patent application (PCT/GB2017/053289) relating to assay technology to detect tumour recurrence. This patent has been licensed to a commercial entity and, under the terms of their employment, C.S. is due a share of any revenue generated from such license(s). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Floris Foijer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Summary of the DDR and CIN loss-of-function screen of genome-doubling-associated drivers from the TRACERx 100 cohort.
a, Unsupervised clustering of the results from the DDR screen of 37 TRACERx drivers in 4 LUAD cell lines. Each row represents a DDR foci readout under a given genotoxic stress. Random forest classifiers were used to identify genes that had feature scores similar to positive controls; known DDR genes such as ATR, CHEK1, RAD51 and TP53BP1 are highlighted. Hierarchical clustering was used to calculate the Euclidean distance between TRACERx drivers and the known DDR positive control genes. b, HAC assay was used to assess the impact of gene depletion and mild replication stress on CIN, through modelling chromosome loss using an HT-1080 reporter system. Cells were processed and data was analysed as described in Methods. Data are displayed as scatter plots of log fold changes (LFCs) and Strictly Standardized Mean Difference (SSMDs), highlighting negative and positive control genes, and genes that passed the hit threshold (green area).
Extended Data Fig. 2 Depletion of several TRACERx driver genes reduces RAD51 foci formation post-ionizing radiation.
a-b, Box plots quantifying RAD51 foci in the presence and absence of 6Gy IR in A549 (A) and H1944 (B) cells. Cells were irradiated and allowed 1h recovery. The boxes represent the interquartile range, horizontal lines represent the median and the whiskers range denotes 1.5 x interquartile ranges, n=3 biological replicates, >150 cells quantified per biological replicate, Kruskal-Wallis test with Dunn’s multiple comparison tests. c, Histograms representing cell cycle profiles following siRNA knockdown of 6 candidate DDR+CIN genes in A549 (left) and H1994 (right) cells, using 2 sets of different siRNAs, n=3 biological repeats, 2-way ANOVA with Sidak corrections. d, Driver mutation distribution of FAT1, and HR-related genes (ATM-CHEK2, ATR-CHEK1, and members of FA/BRCA pathway) in the TRACERx 421 cohort.
Extended Data Fig. 3 FAT1 mutation, copy number loss and hypermethylation are enriched in the TRACERx421 cohort.
a, FAT1 driver mutations are enriched in LUSC tumours in the TRACERx421 LUSC cohort, and mutations are timed early in tumor evolution. b, Graph showing the frequency of FAT1 SCNA loss in the TRACERx 421 cohort. c, GISTIC analysis demonstrating FAT1 genomic loci (4q35.2) SCNA loss is positively selected in both the LUAD and LUSC TRACERx cohort. d, Summary of GISTIC score analysis of LUAD and LUSC TRACERx tumors from Fig. 2b segregated by WGD status. Patients with no WGD and subclonal WGD are included. SCNA loci overlapping with common or rare chromosome fragile sites90,91 were annotated (common fragile sites=blue, rare fragile sites=green). e, FAT1 promoter hypermethylation is enriched in TRACERx LUSC tumors. Notably, FAT1 promoter hypermethylation also co-occurs with FAT1 SCNA loss. f-g, Correlation between FAT1 promoter hypermethylation and expression levels in LUAD (f) and LUSC tumors (g).
Extended Data Fig. 4 FAT1 CRISPR KO cells show reduced RAD51 foci formation post-ionizing.
a, Impact of FAT1 on RAD51/γH2A.X foci formation in polyclonal FAT1 wild-type or CRISPR knockout H1944 cells following 6Gy IR. Cells were irradiated and allowed 1 h to recover. Left, quantification showing RAD51 foci in the presence of 6Gy IR. Red-line = mean. The boxes represent the interquartile range, the lines represent the median and the whiskers range denote 1.5 x interquartile ranges, 2-sided Mann-Whitney test, n=3 biological replicates. Right, representative figures illustrating RAD51/γH2A.X foci following 6Gy IR. Bar = 10 μM. Polyclonal FAT1 CRISPR knockout cells were irradiated and allowed 1h recovery. b, Left, representative figures illustrating RAD51 foci in the presence of 6Gy IR. Scale bar =10 µm. Right: box plots showing FAT1 CRISPR knockout A549 cells displayed a reduction in RAD51 foci formation. The boxes represent the interquartile range, the lines represent the median and the whiskers range denotes 1.5 x interquartile ranges, Dunn’s test, n=3 biological replicates. c, Quantification of 53BP1 foci at various time points post 6h IR irradiation in A549 cells. n=2 biological repeats, over 300 cells were quantified in each condition per time point. Solid line = median, dotted lines = quartile ranges, 2-sided Mann-Whitney tests were carried out between two conditions within the same time point. d, Representative western blot showing cellular fractionation of control and FAT1 CRISPR KO A549 cells. Localization of full-length FAT1 protein and C-terminal isoforms were visualized using a FAT1 antibody targeting the C-terminal of the FAT1 protein. Note the loss of FAT1 full-length and C-terminal (p65) signal in FAT1 KO cells. e, A549 FAT1 KO cells were transfected with the HA-FAT1ICD construct used in Fig. 3b,c. Top, representative western blot showing the level of endogenous FAT1 and HA-FAT1ICD construct in A549 cells following 6Gy IR. Levels of KAP1/TRIM28 phosphorylation, γH2A.X, and ubiquitination of γH2A.X are not affected. Bottom, representative figures illustrating RAD51 foci formation in the presence of 6Gy IR, with or without overexpression of HA-FAT1ICD. Scale bar =10 µm. HD, homozygous deletions; LOH, loss of heterozygosity.
