Abstract
Glycolipid-lectin-driven endocytosis controls the formation of clathrin-independent carriers and the internalization of various cargos such as β1 integrin. Whether this process is regulated in a dynamic manner remained unexplored. Here we demonstrate that, within minutes, the epidermal growth factor triggers the galectin-driven endocytosis of cell-surface glycoproteins, such as integrins, that are key regulators of cell adhesion and migration. The onset of this process—mediated by the Na+/H+ antiporter NHE1 as well as the neuraminidases Neu1 and Neu3—requires the pH-triggered enzymatic removal of sialic acids whose presence otherwise prevents galectin binding. De-sialylated glycoproteins are then retrogradely transported to the Golgi apparatus where their glycan make-up is reset to regulate EGF-dependent invasive-cell migration. Further evidence is provided for a role of neuraminidases and galectin-3 in acidification-dependent bone resorption. Glycosylation at the cell surface thereby emerges as a dynamic and reversible regulatory post-translational modification that controls a highly adaptable trafficking pathway.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Mass spectrometry data have been deposited in ProteomeXchange with the primary accession code PXD041450. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
The adapted version of cmeAnalysis3D software can be found as part of the GitHub repository of LLSM tools at https://github.com/francois-a/llsmtools/.
Change history
25 February 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41556-025-01643-8
References
Lemmon, M. A. & Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010).
Avraham, R. & Yarden, Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 12, 104–117 (2011).
Gao, Y., Luan, X., Melamed, J. & Brockhausen, I. Role of glycans on key cell surface receptors that regulate cell proliferation and cell death. Cells 10, 1252 (2021).
Takahashi, M., Hasegawa, Y., Gao, C., Kuroki, Y. & Taniguchi, N. N-glycans of growth factor receptors: their role in receptor function and disease implications. Clin. Sci. 130, 1781–1792 (2016).
Zhao, Y. Y. et al. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 99, 1304–1310 (2008).
Marsico, G., Russo, L., Quondamatteo, F. & Pandit, A. Glycosylation and integrin regulation in cancer. Trends Cancer 4, 537–552 (2018).
Gilmour, A. M. et al. A novel epidermal growth factor receptor-signaling platform and its targeted translation in pancreatic cancer. Cell Signal. 25, 2587–2603 (2013).
Palmisano, G., Lendal, S. E. & Larsen, M. R. Titanium dioxide enrichment of sialic acid-containing glycopeptides. Methods Mol. Biol. 753, 309–322 (2011).
Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 3, a005199 (2011).
Sorkin, A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr. Opin. Cell Biol. 16, 392–399 (2004).
McMahon, H. T. & Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533 (2011).
Kirchhausen, T., Owen, D. & Harrison, S. C. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 6, a016725 (2014).
Kaksonen, M. & Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 19, 313–326 (2018).
Doherty, G. J. & McMahon, H. T. Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 (2009).
Johannes, L., Parton, R. G., Bassereau, P. & Mayor, S. Building endocytic pits without clathrin. Nat. Rev. Mol. Cell Biol. 16, 311–321 (2015).
Sandvig, K., Kavaliauskiene, S. & Skotland, T. Clathrin-independent endocytosis: an increasing degree of complexity. Histochem. Cell. Biol. 150, 107–118 (2018).
Renard, H. F. & Boucrot, E. Unconventional endocytic mechanisms. Curr. Opin. Cell Biol. 71, 120–129 (2021).
Sigismund, S., Lanzetti, L., Scita, G. & Di Fiore, P. P. Endocytosis in the context-dependent regulation of individual and collective cell properties. Nat. Rev. Mol. Cell Biol. 22, 625–643 (2021).
Cummings, R. D., Liu, F. T., Rabinovich, G. A., Stowell, S. R. & Vasta, G. R. in Essentials of Glycobiology (eds Varki, A. et al.) 491–504 (Cold Spring Harbor Laboratory Press, 2022).
Johannes, L., Jacob, R. & Leffler, H. Galectins at a glance. J. Cell Sci. 131, jcs208884 (2018).
Leffler, H. & Barondes, S. H. Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian β-galactosides. J. Biol. Chem. 261, 10119–10126 (1986).
Stowell, S. R. et al. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 283, 10109–10123 (2008).
Zhuo, Y. & Bellis, S. L. Emerging role of α2,6-sialic acid as a negative regulator of galectin binding and function. J. Biol. Chem. 286, 5935–5941 (2011).
Varki, A. & Gagneux, P. Multifarious roles of sialic acids in immunity. Ann. N. Y. Acad. Sci. 1253, 16–36 (2012).
Läubli, H. & Varki, A. Sialic acid-binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell. Mol. Life Sci. 77, 593–605 (2020).
Partridge, E. A. et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306, 120–124 (2004).
Nabi, I. R., Shankar, J. & Dennis, J. W. The galectin lattice at a glance. J. Cell Sci. 128, 2213–2219 (2015).
Rabinovich, G. A., Toscano, M. A., Jackson, S. S. & Vasta, G. R. Functions of cell surface galectin–glycoprotein lattices. Curr. Opin. Struct. Biol. 17, 513–520 (2007).
Lakshminarayan, R. et al. Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nat. Cell Biol. 16, 595–606 (2014).
Johannes, L., Wunder, C. & Shafaq-Zadah, M. Glycolipids and lectins in endocytic uptake processes. J. Mol. Biol. 428, 4792–4818 (2016).
