Abstract
Autophagic mechanisms that maintain nuclear envelope homoeostasis are bulwarks to ageing and disease. Here we define a quantitative and ultrastructural timeline of nuclear macroautophagy (nucleophagy) in yeast by leveraging four-dimensional lattice light sheet microscopy and correlative light and electron tomography. Nucleophagy begins with a rapid accumulation of the selective autophagy receptor Atg39 at the nuclear envelope and finishes in ~300 s with Atg39-cargo delivery to the vacuole. Although there are several routes to the vacuole, at least one pathway incorporates two consecutive membrane fission steps: inner nuclear membrane (INM) fission to generate an INM-derived vesicle in the perinuclear space and outer nuclear membrane fission to liberate a double-membraned vesicle to the cytosol. Outer nuclear membrane fission occurs independently of phagophore engagement and instead relies surprisingly on dynamin-like protein 1 (Dnm1), which is recruited to sites of Atg39 accumulation by Atg11. Loss of Dnm1 compromises nucleophagic flux by stalling nucleophagy after INM fission. Our findings reveal how nuclear and INM cargo are removed from an intact nucleus without compromising its integrity, achieved in part by a non-canonical role for Dnm1 in nuclear envelope remodelling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All other data supporting the findings presented in this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
The MATLAB and Jupyter Notebook Python scripts are available with no access restrictions via GitHub at https://github.com/LusKingLab/.
References
Mannino, P. J. & Lusk, C. P. Quality control mechanisms that protect nuclear envelope identity and function. J. Cell Biol. 221, e202205123 (2022).
Mucino-Hernandez, G. et al. Nucleophagy contributes to genome stability through degradation of type II topoisomerases A and B and nucleolar components. J. Cell Sci. 136, jcs.260563 (2023).
Hasper, J. et al. Long lifetime and tissue-specific accumulation of lamin A/C in Hutchinson–Gilford progeria syndrome. J. Cell Biol. 223, jcb.202307049 (2024).
Tsai, P. L., Zhao, C., Turner, E. & Schlieker, C. The lamin B receptor is essential for cholesterol synthesis and perturbed by disease-causing mutations. eLife. 5, e16011 (2016).
Papandreou, M. E. & Tavernarakis, N. Nucleophagy: from homeostasis to disease. Cell Death Differ. 26, 630–639 (2019).
Natarajan, N., Foresti, O., Wendrich, K., Stein, A. & Carvalho, P. Quality control of protein complex assembly by a transmembrane recognition factor. Mol Cell. 77, 108–119.e9 (2020).
Khmelinskii, A. et al. Protein quality control at the inner nuclear membrane. Nature 516, 410–413 (2014).
Smoyer, C. J. et al. Distribution of proteins at the inner nuclear membrane is regulated by the Asi1 E3 ligase in Saccharomyces cerevisiae. Genetics. 211, 1269–1282 (2019).
Dou, Z. et al. Autophagy mediates degradation of nuclear lamina. Nature 527, 105–109 (2015).
King, G. A. et al. Meiotic cellular rejuvenation is coupled to nuclear remodeling in budding yeast. eLife. 8, e47156 (2019).
King G. A. et al. Meiotic nuclear pore complex remodeling provides key insights into nuclear basket organization. J. Cell Biol. https://doi.org/10.1083/jcb.202204039 (2023).
Mizuno, T. & Irie, K. Msn2/4 transcription factors positively regulate expression of Atg39 ER-phagy receptor. Sci. Rep. 11, 11919 (2021).
Papandreou, M. E., Konstantinidis, G. & Tavernarakis, N. Nucleophagy delays aging and preserves germline immortality. Nat. Aging 3, 34–46 (2023).
Wu N. et al. Autophagy in aging-related diseases and cancer: principles, regulatory mechanisms and therapeutic potential. Ageing Res. Rev. https://doi.org/10.1016/j.arr.2024.102428 (2024).