Extended Data Fig. 5 FAT1 KO A549 cells display mild sensitivity towards hydroxyurea, cisplatin and Olaparib.
a, Comparison of growth rate trajectories of CTRL, FAT1 and BRCA1 knockdown in HCT116-iRFP cells75 in the presence and absence of Olaparib. b, Representative images and quantification of clonogenic assays (left: hydroxyurea, replication stress inducer; middle: Olaparib, PARP inhibitor; right: cisplatin, DNA crosslinker). c-d, Genomics England mutational signature analysis showing the signature proportion (left) and signature count (right) of the mutational profile of COSMIC ID6 (c) and SBS3 (d) mutational signatures, both dependent on indels as a result of end-joining activity.
Extended Data Fig. 6 FAT1 loss results in chromosomal instability in lung cancer cells.
a, Transient FAT1 siRNA knockdown induces the formation of 53BP1 nuclear bodies in T2P cells. The boxes represent the interquartile range, the lines represent the median and the whiskers range denotes 1.5 x interquartile ranges, Dunn’s test, red bar = mean, n=3 biological replicates. Scale bar =10 µm. b, Transient FAT1 siRNA significantly increases the number of lagging chromosomes per cell after replication stress (left, centromeric; right, acentric), Dunn’s test. Over 100 mitotic cells were scored across 3 biological replicates. Red bar = mean. c, Representative metaphase spreads of FAT1 WT and KO A549 cells after 24 hours of aphidicolin treatment. Scale bar = 5µM d, Metaphase chromosome number following transient FAT1 knockdown in H1944 cells. A significant increase in metaphase chromosome number is observed in H1944 cells following FAT1 knockdown. N=3 biological repeats, red bar = mean. 2-sided Welch’s T-test. e, Mitotic error rate in PC9 cells following transient FAT1 knockdown in PC9 cells. one-way ANOVA, N=4 biological repeats, mean±SEM. f, Western blot showing FAT1 knockdown efficiency in PC9 cells. FANCD2 monoubiquitylation and γH2A.X, or expression level of E2F7 are not significantly affected by FAT1 ablation. g, FAT1 knockdown leads to more replication fork stalling in genome-doubled PC9 cells. >600 forks were counted across 3 biological repeats, 2-sided Paired T-test, scale bar =20 µM. h–j, FAT1 knockdown significantly reduces interphase FANCD2 foci (h, histogram) and mitotic FANCD2 foci (i and j, histogram and representative image, respectively) despite the increased rate of fork collapse. The boxes represent the interquartile range, black horiziontal bar represent the median and the whiskers range denotes 1.5 x interquartile ranges, red line= mean, N=3 biological replicates, >1200 interphase and >120 mitotic cells scored per condition, Dunn’s test.
Extended Data Fig. 7 FAT1 loss promotes WGD and multinucleation.