Renard, H. F. et al. Endophilin-A3 and galectin-8 control the clathrin-independent endocytosis of CD166. Nat. Commun. 11, 1457 (2020).
Mathew, M. P. & Donaldson, J. G. Distinct cargo-specific response landscapes underpin the complex and nuanced role of galectin-glycan interactions in clathrin-independent endocytosis. J. Biol. Chem. 293, 7222–7237 (2018).
Zhang, C. et al. SLC3A2 N-glycosylation and Golgi remodeling regulate SLC7A amino acid exchangers and stress mitigation. J. Biol. Chem. 299, 105416 (2023).
Shafaq-Zadah, M. et al. Exploration into galectin-3 driven endocytosis and lattices. Biomolecules 14, 1169 (2024).
Arabkhari, M. et al. Desialylation of insulin receptors and IGF-1 receptors by neuraminidase-1 controls the net proliferative response of L6 myoblasts to insulin. Glycobiology 20, 603–616 (2010).
Amith, S. R. et al. Dependence of pathogen molecule-induced toll-like receptor activation and cell function on Neu1 sialidase. Glycoconj. J. 26, 1197–1212 (2009).
Moreno-Layseca, P., Icha, J., Hamidi, H. & Ivaska, J. Integrin trafficking in cells and tissues. Nat. Cell Biol. 21, 122–132 (2019).
Bridgewater, R. E., Norman, J. C. & Caswell, P. T. Integrin trafficking at a glance. J. Cell Sci. 125, 3695–3701 (2012).
Pretzlaff, R. K., Xue, V. W. & Rowin, M. E. Sialidase treatment exposes the β1-integrin active ligand binding site on HL60 cells and increases binding to fibronectin. Cell Adhes. Commun. 7, 491–500 (2000).
Seales, E. C. et al. Hypersialylation of β1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 65, 4645–4652 (2005).
Pan, D. & Song, Y. Role of altered sialylation of the I-like domain of β1 integrin in the binding of fibronectin to β1 integrin: thermodynamics and conformational analyses. Biophys. J. 99, 208–217 (2010).
Hou, S. et al. Importance of membrane-proximal N-glycosylation on integrin β1 in its activation and complex formation. FASEB J. 30, 4120–4131 (2016).
de Melker, A. A., van der Horst, G., Calafat, J., Jansen, H. & Borst, J. c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J. Cell Sci. 114, 2167–2178 (2001).
Stang, E. et al. Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits. Mol. Biol. Cell 15, 3591–3604 (2004).
Pruss, R. M. & Herschman, H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc. Natl Acad. Sci. USA 74, 3918–3921 (1977).
Yu, H. A. & Pao, W. Targeted therapies: Afatinib–new therapy option for EGFR-mutant lung cancer. Nat. Rev. Clin. Oncol. 10, 551–552 (2013).
Byyny, R. L., Orth, D. N., Cohen, S. & Doyne, E. S. Epidermal growth factor: effects of androgens and adrenergic agents. Endocrinology 95, 776–782 (1974).
Glanz, V. Y., Myasoedova, V. A., Grechko, A. V. & Orekhov, A. N. Inhibition of sialidase activity as a therapeutic approach. Drug Des. Devel. Ther. 12, 3431–3437 (2018).
Moons, S. J. et al. Structure-activity relationship of metabolic sialic acid inhibitors and labeling reagents. ACS Chem. Biol. 17, 590–597 (2022).
Jones, M. C., Caswell, P. T. & Norman, J. C. Endocytic recycling pathways: emerging regulators of cell migration. Curr. Opin. Cell Biol. 18, 549–557 (2006).
Furtak, V., Hatcher, F. & Ochieng, J. Galectin-3 mediates the endocytosis of β-1 integrins by breast carcinoma cells. Biochem. Biophys. Res. Commun. 289, 845–850 (2001).
Shafaq-Zadah, M. et al. Persistent cell migration and adhesion rely on retrograde transport of β1 integrin. Nat. Cell Biol. 18, 54–64 (2016).
Monti, E. et al. Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv. Carbohydr. Chem. Biochem. 64, 403–479 (2010).
Maurice, P. et al. New insights into molecular organization of human neuraminidase-1: transmembrane topology and dimerization ability. Sci Rep. 6, 38363 (2016).
Seyrantepe, V. et al. Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells. J. Biol. Chem. 279, 37021–37029 (2004).
Zanchetti, G. et al. Sialidase NEU3 is a peripheral membrane protein localized on the cell surface and in endosomal structures. Biochem. J. 408, 211–219 (2007).
Monti, E. et al. Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane. Biochem. J. 349, 343–351 (2000).
Monti, E., Preti, A., Nesti, C., Ballabio, A. & Borsani, G. Expression of a novel human sialidase encoded by the NEU2 gene. Glycobiology 9, 1313–1321 (1999).
Bonten, E., van der Spoel, A., Fornerod, M., Grosveld, G. & d’Azzo, A. Characterization of human lysosomal neuraminidase defines the molecular basis of the metabolic storage disorder sialidosis. Genes Dev. 10, 3156–3169 (1996).
Sphranger, J., Gehler, J. & Cantz, M. Mucolipidosis I–a sialidosis. Am. J. Med. Genet. 1, 21–29 (1977).