Bahmanyar, S. et al. Spatial control of phospholipid flux restricts endoplasmic reticulum sheet formation to allow nuclear envelope breakdown. Genes Dev. 28, 121–126 (2014).
Campbell, J. L. et al. Yeast nuclear envelope subdomains with distinct abilities to resist membrane expansion. Mol. Biol. Cell 17, 1768–1778 (2006).
Romanauska, A. & Kohler, A. Lipid saturation controls nuclear envelope function. Nat. Cell Biol. 25, 1290–1302 (2023).
Santos-Rosa, H., Leung, J., Grimsey, N., Peak-Chew, S. & Siniossoglou, S. The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 24, 1931–1941 (2005).
Barbosa, A. D. et al. Compartmentalized synthesis of triacylglycerol at the inner nuclear membrane regulates nuclear organization. Dev. Cell 50, 755–766 e6 (2019).
Romanauska, A. & Kohler, A. The inner nuclear membrane is a metabolically active territory that generates nuclear lipid droplets. Cell. 174, 700–715 e18 (2018).
Romanauska, A. & Kohler, A. Reprogrammed lipid metabolism protects inner nuclear membrane against unsaturated fat. Dev. Cell 56, 2562–2578 e3 (2021).
Melia T. J., Lystad A. H. & Simonsen A. Autophagosome biogenesis: from membrane growth to closure. J. Cell Biol. https://doi.org/10.1083/jcb.202002085 (2020).
Eickhorst, C., Licheva, M. & Kraft, C. Scaffold proteins in bulk and selective autophagy. Prog. Mol. Biol. Transl. Sci. 172, 15–35 (2020).
Kucinska, M. K. et al. TMX4-driven LINC complex disassembly and asymmetric autophagy of the nuclear envelope upon acute ER stress. Nat. Commun. 14, 3497 (2023).
Roberts, P. et al. Piecemeal microautophagy of nucleus in Saccharomyces cerevisiae. Mol. Biol. Cell 14, 129–141 (2003).
Allegretti, M. et al. In-cell architecture of the nuclear pore and snapshots of its turnover. Nature 586, 796–800 (2020).
Lee, C. W. et al. Selective autophagy degrades nuclear pore complexes. Nat. Cell Biol. 22, 159–166 (2020).
Tomioka, Y. et al. TORC1 inactivation stimulates autophagy of nucleoporin and nuclear pore complexes. J. Cell Biol. 219, e201910063 (2020).
Mochida, K. et al. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature 522, 359–362 (2015).
Mizuno, T., Muroi, K. & Irie, K. Snf1 AMPK positively regulates ER-phagy via expression control of Atg39 autophagy receptor in yeast ER stress response. PLoS Genet. 16, e1009053 (2020).
Otto, F. B. & Thumm, M. Mechanistic dissection of macro- and micronucleophagy. Autophagy. 17, 626–639 (2021).
Chandra, S. et al. Atg39 selectively captures inner nuclear membrane into lumenal vesicles for delivery to the autophagosome. J. Cell Biol. 220, jcb.202103030 (2021).
Mochida, K. et al. Atg39 links and deforms the outer and inner nuclear membranes in selective autophagy of the nucleus. J. Cell Biol. 221, jcb.202103178 (2022).
Otsuga, D. et al. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J. Cell Biol. 143, 333–349 (1998).
Kuravi, K. et al. Dynamin-related proteins Vps1p and Dnm1p control peroxisome abundance in Saccharomyces cerevisiae. J. Cell Sci. 119, 3994–4001 (2006).
Gonzalez, A. et al. Ubiquitination regulates ER-phagy and remodelling of endoplasmic reticulum. Nature 618, 394–401 (2023).
Rosado, C. J., Mijaljica, D., Hatzinisiriou, I., Prescott, M. & Devenish, R. J. Rosella: a fluorescent pH-biosensor for reporting vacuolar turnover of cytosol and organelles in yeast. Autophagy 4, 205–213 (2008).
Klionsky D. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy https://doi.org/10.1080/15548627.2015.1100356 (2021).