a, Proportion of FAT1 driver mutations in genome-doubled TRACERx421 tumors. Fisher’s Exact Test, p=0.179. b, FAT1 CRISPR KO leads to elevated MCM7 reloading rate >6N in TP53 mutant PC9 cells, 2-sided Paired T-test, mean±SD, n=3 biological replicates. c, FAT1 ablation leads to elevated MCM7 reloading rate >4N in TP53 WT RPE1 cells with transient FAT1 siRNA transfection, mean±SEM, 2-sided paired T-test, n=3 biological repeat. d,e, Cyclin B1 levels in the G2/M population following CTRL or FAT1 depletion in TP53 WT RPE1 cells. The histogram in d shows no difference in G2/M cyclin B1 levels, mean±SEM, 2-sided paired T-test, n=3 biological repeats. The representative dot plots in e show cyclin B1 levels of the G2 population. f, Nocodazole treatment arrests PC9 cells at mitosis and causes endoreplication (EdU positive cells >6N). FAT1 knockdown does not significantly further increase EdU-positive cells >6N when treated with nocodazole, mean±SEM, 2-sided paired T-Test, n=4 biological replicates. g, Live cell tracking data showing mitotic error rate of RPE1-TERT-FUCCI cells. 2 different FAT1 siRNAs produced a significant increase in mitotic error rate. One-way ANOVA with Sidak correction, mean±SD, n=3 biological repeats. h, Quantification of fixed microscopy images showing that FAT1 siRNA knockdown increases the rate of multinucleated U2OS cells, with or without replication stress induced with hydroxyurea. Left, multinucleation was quantified using phalloidin as a marker for cell boundaries and nuclear envelope stain emerin was used to mark the number of nuclei per cell. Mean±SD, 2-sided paired T-test, Biological repeats: n=4 without damage, n=6 with HU. Right, representative image of cells, scale bar = 10 μM, white arrows = multinucleated cells. i, Quantification of fixed microscopy images showing FAT1 knockdown elevates EdU incorporation rate in multinucleated TP53 KO RPE1-TERT cells, but not in the TP53 WT counterpart. Following aphidicolin-induced replication stress, FAT1 depletion increases EdU incorporation rate in both TP53 WT and TP53 KO cells, mean±SD, one-way ANOVA with Holm-Sidak correction. Biological repeats, no damage n=3; Ctrl siRNA with aphidicolin n=3, FAT1 siRNA with aphidicolin n=4.
Extended Data Fig. 8 FAT1 loss results in nuclear shape deformation and exacerbates targeted therapy resistance.
a, Nuclear morphometric measurements of PC9 cells following CTRL and FAT1 siRNA knockdown. Genome-doubled PC9 cells were treated for 24h with replication stress inducer aphidicolin. Z-project images were segmented and analysed for the difference in nuclear morphology. More than 1680 cells were analysed per condition over 3 biological repeats. 2-sided Mann-Whitney test, p<0.001. b, Live-cell imaging demonstrated higher chromatin bridge formation rate following transient FAT1 siRNA knockdown in RPE1-TERT cells. In addition, the daughter cells are more likely to display nuclear shape deformation morphologies following chromatin bridge formation when FAT1 is depleted. c, Mitotic outcomes of RPE1-TERT cells monitored after initial nuclear shape deformation. Among FAT1 depleted cells, those cells with nuclear shape deformation were less likely to undergo normal mitotic segregation in the following mitosis. 60x magnification live cell microscopy performed using 5 minute intervals, 25 z-steps. scale bar = 5 μM, Chi-squared test. At least 50 mitotic events were tracked per condition over 8 biological repeats. d, Estimation of the effect of FAT1 depletion on mitotic timing in RPE1-TERT-FUCCI cells. At least 100 cells were scored per siRNA knockdown across 3 biological repeats. Cells were imaged at 5-minute intervals over 12 h. Black bar = mean, Kruskal–Wallis test with False Discovery Rate Correction. e, IC90 determination of osimertinib treatment in PC9 cells. f, Experimental flowchart showing the generation of osimertinib-resistant PC9 subclones. g,h, Graphs demonstrating the impact of FAT1 CRISPR KO on the acquisition of osimertinib resistance, as indicated by clone survival (g; 2-sided Mann-Whitney Test) and the number of long-term derivation of resistant subclones (h; Fisher’s exact test). i, Among Osimertinib-resistant subclones, FAT1 CRISPR KO cells exhibited significantly variable DNA ploidy compared with non-targeting controls. 2-sided Welch’s T-test, non-targeting = 6 clones, FAT1 KO = 31 clones.
Extended Data Fig. 9 FAT1 loss results in dysregulation of YAP1 nuclear localization.
a,b Transient siRNA depletion of FAT1 increases the relative YAP1 nuclear localization ratio in RPE1-FUCCI cells (a; PTEMF fixation) and T2P cells (b (left panel); PFA fixation). Scale bars =10 µm. Representative images are shown. The right panel in b shows quantification of the results in T2P cells. Red bar =mean, 2-sided Mann-Whitney test. c, Transient siRNA depletion of FAT1 and LATS1 does not consistently alter the phosphorylation level of YAP1 in RPE1 and T2P cells. d, Plot showing the incidences of amplification and deep deletions of Hippo pathway members in the TRACERx 421 LUSC cohort. The effectors TEAD4 and WWTR1/TAZ are amplified in >10% and >35% of cases, respectively. e, The localization of the HA-epitope tag does not affect the ability to repress TEAD reporter transcriptional activity. Left, Scheme showing the location of the HA-epitope tag in different FAT1 expression constructs. The HA-epitope tag was located either at the N terminus or internally within the FAT1 construct. Right, Histogram showing normalized TEAD transcriptional activity upon overexpression of different FAT1 construct. The edge of histograms denotes the mean values, One-way ANOVA with Holm-Sidak multiple comparisons test, for each condition >95 cells were scored across 3 biological repeats.