Lukong, K. E. et al. Intracellular distribution of lysosomal sialidase is controlled by the internalization signal in its cytoplasmic tail. J. Biol. Chem. 276, 46172–46181 (2001).
Albohy, A., Li, M. D., Zheng, R. B., Zou, C. & Cairo, C. W. Insight into substrate recognition and catalysis by the human neuraminidase 3 (NEU3) through molecular modeling and site-directed mutagenesis. Glycobiology 20, 1127–1138 (2010).
Nunez, M., Mayo, K. H., Starbuck, C. & Lauffenburger, D. pH sensitivity of epidermal growth factor receptor complexes. J. Cell. Biochem. 51, 312–321 (1993).
Sardet, C., Counillon, L., Franchi, A. & Pouysségur, J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science 247, 723–726 (1990).
Koivusalo, M. et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 188, 547–563 (2010).
Stock, C. & Pedersen, S. F. Roles of pH and the Na+/H+ exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective? Semin. Cancer Biol. 43, 5–16 (2017).
Fliegel, L. Role of genetic mutations of the Na+/H+ exchanger isoform 1, in human disease and protein targeting and activity. Mol. Cell. Biochem. 476, 1221–1232 (2021).
Stüwe, L. et al. pH dependence of melanoma cell migration: protons extruded by NHE1 dominate protons of the bulk solution. J. Physiol. 585, 351–360 (2007).
Takahashi, E. et al. p90RSK is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J. Biol. Chem. 274, 20206–20214 (1999).
Scholz, W. et al. Hoe 694, a new Na+/H+ exchange inhibitor and its effects in cardiac ischaemia. Br. J. Pharmacol. 109, 562–568 (1993).
Lawrence, S. P., Holman, G. D. & Koumanov, F. Translocation of the Na+/H+ exchanger 1 (NHE1) in cardiomyocyte responses to insulin and energy-status signalling. Biochem. J. 432, 515–523 (2010).
Liu, L., Schlesinger, P. H., Slack, N. M., Friedman, P. A. & Blair, H. C. High capacity Na+/H+ exchange activity in mineralizing osteoblasts. J. Cell. Physiol. 226, 1702–1712 (2011).
Iacobini, C., Fantauzzi, C. B., Pugliese, G. & Menini, S. Role of galectin-3 in bone cell differentiation, bone pathophysiology and vascular osteogenesis. Int. J. Mol. Sci. 18, 2481 (2017).
Iacobini, C. et al. Galectin-3 is essential for proper bone cell differentiation and activity, bone remodeling and biomechanical competence in mice. Metabolism 83, 149–158 (2018).
Chabadel, A. et al. CD44 and β3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol. Biol. Cell 18, 4899–4910 (2007).
Stegmayr, J. et al. Extracellular and intracellular small-molecule galectin-3 inhibitors. Sci. Rep. 9, 2186 (2019).
Salameh, B. A., Cumpstey, I., Sundin, A., Leffler, H. & Nilsson, U. J. 1H-1,2,3-Triazol-1-yl thiodigalactoside derivatives as high affinity galectin-3 inhibitors. Bioorg. Med. Chem. 18, 5367–5378 (2010).
Roy, M. et al. Galectin-8 modulates human osteoclast activity partly through isoform-specific interactions. Life Sci. Alliance 7, e202302348 (2024).
Howes, M. T. et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 190, 675–691 (2010).
Aguet, F. et al. Membrane dynamics of dividing cells imaged by lattice light-sheet microscopy. Mol. Biol. Cell 27, 3418–3435 (2016).
Kural, C. et al. Asymmetric formation of coated pits on dorsal and ventral surfaces at the leading edges of motile cells and on protrusions of immobile cells. Mol. Biol. Cell 26, 2044–2053 (2015).
Sottile, J. & Chandler, J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol. Biol. Cell 16, 757–768 (2005).
Ezratty, E. J., Partridge, M. A. & Gundersen, G. G. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7, 581–590 (2005).
Chao, W. T. & Kunz, J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 583, 1337–1343 (2009).
Ezratty, E. J., Bertaux, C., Marcantonio, E. E. & Gundersen, G. G. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J. Cell Biol. 187, 733–747 (2009).
Shi, F. & Sottile, J. MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. J. Cell Sci. 124, 4039–4050 (2011).
Almeida-Souza, L. et al. A flat BAR protein promotes actin polymerization at the base of clathrin-coated pits. Cell 174, 325–337 (2018).
Moreno-Layseca, P. et al. Cargo-specific recruitment in clathrin- and dynamin-independent endocytosis. Nat. Cell Biol. 23, 1073–1084 (2021).
Renard, H. F. et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 517, 493–496 (2015).
Simunovic, M. et al. Friction mediates scission of tubular membranes scaffolded by BAR proteins. Cell 170, 172–184 (2017).
Shafaq-Zadah, M., Dransart, E. & Johannes, L. Clathrin-independent endocytosis, retrograde trafficking, and cell polarity. Curr. Opin. Cell Biol. 65, 112–121 (2020).
Johannes, L. & Shafaq-Zadah, M. SNAP-tagging the retrograde route. Methods Cell Biol. 118, 139–155 (2013).
Gautier, A. et al. An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 15, 128–136 (2008).
Blay, J. & Brown, K. D. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J. Cell. Physiol. 124, 107–112 (1985).