Kim, J. et al. Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole. J. Cell Biol. 153, 381–396 (2001).
Kirisako, T. et al. Formation process of autophogosome is traced with Apg8/Aut7p in yeast. J. Cell Biol. 147, 435–446 (1999).
Kvam, E. & Goldfarb, D. S. Nvj1p is the outer-nuclear-membrane receptor for oxysterol-binding protein homolog Osh1p in Saccharomyces cerevisiae. J. Cell Sci. 117, 4959–4968 (2004).
Borah, S. et al. Heh2/Man1 may be an evolutionarily conserved sensor of NPC assembly state. Mol. Biol. Cell 32, 1359–1373 (2021).
Thaller, D. J. et al. An ESCRT-LEM protein surveillance system is poised to directly monitor the nuclear envelope and nuclear transport system. eLife. 8, e45284 (2019).
Webster, B. M., Colombi, P., Jager, J. & Lusk, C. P. Surveillance of nuclear pore complex assembly by ESCRT-III/Vps4. Cell 159, 388–401 (2014).
Webster, B. M. et al. Chm7 and Heh1 collaborate to link nuclear pore complex quality control with nuclear envelope sealing. EMBO J. 35, 2447–2467 (2016).
Chen, S., Cui, Y., Parashar, S., Novick, P. J. & Ferro-Novick, S. ER-phagy requires Lnp1, a protein that stabilizes rearrangements of the ER network. Proc. Natl Acad. Sci. USA 115, E6237–E6244 (2018).
Chen, S. et al. Vps13 is required for the packaging of the ER into autophagosomes during ER-phagy. Proc. Natl Acad. Sci. USA 117, 18530–18539 (2020).
Cui, Y. et al. A COPII subunit acts with an autophagy receptor to target endoplasmic reticulum for degradation. Science 365, 53–60 (2019).
Liu D. et al. ER-phagy requires the assembly of actin at sites of contact between the cortical ER and endocytic pits. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2117554119 (2022).
Anwar, K. et al. The dynamin-like GTPase Sey1p mediates homotypic ER fusion in S. cerevisiae. J. Cell Biol. 197, 209–217 (2012).
Voeltz, G. K., Prinz, W. A., Shibata, Y., Rist, J. M. & Rapoport, T. A. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124, 573–586 (2006).
Mozdy, A. D., McCaffery, J. M. & Shaw, J. M. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J. Cell Biol. 151, 367–380 (2000).
Sesaki, H., Southard, S. M., Yaffe, M. P. & Jensen, R. E. Mgm1p, a dynamin-related GTPase, is essential for fusion of the mitochondrial outer membrane. Mol Biol Cell. 14, 2342–2356 (2003).
Smaczynska-de, R. II et al. A role for the dynamin-like protein Vps1 during endocytosis in yeast. J. Cell Sci. 123, 3496–3506 (2010).
Ferguson, S. M. & De Camilli, P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 13, 75–88 (2012).
Mao, K., Liu, X., Feng, Y. & Klionsky, D. J. The progression of peroxisomal degradation through autophagy requires peroxisomal division. Autophagy 10, 652–661 (2014).
Motley, A. M., Ward, G. P. & Hettema, E. H. Dnm1p-dependent peroxisome fission requires Caf4p, Mdv1p and Fis1p. J. Cell Sci. 121, 1633–1640 (2008).
Naylor, K. et al. Mdv1 Interacts with assembled Dnm1 to promote mitochondrial division. J. Biol. Chem. 281, 2177–2183 (2006).
Mao, K., Wang, K., Liu, X. & Klionsky, D. J. The scaffold protein Atg11 recruits fission machinery to drive selective mitochondria degradation by autophagy. Dev. Cell 26, 9–18 (2013).
Hu, C., Chinenov, Y. & Kerppola, T. K. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9, 789–798 (2002).
Ader, N. R. et al. An ESCRT grommet cooperates with a diffusion barrier to maintain nuclear integrity. Nat. Cell Biol. 25, 1465–1477 (2023).