Extended Data Fig. 10 Loss of LATS2 is enriched in the TRACERx LUSC cohort.
a, Representative western blot following LATS1/2 knockdown in the U2OS-AsiSI DIvA system reveals that DNA damage markers such as γH2A.X and phospho-KAP1 are activated proficiently upon damage. b, Middle, representative gel showing that transient knockdown of LATS2, but not LATS1, induces an illegitimate repair product in U2OS-AsiSI DIvA cells. PCR products generated from the legitimate repair product were used as the loading control (bottom). n=3 biological repeats. c, Plots of GISTIC scores illustrating that the SCNA loss of the LATS2 genomic loci (13q12.11) is positively selected in the LUSC but not the LUAD TRACERx421 cohort. Red lines denote q value <0.2. d,e, Histograms representing cell cycle profiles following loss of FAT1, YAP1 or combined loss of both FAT1 and YAP1 by siRNA in U2OS-AsiSI cells (d) and A549 FAT1 WT/KO cells (e), n=3 biological repeats, one-way ANOVA with Sidak corrections. f, Histogram quantifying the percentage of FAT1 WT or KO A549 cells with lagging chromosomes after aphidicolin treatment following transient depletion of YAP1 using RNAi, mean±SD, one-way ANOVA with Holm-Sidak multiple correction, biological repeats: FAT1 WT =3, FAT1 KO =4. g, Representative western blot showing YAP1 and FAT1 knockdown efficiency in the RPE1-FUCCI and A549 cells. h, Proposed model showing potential interaction between FAT1 and the Hippo pathway.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10, uncropped blots for Supplementary Figs. 2 and 6, Supplementary Methods, full plasmid maps and sequences.
Supplementary Video 1
Live-cell imaging showing an example of a typical tracking experiment with control siRNA knockdown in FUCCI–RPE-1 cells, without exogenous damage. Video tracking length: 56 h (1 frame is 20 min). The mitotic outcome was tracked manually. Blue, H2B; red, CDT1; green, geminin.
Supplementary Video 2
Live-cell imaging showing an example of the failed cytokinesis phenotype following FAT1 siRNA knockdown in FUCCI–RPE-1 cells. Blue, H2B; red, CDT1; green, geminin.
Supplementary Video 3
Live-cell imaging showing an example of the nuclear morphology deformation phenotype following FAT1 siRNA knockdown in FUCCI–RPE-1 cells. The cell proceeded to finish the second cell cycle and failed cytokinesis. Blue, H2B; red, CDT1; green, geminin.
Supplementary Video 4
Video showing control siRNA RPE-1 cells going through mitosis. The cells recovered normally after mitotic bridge formation.
Supplementary Video 5
Video showing FAT1 siRNA-depleted RPE-1 cells going through mitosis. The cells displayed aberrant morphology after mitotic bridge formation.
Supplementary Tables 1–3
Supplementary Table 1. Antibodies. Supplementary Table 2. siRNA sequences. Supplementary Table 3. Full list of members of the TRACERx consortium.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Source Data Fig. 5
Unprocessed gels or blots.
Source Data Fig, 6
Unprocessed gels or blots.
Source Data Fig. 7
Unprocessed gels or blots.
Source Data Extended Fig. 4
Unprocessed gels or blots.
Source Data Extended Fig. 6
Unprocessed gels or blots.
Source Data Extended Fig. 9
Unprocessed gels or blots.
Source Data Extended Fig. 10
Unprocessed gels or blots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, WT., Zalmas, LP., Bailey, C. et al. TRACERx analysis identifies a role for FAT1 in regulating chromosomal instability and whole-genome doubling via Hippo signalling. Nat Cell Biol 27, 154–168 (2025). https://doi.org/10.1038/s41556-024-01558-w
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41556-024-01558-w
This article is cited by
-
Targeting the Hippo pathway in cancer
Nature Reviews Drug Discovery (2025)
-
Prospective validation of ORACLE, a clonal expression biomarker associated with survival of patients with lung adenocarcinoma
Nature Cancer (2025)