Kaukonen, R., Jacquemet, G., Hamidi, H. & Ivaska, J. Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat. Protoc. 12, 2376–2390 (2017).
Hennigan, R. F., Hawker, K. L. & Ozanne, B. W. Fos-transformation activates genes associated with invasion. Oncogene 9, 3591–3600 (1994).
Elices, M. J., Urry, L. A. & Hemler, M. E. Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J. Cell Biol. 112, 169–181 (1991).
Chen, Y. et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell 40, 818–834 (2022).
Carpier, J. M. et al. Rab6-dependent retrograde traffic of LAT controls T cell activation. J. Exp. Med. 215, 1245–1265 (2018).
Blangy, A. et al. The osteoclast cytoskeleton—current understanding and therapeutic perspectives for osteoporosis. J. Cell Sci. 133, jcs244798 (2020).
de Geest, N. et al. Systemic and neurologic abnormalities distinguish the lysosomal disorders sialidosis and galactosialidosis in mice. Hum. Mol. Genet. 11, 1455–1464 (2002).
Amith, S. R. & Fliegel, L. Regulation of the Na+/H+ exchanger (NHE1) in breast cancer metastasis. Cancer Res. 73, 1259–1264 (2013).
Boll, I., Jensen, P., Schwämmle, V. & Larsen, M. R. Depolarization-dependent induction of site-specific changes in sialylation on N-linked glycoproteins in rat nerve terminals. Mol. Cell. Proteomics 19, 1418–1435 (2020).
Abe, C., Yi, Y., Hane, M., Kitajima, K. & Sato, C. Acute stress-induced change in polysialic acid levels mediated by sialidase in mouse brain. Sci Rep. 9, 9950 (2019).
Cohen, S. & Elliott, G. A. The stimulation of epidermal keratinization by a protein isolated from the submaxillary gland of the mouse. J. Invest. Dermatol. 40, 1–5 (1963).
Sigismund, S. et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl Acad. Sci. USA 102, 2760–2765 (2005).
Shafaq-Zadah, M. et al. Spatial N-glycan rearrangement on a5b1 integrin nucleates galectin-3 oligomers to determine endocytic fate. Preprint at bioRxiv https://doi.org/10.1101/2023.10.27.564026 (2023).
Stowell, S. R., Ju, T. & Cummings, R. D. Protein glycosylation in cancer. Annu. Rev. Pathol. 10, 473–510 (2015).
Willems, A. P., van Engelen, B. G. & Lefeber, D. J. Genetic defects in the hexosamine and sialic acid biosynthesis pathway. Biochim. Biophys. Acta 1860, 1640–1654 (2016).
Smith, B. A. H. & Bertozzi, C. R. The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 20, 217–243 (2021).
Hayashi, T. & Sakamoto, S. Radioimmunoassay of human epidermal growth factor–hEGF levels in human body fluids. J. Pharmacobiodyn. 11, 146–151 (1988).
Beardmore, J. M. & Richards, R. C. Concentrations of epidermal growth factor in mouse milk throughout lactation. J. Endocrinol. 96, 287–292 (1983).
Grau, M., Rodriguez, C., Soley, M. & Ramirez, I. Relationship between epidermal growth factor in mouse submandibular glands, plasma, and bile: effects of catecholamines and fasting. Endocrinology 135, 1854–1862 (1994).
Gann, P. H., Chatterton, R., Vogelsong, K., Grayhack, J. T. & Lee, C. Epidermal growth factor-related peptides in human prostatic fluid: sources of variability in assay results. Prostate 32, 234–240 (1997).
Poullet, P., Carpentier, S. & Barillot, E. myProMS, a web server for management and validation of mass spectrometry-based proteomic data. Proteomics 7, 2553–2556 (2007).
The, M., MacCoss, M. J., Noble, W. S. & Käll, L. Fast and accurate protein false discovery rates on large-scale proteomics data sets with Percolator 3.0. J. Am. Soc. Mass Spectrom. 27, 1719–1727 (2016).
Valot, B., Langella, O., Nano, E. & Zivy, M. MassChroQ: a versatile tool for mass spectrometry quantification. Proteomics 11, 3572–3577 (2011).
Supek, F., Bošnjak, M., Škunca, N. & Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6, e21800 (2011).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–d552 (2022).
Lonowski, L. A. et al. Genome editing using FACS enrichment of nuclease-expressing cells and indel detection by amplicon analysis. Nat. Protoc. 12, 581–603 (2017).
Cukierman, E., Pankov, R., Stevens, D. R. & Yamada, K. M. Taking cell-matrix adhesions to the third dimension. Science 294, 1708–1712 (2001).
Chen, B. C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014).
Sofroniew, N. et al. napari: A multi-dimensional image viewer for Python. Zenodo https://doi.org/10.5281/zenodo.3555620 (2025).
Jaqaman, K. et al. Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods 5, 695–702 (2008).
Boulanger, J. et al. Patch-based nonlocal functional for denoising fluorescence microscopy image sequences. IEEE Trans. Med. Imaging 29, 442–454 (2010).
Prigent, S. et al. BioImageIT: open-source framework for integration of image data management with analysis. Nat. Methods 19, 1328–1330 (2022).
Wang, Z. et al. Syngeneic animal models of tobacco-associated oral cancer reveal the activity of in situ anti-CTLA-4. Nat. Commun. 10, 5546 (2019).
Vitale-Cross, L. et al. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev. Res. 5, 562–573 (2012).