Bailey, M. L. P. et al. Loops and the activity of loop extrusion factors constrain chromatin dynamics. Mol. Biol. Cell 34, ar78 (2023).
Crocker, J. C. & Grier, D. G. Methods of digital video microscopy for colloidal studies. J. Colloid Interface Sci. 179, 298–310 (1996).
Lawrimore, J., Bloom, K. S. & Salmon, E. D. Point centromeres contain more than a single centromere-specific Cse4 (CENP-A) nucleosome. J. Cell Biol. 195, 573–582 (2011).
Campbell, B. C., Paez-Segala, M. G., Looger, L. L., Petsko, G. A. & Liu, C. F. Chemically stable fluorescent proteins for advanced microscopy. Nat. Methods 19, 1612–1621 (2022).
Villinger, C., Neusser, G., Kranz, C., Walther, P. & Mertens, T. 3D Analysis of HCMV induced-nuclear membrane structures by FIB/SEM tomography: insight into an unprecedented membrane morphology. Viruses 7, 5686–5704 (2015).
Speese, S. D. et al. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell. 149, 832–846 (2012).
Fujioka, Y. et al. Phase separation promotes Atg8 lipidation for autophagy progression. Preprint at bioRxiv https://doi.org/10.1101/2024.08.29.610189 (2024).
Schneider, B. L., Seufert, W., Steiner, B., Yang, Q. H. & Futcher, A. B. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast 11, 1265–1274 (1995).
Longtine, M. S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 (1998).
Zhang, Y., Serratore, N. D. & Briggs, S. D. N-ICE plasmids for generating N-terminal 3 × FLAG tagged genes that allow inducible, constitutive or endogenous expression in Saccharomyces cerevisiae. Yeast 34, 223–235 (2017).
Baudin, A., Ozier-Kalogeropoulos OD, A., Lacroute, F. & Cullin, C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 21, 3329–3330 (1993).
Guldener, U., Heck, S., Fiedler, T., Beinhauer, J. & Hegemann, J. H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24, 2519–2524 (1996).
Lacy, M. M., Baddeley, D. & Berro, J. Single-molecule imaging of the BAR-domain protein Pil1p reveals filament-end dynamics. Mol. Biol. Cell 28, 2251–2259 (2017).
Botman, D., de Groot, D. H., Schmidt, P., Goedhart, J. & Teusink, B. In vivo characterisation of fluorescent proteins in budding yeast. Sci. Rep. 9, 2234 (2019).
Smoyer, C. J. et al. Analysis of membrane proteins localizing to the inner nuclear envelope in living cells. J. Cell Biol. 215, 575–590 (2016).
Chen, B. C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014).
Adell, M. A. Y. et al. Recruitment dynamics of ESCRT-III and Vps4 to endosomes and implications for reverse membrane budding. eLife. 6, e31652 (2017).
Kukulski, W. et al. Precise, correlated fluorescence microscopy and electron tomography of lowicryl sections using fluorescent fiducial markers. Methods Cell Biol. 111, 235–257 (2012).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Kremer JRM, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Mastronarde, D. N. & Held, S. R. Automated tilt series alignment and tomographic reconstruction in IMOD. J. Struct. Biol. 197, 102–113 (2017).
Flajolet, P., Gardy, D. & Thimonier, L. Birthday paradox, coupon collectors, caching algorithms and self-organizing search. Discrete Appl. Math. 39, 207–229 (1992).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Bolte, S. & Cordelieres, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006).
Acknowledgements
We thank the Center for Cellular and Molecular Imaging for assistance with electron microscopy, particularly M. Graham, Z. Zuo and X. Liu. We are grateful to C. Kraft, M. Graef and L. Lackner for yeast strains. We thank K. Li for technical support. This work was funded by the following grants from National Institutes of Health: R01 AG090465, R56 AG071201 and R21 AG058033 to C.P.L. and T.J.M.; R01 GM105672 to C.P.L.; F32 GM139285 to N.R.A.; and F31 AG069490 to P.J.M.