Sequeira, I. et al. Genomic landscape and clonal architecture of mouse oral squamous cell carcinomas dictate tumour ecology. Nat. Commun. 11, 5671 (2020).
Padilla-Rodriguez, M. et al. The actin cytoskeletal architecture of estrogen receptor positive breast cancer cells suppresses invasion. Nat. Commun. 9, 2980 (2018).
Maurin, J. et al. The β-tubulin isotype TUBB6 controls microtubule and actin dynamics in osteoclasts. Front. Cell. Dev. Biol. 9, 778887 (2021).
Acknowledgements
We thank L. Fliegel (University of Alberta, Edmonton, Canada), T. Boltje, C. Büll (Radboud University, Radboud Consortium for Glycoscience, Nijmegen, The Netherlands), T. L. Kirchhausen (Harvard Medical School, Boston, USA) and U. J. Nilsson (Lund University, Sweden) for generously providing reagents and cell lines; Z. Yang and Y. Narimatsu (Copenhagen Center for Glycomics, Denmark) for advice and help on the generation of neuraminidase-knockout CRISPR cells; S. Sigismund (European Institute of Oncology, Milan, Italy) for discussions on EGFR trafficking and signalling; P. Maurice (Université de Reims Champagne–Ardenne, France) for advice on sialidase assays; T. V. v. Zanten and G. Pradeep (NCBS, Bangalore, India) for their microscopy support; J. Salamero (Institut Curie, Paris, France) for managing and funding of the lattice light-sheet microscope and J. Dhayni (CRBM, CNRS, Montpellier, France) for help with the osteoclast experiments. We acknowledge the CurieCoreTech facilities for Cytometry, Recombinant Proteins, and the Cell and Tissue Imaging (PICT-IBiSA) and the Nikon Imaging Centre at Institut Curie are acknowledged. This work was supported by grants from Labex Cell(n)Scale (11-LABX-0038) and Idex Paris Sciences et Lettres (ANR-10-IDEX-0001-02 PSL; L.J. and SERPICO); the Mizutani Foundation for Glycosciences, (grant number 200014; L.J.); Q-Life ANR-17-CONV-0005 (L.J.); Agence Nationale pour la Recherche ANR-16-CE23-0005 (L.J.), ANR-19-CE13-0001-01 (L.J.), ANR-20-CE15-0009-01 (L.J.), ANR-22-CE11-0030-03 (L.J.), and ANR-23-CE13-0033-01 (A.B.); Fondation pour la Recherche Médicale EQU202103012926 (L.J.); Labex DCBiol ANR-11-LABX-0043 (L.J.); ITMO Cancer 18CQ091 (L.J. and SERPICO); A.F. is an F.R.S.–FNRS Chercheur Qualifiée and member of the WEL Research Institute; France–BioImaging National Infrastructure ANR-10-INBS-04-07 (SERPICO); ‘La Région Île-de-France’ grant number EX061034 (D.L.); ITMO Cancer of Aviesan and INCa on funds administered by Inserm (grant number 21CQ016-00; D.L.); Department of Atomic Energy, Government of India (Project Identification number RTI 4006; S.M.); JC Bose fellowship from the Department of Science and Technology (GoI; S.M.); ARC-Passerelle fellowship (E.M.); Margadarshi Fellowship (IA/M/15/1/502018; S.M.); Leverhulme International Professorship award (LIP-2021-017; S.M.); Curie–NCBS Campus fellowship (E.M.); Novo Nordisk Foundation Grant NNF0067602 (T.D.M.); The Novo Nordisk Foundation and Danish National Research Foundation (DNRF107; H.C.); University Grants Commission (UGC) of India for graduate fellowship (R.G.); Cancer Research UK (DCRPGF\100002; P.C.); the Wellcome Trust (203128/A/16/Z and 226804/Z/22/Z; P.C.); European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska–Curie grant agreement number 847718847718 (D.G.); French National Research Infrastructure France–BioImaging (ANR-10-INBS-01; Cell and Tissue Imaging (PICT-IBiSA), Nikon Imaging Centre at Institut Curie); R.W. and Y.N. are supported by the NCI intramural program (ZIA BC 011682); and Netherlands Organization for Scientific Research (VICI grant number 09150182010010; D.J.L.).
Author information
Authors and Affiliations
Contributions
Conceptualization: L.J., C.W., E.M., M.R.L. and H.L. Methodology: L.J., C.W., E.M., A.F., E.D., M.S.-Z., T.D.M., R.W., J.H.R.H., C.A.V.-C., R.G., S.M. and A.B. Critical advice on the conceptualization and materials: H.C. and D.J.L. Investigation: E.M., C.W., A.F., J.H.R.H., C.A.V.-C., L.L., E.D., M.S.-Z., D.B., T.D.M., D.G., A.P. and Y.N. Visualization: E.M., C.W., A.F., J.H.R.H., C.A.V.-C., V.C., A.A.S., B.L., D.L., E.D. and M.S.-Z. Funding acquisition: L.J., S.M., R.W., E.M. and P.C. Project administration: L.J., C.W., S.M., R.W. and P.C. Writing of the original draft: L.J., E.M. and C.W. All authors edited and agreed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.L. is a shareholder in Galecto Biotech AB, a company that is developing galectin inhibitors. The other authors declare no conflicts of interest.
Peer review
Peer review information
Nature Cell Biology thanks Benjamin Schumann, Derek Toomre and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 EGF induces Gal3 binding to cells in vitro and in vivo.
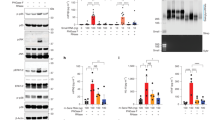
a, Serum-starved MDA-MB-231 cells were incubated for 1 h on ice with 200 nM Alexa488-labelled Gal3 in the presence of EGF at the indicated concentrations. Fluorescence intensity was determined for 4 independent experiments (≈100 cells per condition). One-way ANOVA with Dunnett’s multiple comparison test. b, Gal3 (green) binding to HN12 cells. HN12 cells were incubated for 30 min at 37 °C with 200 nM AFT or 1 mM DANA in serum-free media before incubation for 1 h on ice in serum-free media with 200 nM Alexa488-labelled Gal3 in the presence or absence of 100 ng/ml EGF. Fluorescence intensity was determined from 6 independent experiments (≈200 cells per condition) and represented as %CTRL. One-way ANOVA with Dunnett’s multiple comparison test. SUM intensity confocal images are shown. Scale bar, 10 µm. Nuclei in blue. c, Gal3 binding to mouse embryonic fibroblasts (MEFs). MEFs were serum starved for 30 min at 37 °C in the presence or absence of sialidase (1 unit) or 1 mM DANA. In the continued presence or absence of sialidase or DANA, the cells were then incubated for 30 min on ice with 200 nM Alexa488-labelled Gal3 in the presence or absence of 100 ng/ml EGF. Total fluorescence/area from SUM confocal images was quantified from one representative experiment (≈30 cells per condition) out of two and represented as %CTRL. One-way ANOVA with Turkey’s multiple comparison test. Maximum intensity images are shown. Scale bar, 10 µm. d, Gal3 binding to NR6 cells. NR6 cells (mutant EGFR) and NR6 expressing functional EGFR were serum starved for 30 min at 37 °C before incubation for 30 min on ice with 200 nM Alexa488-labelled Gal3 in the presence or absence of 100 ng/ml EGF. Total fluorescence/area from SUM confocal images was quantified from one representative experiment (≈25 cells per condition) out of two and represented as %CTRL. One-way ANOVA with Turkey’s multiple comparison test. e, In vivo experiment in mice. 6 h after injection of 30 mg/kg AFT into mice, tongues were excised and lysed in RIPA buffer, lysates from each of the mice were probed with antibodies against pEGFR (Y1068) and tubulin. Data were normalized to first lane (cohort 1 orange triangle, cohort 2 blue triangles). Mann–Whitney test. f, EGF-induced signalling. MDA-MB-231 cells were serum starved with the indicated inhibitors for 1 h at 37 °C before incubation for 10 min at 37 °C in the continued presence or absence of the inhibitors and of 100 ng/ml EGF, as indicated. Cells were lysed in RIPA buffer, and lysates probed with the indicated antibodies. g,h, Quantification of 3 independent experiments as in (f). Determination of pERK signal (g) or the pRSK signal (h). One-way ANOVA with Tukey’s multiple comparison test. i, General inhibition of sialylation. MDA-MB-231 cells were incubated for 72 h at 37 °C with 10 µM sialostatin (STI) or DMSO, serum starved for 30 min at 37 °C and incubated for 1 h on ice with 200 nM Alexa488-labelled Gal3 in the presence or absence of 100 ng/ml EGF. Total fluorescence/area from SUM confocal images was quantified from 3 independent experiments (≈75 cells per condition) and represented as %CTRL. One-way ANOVA with Holm-Šídák’s multiple comparisons test. j, EGF effect on SNA binding. MDA-MB-231 cells were serum starved for 1 h at 37 °C before incubation for 1 h on ice with 5 µg/ml of SNA-Cy5 in the presence or absence of 100 ng/ml EGF. Total fluorescence/area from SUM confocal images was quantified from 3 independent experiments (≈75 cells per condition) and represented as %CTRL. One-way ANOVA with Dunnett’s multiple comparison test. k, EGF effect on Lectenz binding. MDA-MB-231 cells were serum starved for 1 h at 37 °C, incubated for 1 h on ice with 5 µg/ml of the α2-3 Sia linkage-specific biotinylated Lectenz reagent in the presence or absence of 100 ng/ml EGF, fixed and labelled with neutravidin-488. Total fluorescence/area from SUM confocal images was quantified from one representative experiment (≈50 cells per condition) out of 2 and represented as %CTRL. Unpaired t-test. l, EGF effect on RCA lectin binding. MDA-MB-231 cells were serum starved for 30 min in the presence of DANA before incubation on ice with 5 µg/ml biotin coupled RCA lectin in the presence or absence of EGF (100 ng/ml) and/or DANA. Total fluorescence/area from SUM confocal images was quantified from 4 independent experiments (≈100 per condition). One-way ANOVA, Dunnett’s multiple comparison test. For all quantifications except (e), means ± SEM are shown. For (e) the solid line indicates median of all data points. In (a,b,i–l), means from separate experiments are indicated by solid dots, and measurements of individual cells have different coloured symbols for each experiment. ns = p > 0.05, * p ≤ 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Source numerical data and unprocessed blots are provided.