Author information
Authors and Affiliations
Contributions
Conceptualization: P.J.M. and C.P.L. Methodology: P.J.M., A.P., I.V.S., N.R.A., L.S. and E.C.R. Investigation: P.J.M., A.P., I.V.S. and N.R.A. Validation: P.J.M. and A.P. Data curation: P.J.M., A.P., I.V.S. and N.R.A. Formal analysis: P.J.M., A.P., I.V.S. and N.R.A. Funding acquisition: P.J.M., T.J.M. and C.P.L. Visualization: P.J.M. and A.P. Project administration: P.J.M., M.C.K. and C.P.L. Resources: L.S. and C.P.L. Software: A.P., I.V.S. and L.S. Supervision: T.J.M., M.C.K. and C.P.L. Writing—original draft: P.J.M. and C.P.L. Writing—review and editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Nektarios Tavernarakis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Models of nucleophagy and characterization of Atg39 expression levels.
a, b) Schematic of two models of nucleophagy incorporating either sequential (Model 1) or simultaneous (Model 2) INM and ONM fission events. Model 1 would also lead to the formation of a lumenal vesicle/INMDV intermediate. ONM, outer nuclear membrane; INM, inner nuclear membrane; PNS, perinuclear space; INMDV, inner nuclear membrane-derived vesicle; NDV, nucleus-derived vesicle. c) RT-qPCR on indicated autophagy gene transcripts was performed in WT cells either treated with rapamycin for 2 h or cultured in medium lacking nitrogen for 2 h. Results displayed as a bar graph comparing the mean fold change of the transcripts between the rapamycin and -Nitrogen condition. Mean and standard deviation (error bars) of three biological replicates (black dots). Unpaired two-sided t tests.**** is P < 0.0001; ** is P = 0.0019; ns, not significant (P > 0.05). d) Protein levels of Atg39-3xHA fusions derived from transcripts including either the ADH1 3′UTR or the endogenous ATG39 3′UTR were assessed by western blot with anti-HA antibody directly coupled to HRP (α-HA-HRP) and ECL. Positions of molecular weight (MW) standards (kD) at left. At bottom, membranes were stained with Ponceau to evaluate total protein loads. e) Fluorescence micrographs of Atg39-GFP produced from transcripts with 3′UTRs from the ADH1 or ATG39 genes after 4 h in medium lacking nitrogen. Scale bar is 5 µm. Data are from one experiment. f) Western blot of whole protein extracts from cells expressing Atg39-pHn-mCh with fall out fragment (‘pHn) in the indicated genetic backgrounds and PMSF treatment after 24 h in sd-N. Position ‘pHn indicated on the right. An anti-GFP antibody and anti-mouse-HRP secondary followed by ECL was used to detect proteins. Positions of molecular weight (MW) standards (kD) at left. * is a non-specific band. # is the expected position of Atg39-pHn-mCh, which is undetectable in most backgrounds due to its low abundance and lack of sensitivity of the anti-GFP antibody. Numerical source data and unprocessed blots are provided.
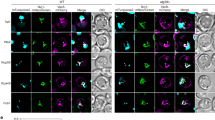
Extended Data Fig. 2 Dnm1 colocalizes with Atg39 in the absence of Fis1.
a–c) Inverted fluorescence micrographs of a timelapse series of fis1Δ cells expressing Atg39-GFP and Dnm1-mCh with and without either an extra copy of DNM1 or the dominant-negative dnm1-K41A allele under conditions of nitrogen starvation. Green and red channels shown with merge at indicated times (T) with T = 0 s being the first detection of a Atg39-GFP focus (arrow). Arrowheads point to colocalization between Atg39-GFP and Dnm1-mCh. Numbers in merge are the z-position (in μm) of the image shown in reference to the midplane. Scale bars are 5 µm. Data shown are representative of three independent experiments.