Extended Data Fig. 2 EGF induces de-sialylation via MAPK–NHE1–neuraminidase activation.
a, Neuraminidase screen. MEFs were transfected for 72 h at 37 °C with 100 nM siRNA against individual neuraminidases (25 nM of each oligonucleotide). Cells were then serum starved for 30 min at 37 °C, before incubation on ice with 5 µg/ml Alexa488-labelled Gal3 in the presence or absence of 100 ng/ml EGF. Results of one experiment in duplicate. Data from 30 randomly picked cells in each set were analysed and quantified. One-way ANOVA with Turkey’s multiple comparison test. b, Screen of multiple clones for Neu1, Neu3, and Neu1/3 CRISPR-edited MDA-MB-231 cells. Cells were serum starved for 1 h at 37 °C before incubation for 1 h on ice with 200 nM Alexa488-labelled Gal3 in the presence or absence of 100 ng/ml EGF. SUM intensity confocal images shown. Scale bar, 10 µm. c, Quantification of total fluorescence/area from SUM confocal images from one representative experiment as in (b) are shown as %CTRL. d, Autophosphorylation of EGFR. MDA-MB-231 cells were serum starved for 30 min at 37 °C before incubation for 10 min at 37 °C in the presence or absence of 100 ng/ml EGF. Cells were lysed in RIPA buffer and western blotting performed with anti-pEGFRY1068 antibodies. Right: Quantification of 3 independent experiments. One-way ANOVA Tukey’s multiple comparison test. e, Neuraminidase activity on ice. MDA-MB-231 cell extracts were prepared by sonication. Extracts were supplemented with or without 1 mM DANA, then 200 mM 4-MUNANA was added to each triplicate and incubated in the continued presence of DANA for 2 h on ice or at 37 °C. DANA treated extract values were subtracted from matched 37 °C and 4 °C samples. Remaining activity was expressed as a percentage of 37 °C. 3 independent experiments. Unpaired t-test. f, Electrophoretic mobility of β1 integrin. Patient fibroblast harbouring mutations in B4GALT1, MGAT2, or NEU1 were lysed, and lysates were analysed by western blotting with anti-β1 integrin antibodies. Tubulin served as loading control. g, pERK signalling. Patient fibroblasts were serum starved for 30 min at 37 °C and then incubated for 10 min at 37 °C in the presence of absence of 100 ng/ml EGF. Lysates of these cells were analysed by western blotting with the indicated antibodies. Note that pERK signals were increased by EGF treatment in all conditions. Tubulin served as loading control. Tubulin band is indicated by star. h, Gal3 binding under NHE1 interference conditions. CRISPR-edited NHE1 knockout MDA-MB-231 cells and GFP-NHE1 mutant addback cells were serum starved for 30 min at 37 °C before incubation for 1 h on ice with or without 100 ng/ml EGF. Total fluorescence/area from SUM confocal images were quantified from 4 independent experiments and represented as %CTRL for each cell line. One-way ANOVA with Tukey’s multiple comparison test. i, MAPK signalling is required for EGF-induced Gal3 binding. MDA-MB-231 cells were serum starved for 30 min at 37 °C, and then incubated for 1 h on ice with 200 nM Alexa488-labelled Gal3 in the presence or absence of the indicated inhibitors and of 100 ng/ml EGF. SUM intensity confocal images are shown. Scale bar, 10 µm. Nuclei in blue. j, MDA-MB-231 cells were serum starved for 30 min at 37 °C in the presence or absence of the indicated inhibitors (250 nM wortmannin, 1 µM AKTi, 200 nM PI-103, 200 nM RSKi), before incubation for 10 min with or without 100 ng/ml EGF in the continued presence of the inhibitors. Cells were lysed and analysed via western blotting with the indicated antibodies. Top: Representative blot of one out of 4 independent experiments. Bottom: Quantification of 4 independent experiments. One-way ANOVA Dunnett’s multiple comparison test. k, Gal3 binding in the presence of AKT and RSK inhibitors. MDA-MB-231 cells were treated with the indicated inhibitors as in (j), followed by incubation for 1 h on ice in the presence of 200 nM Alexa488-Gal3. Quantification of 6 independent experiments (≈150 cells per condition). One-way ANOVA Dunnett’s multiple comparison test. Note that AKTi did not interfere with the EGF-induced increase in Gal3 binding. Wortmannin was excluded from analysis as it produced highly variable results on Gal3 binding. l, NHE1 cell-surface levels. MDA-MB-231 cells were serum starved for 10 min at 37 °C, incubated for 10 min on ice or at 37 °C in the presence or absence of 100 ng/ml EGF, followed by surface labelling for 1 h on ice with 800 μM Sulfo-NHS-biotin. Cells were lysed with RIPA buffer on ice, biotinylated proteins were pulled down overnight at 4 °C with streptavidin beads, followed by western analysis with the indicated antibodies. Note that plasma membrane levels of NHE1 remain unchanged in all conditions. Right: Quantification of 3 independent experiments. One-way ANOVA Dunnett’s multiple comparison test. For all quantifications, means ± SEM are shown. In (h,k), means from separate experiments are indicated by solid dots, and measurements of individual cells have different coloured symbols for each experiment. ns = p > 0.05, * p ≤ 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Source numerical data and unprocessed blots are provided.