Extended Data Fig. 3 Atg11 recruits Dnm1 to the NE.
a) Fluorescence micrographs of fis1Δ cells and fis1Δatg11Δ cells expressing Atg39-pHn, Dnm1-mCh with and without an extra copy of DNM1 or the dnm1-K41A allele under conditions of nitrogen starvation. Green and red channels shown with merge. Scale bar is 5 µm. Data shown are representative of three independent experiments. b) Plot of the mean percentage of Atg39-pHn foci that colocalize with Dnm1-mCh from experiments in A. Mean and standard deviation (error bars) from three biological replicates (black dots). For fis1Δ cells, n = 79 Atg39-pHn foci. For fis1Δ cells expressing DNM1, n = 104 Atg39-pHn foci. For fis1Δ cells expressing dnm-K41A, n = 166 Atg39-pHn foci. For fis1Δatg11Δ cells, n = 78 Atg39-pHn foci. For fis1Δatg11Δ cells expressing DNM1, n = 96 Atg39-pHn foci. For fis1Δatg11Δ cells expressing dnm1-K41A, n = 165 Atg39-pHn foci. Unpaired two-sided t test. *** is P = 0.0003. ns, not significant (P > 0.05). c) Schematic of BiFC. VN and VC are the N- and C- terminal fragments of Venus, respectively. An interaction between Dnm1-VN and VC-Atg11 leads to the reconstitution of Venus and its fluorescence. d) Fluorescence micrographs of cells expressing Atg39-ymTurquoise2 (Atg39-ymTq2), VC-Atg11, and Dnm1-VN under conditions of nitrogen starvation. Yellow (magenta) and blue (green) channels shown with merge. The arrow points out colocalization between the Venus and Atg39-ymTq2 foci. Scale bar is 5 µm. Data shown are representative of three independent experiments. e) Plot of the mean percentage of Atg39-ymTq2 foci that colocalize with Venus foci from experiments in d. Mean and standard deviation (error bars) from three biological replicates (black dots). n = 84 Atg39-ymTq2 foci. Numerical source data are provided.
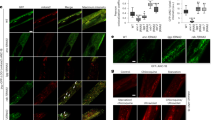
Extended Data Fig. 4 Atg8 recruitment, copy number, and Atg39 dynamics during nucleophagy.
a) Fluorescence micrographs of cells expressing Atg39-GFP and 2xmCh-Atg8 imaged every 10 seconds under conditions of nitrogen starvation. Green and red channels shown with merge. Numbers in merge are z-position (in μm) of the displayed image in reference to the midplane. T (s) indicates the time since the first detection of the Atg39-GFP focus (indicated above, arrowhead). Arrows point to colocalization between Atg39-GFP and 2xmCh-Atg8. * indicates fluorescence from the vacuole in the red channel. Scale bar is 3 µm. Data shown are representative of three independent experiments. b) Histogram of the number of colocalization instances at the indicated time after the appearance of an Atg39-GFP focus. Data are from the experiments in A; n = 73 cells, 74 tracks, three biological replicates. c) To calculate copy number of Atg39-GFP within individual foci, we imaged budding yeast expressing Atg39-GFP next to S. pombe expressing Fta3-GFP, a kinetochore protein which has 111 molecules/focus in anaphase B61. By directly relating the fluorescence intensities, we calculated Atg39-GFP copy number/focus as presented in Fig. 3d. Scale bar is 5 µm for the large image and 500 nm for the insets. Data shown are representative of three independent experiments. d) Inverted fluorescence micrographs of WT cells expressing Atg39-GFP and Vph1-mCh imaged every two seconds under conditions of nitrogen starvation. Green and red channels shown with merge. Numbers in merge are z-position (in μm) of the displayed image in reference to the midplane. T (s) indicates the time in seconds since the first detection of the Atg39-GFP focus (green arrowhead). Another less intense focus of Atg39-GFP is visible merging with the initial particle (pink arrowhead). Scale bar is 3 µm. Data is from one experiment. Numerical source data are provided.
Extended Data Fig. 5 Atg11 dependence during nucleophagy.
a) Fluorescence micrographs of a time series of atg11Δ cells expressing Atg39-GFP under conditions of nitrogen starvation. Numbers in merge are z-position (in μm) of the displayed image in reference to the midplane. T (s) indicates the time in seconds since the first detection of the Atg39-GFP focus (indicated above, arrowhead). Cells shown are representative examples of two classifications of the Atg39-GFP foci dynamics that either disappear prior to internalization in the vacuole (aborted) or don’t (not aborted). Scale bar is 3 µm. Data shown are representative of three independent experiments. b) Plot of the mean number of focal accumulations divided by the total number of cells divided by the total time of the experiment (h) for the indicated genotype. Data are from three biological replicates (black dots) where n = 294, n = 557 and n = 522 cells were assessed for WT, dnm1Δ and atg11Δ, respectively. Ordinary one-way ANOVA with multiple comparisons. ** is P = 0.0032; ns, not significant (P > 0.05). c) Fluorescence intensity of Atg39-GFP foci in atg11Δ cells plotted versus time after their first detection. Data are split into two groups, aborted (red, squares) and not aborted (blue, circles) from three biological replicates. The ‘aborted’ group comprised n = 40 cells, 42 tracks and the ‘not aborted’ group, n = 37 cells, 38 tracks. Numerical source data are provided.
Extended Data Fig. 6 Characteristics of Atg39-GFP mobility and cargo capture.
a) Fluorescence micrographs of WT cells expressing Atg39-GFP and a model INM cargo comprising the INM targeting and transmembrane domain of Heh1 fused to two tandem mCh fluorophores (H1-2xmCh) under conditions of nitrogen starvation. Green and red channels shown with merge. Scale bar is 3 µm. b) Inverted fluorescence micrographs of a time series of WT cells expressing Atg39-GFP and H1-2xmCh under conditions of nitrogen starvation. Time (T) 0 is the first detected point of Atg39-GFP accumulation. Green and red channels shown with merge. Arrowheads point to colocalization between Atg39-GFP and H1-2xmCh. Cell boundaries are outlined. Numbers in merge are z-position (in μm) of the image in reference to the midplane. Scale bar is 3 µm. Data shown are representative of two independent experiments. c) Scatter plot of the elapsed time from the first detection of an Atg39-GFP focus and the first instance of colocalization with H1-2xmCh from experiments as in B. The median is the black bar. Data are from n = 36 cells in which Atg39-GFP and H1-2xmCh were colocalized outside of the nucleus out of n = 42 total cells from two biological replicates. d) Bar graph of the percentage of Atg39-GFP foci tracked from first appearance that do not enter the vacuole over a 20-minute timelapse experiment as noted in Fig. 3f for the indicated genotype. Mean and standard deviation (error bars) from 5 (WT cells) or 4 (dnm1∆ cells) biological replicates (black dots). n = 114 tracks, 99 cells for WT cells and n = 95 tracks, 82 cells for dnm1∆ cells. Unpaired two-sided t test. *** is P = 0.0008. e) Plot of the percentage of time that Atg39-GFP foci spend in each phase as per color code in Fig. 3e and g in WT and dnm1Δ cells. The latter are also segregated based on those that enter the vacuole (unperturbed) and those that don’t (stalled). Numerical source data are provided.
Extended Data Fig. 7 Ultrastructure of sites of Atg39 accumulation during nucleophagy.
a–e) CLEM/tomography of sites of Atg39-hfYFP foci. From left to right: fluorescence image of WT cells expressing Atg39-hfYFP (green), Vph1-mCh and Nup170-mCh (magenta). Scale bar is 3 µm. EM tomogram acquired of boxed region in fluorescence image and tomogram virtual slice without and with annotation shown alongside a snapshot of a 3D model. Arrows point to INMDVs. ONM is green, INM is light blue, phagophore/autophagosome is orange, vacuole membrane is magenta. Scale bar is 200 nm. C, cytosol; N, nucleus; V, vacuole; ONM, outer nuclear membrane; INM, inner nuclear membrane; ER, endoplasmic reticulum; INMDV, inner nuclear membrane derived vesicle; AM, autophagic membrane. Numbers at right refer to classification scheme in Fig. 4f. Data are from one experiment.
Extended Data Fig. 8 Ultrastructure of sites of Atg39 accumulation in dnm1Δ cells.
a–e) CLEM/tomography of sites of Atg39-hfYFP foci in dnm1Δ cells. From left to right: fluorescence image of WT cells expressing Atg39-hfYFP (green), Vph1-mCh and Nup170-mCh (magenta). Scale bar is 3 µm. EM tomogram acquired of boxed region in fluorescence image and tomogram virtual slice without and with annotation shown alongside a snapshot of a 3D model. Arrows point to INMDVs. ONM is green, INM is light blue, phagophore/autophagosome is orange, vacuole membrane is magenta. Scale bar is 200 nm. C, cytosol; N, nucleus; V, vacuole; ONM, outer nuclear membrane; INM, inner nuclear membrane; ER, endoplasmic reticulum; INMDV, inner nuclear membrane derived vesicle; AM, autophagic membrane; LD, lipid droplet. Numbers at right refer to classification scheme in Fig. 4f. Data are from one experiment.
Extended Data Fig. 9 Proposed model of nucleophagy.
Illustrative model of the molecular and morphological steps of nucleophagy. Atg39 locally accumulates adjacent to the NVJ in a mechanism supported by Atg11 (blue cylinders). It is likely that this clustering coincides with cargo capture and the evagination and fission of the INM to generate an INMDV. Initial small (50-100 nm INMDVs) may grow by fusion to generate larger (200–300 nm) INMDVs that are ultimately released from the NE through a Dnm1-dependent ONM fission step, or from the cortical ER through a proposed Scs2-dependent mechanism. Engagement with the phagophore occurs at or just after ONM fission. Autophagosomes containing double membrane nuclear derived vesicles (NDVs) fuse with vacuoles. Some INMDVs may also be capable of transiting through the broader ER but their fate remains uncertain.
Supplementary information
Supplementary Table 1
Lists of yeast strains, plasmids and primers used in this study.
Supplementary Video 1
A 5-minute time series of cells expressing Atg39–GFP and Vph1–mCh imaged every 10 s by LLSM. Vph1–mCh signal is rendered as a surface (magenta) and Atg39–GFP foci are rendered as balls (green). Scale bar, 10 µm. Frame rate is 24 frames per second.
Supplementary Video 2
Electron tomogram of a cell expressing Atg39–hfYFP, Nup170–mCh and Vph1–mCh obtained at a site of Atg39–hfYFP accumulation shown in Fig. 4d. Note that the outer membrane of the autophagosome and the vacuole extend towards each other but do not touch. However, content with similar electron density of the vacuole lumen is observed between the inner and outer membrane of the autophagosome suggesting prior fusion with the vacuole. NE, green; INMDV, blue; autophagic membranes, orange; vacuole, magenta. Scale bar, 100 nm.
Supplementary Video 3
Electron tomogram of a cell expressing Atg39–hfYFP, Nup170–mCh and Vph1–mCh in a dnm1Δ cell obtained at a region of Atg39–hfYFP accumulation shown in Fig. 5d. NE, green; INMDV, blue; autophagic membranes, orange; vacuole, magenta. Scale bar, 100 nm.
Source data
Source Data Figs. 1–4 and Extended Data Figs. 1 and 3–6
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots and Ponceau.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mannino, P.J., Perun, A., Surovtsev, I.V. et al. A quantitative ultrastructural timeline of nuclear autophagy reveals a role for dynamin-like protein 1 at the nuclear envelope. Nat Cell Biol 27, 464–476 (2025). https://doi.org/10.1038/s41556-025-01612-1
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41556-025-01612-1