Extended Data Fig. 3 EGF drives clathrin-independent endocytosis and triggers formation of CLICs.
a, The anti-α3 integrin antibody by itself does not stimulate α3 integrin internalization. MDA-MB-231 cells were incubated on ice at sub-saturating concentrations (0.2 µg/ml) with fluorescent anti-α3 integrin antibody (PE in red) and washed. Uptake was measured at 37 °C for 10 min in the presence of increasing concentrations of non-fluorescence tagged anti-α3 integrin antibody (0 to 100 µg/ml). Representative images of 3 independent experiments. Scale bar, 20 µm. b, Quantification of data as in (b). Fluorescence intensity of anti-α3 integrin–PE antibody uptake in the presence of non-fluorescence tagged anti-α3 integrin antibody (≈150 cells per condition). One-way ANOVA Dunnett’s multiple comparison test. ns p > 0.05. c, EGF-induced β1 integrin uptake (quantification in Fig. 4c). MDA-MB-231 cells were serum starved for 30 min at 37 °C and then incubated for 10 min at 37 °C with 1 µg/ml of anti-β1 integrin antibody K20 in the presence or absence of 100 ng/ml EGF. Note that EGF stimulates β1 integrin uptake. SUM intensity confocal images are shown. Scale bar, 10 µm. d, Investigation of caveolar function in EGF-stimulated β1 integrin uptake. Wild-type and caveolin-1 (CAV1) knockout Hela cells were serum starved for 30 min at 37 °C and then incubated for 10 min at 37 °C with 1 µg/ml of anti-β1 integrin antibody K20 in the presence or absence of EGF. Note that EGF stimulated β1 integrin uptake persists in the absence of caveolin-1 expression. One-way ANOVA Dunnett’s multiple comparison test on all data points. e, TNF-induced β1 integrin uptake (quantification in Fig. 4c). MDA-MB-231 cells were serum starved for 30 min at 37 °C and then incubated for 10 min at 37 °C with 1 µg/ml of anti-β1 integrin antibody K20 in the presence or absence of 100 ng/ml TNF. Note that TNF stimulates β1 integrin uptake. SUM intensity confocal images are shown. Scale bar, 10 µm. f, Monitoring pH at the cell surface next to α3 integrin. MDA-MB-231 cells were continuously incubated with AcidiFluor-coupled anti-α3 integrin antibody at 37 °C and imaged in TIRF modalities. pH 4 buffer was added at the indicated timepoint. Note the acidification-induced increase in fluorescence. g,h, Lattice light-sheet microscopy imaging. Mean intensity (g) and maximum intensity (h) of β1 integrin endocytic tracks are shown from 3 independent experiments as in Fig. 5c–e. 1,802 tracks from 16 CTRL cells, and 2,157 tracks from 18 EGF-treated cells. Unpaired t-test. Note that mean and maximum intensities decreased after EGF stimulation. i, Monitoring pH responsiveness of Tf-AcidiFluor Orange. MDA-MB-231 cells were incubated with 10 µg/ml Tf-AcidiFluor Orange for 10 min before fixation and acid stripping. Images were acquired in pH 7.4 imaging buffer before switching to pH 5 imaging buffer. j, Monitoring functionality of Tf-AcidiFluor Orange. MDA-MB-231 cells were either directly imaged (autofluorescence) or pulsed with Tf-AcidiFluor Orange (10 µg/ml) and unlabelled Tf at 1 mg/ml, or with Tf-AcidiFluor Orange (10 µg/ml) in the absence of competing unlabelled Tf. k–m, Representative electron micrographs. (k) CLICs containing the indicated HRP-coupled cargo proteins. Scale bars, 200 nm. (l) Vesicular structure containing HRP-coupled anti-α3 integrin antibody (ASC-1). Scale bar, 200 nm. (m) Macropinocytic structure containing HRP-coupled anti-β1 integrin antibody (K20). Scale bar, 1 µm. Representative images of quantifications in Fig. 5b. n, Workflow for the classification of AP2-positive and AP2-negative uptake events, as imaged by LLSM. For all quantifications in this figure, means ± SEM are shown. In (b), means from separate experiments are indicated by solid dots, and measurements of individual cells have different coloured symbols for each experiment. ns p > 0.05, **** p < 0.0001. Source numerical data are provided.
Extended Data Fig. 4 EGF-induced de-sialylation triggers retrograde trafficking to the Golgi.
a, Retrograde trafficking of Gal3. HeLa cells stably expressing GalT–GFP-SNAP were continuously incubated for 4 h at 37 °C with benzylguanine (BG)-labelled Gal3 in the presence or absence of 100 ng/ml EGF. GFP-trap pulldown samples were analysed by western blotting with the indicated antibodies. Note the reduced electrophoretic mobility of the conjugate between Gal3 and GalT–GFP-SNAP (red dashed box). Representative blot of one out of 3 independent experiments. b, Retrograde trafficking analysis by immunofluorescence. HeLa cells stably expressing GalT–GFP-SNAP were continuously incubated for 1 h at 37 °C with benzylguanine (BG) and Seta555-labelled Gal3. Cells were fixed and analysed by confocal microscopy. Note that the fraction of internalized Gal3 in perinuclear Golgi increases upon incubation with 100 ng/ml of EGF. Representative maximum intensity images of 3 independent experiments are shown. Unpaired t-test, minimum of 50 cells per condition. c, Cluster analysis of the summarized list of biological process gene ontology phrases associated with the list of 26 Gal3 binders that were significantly enriched after EGF stimulation. Spot size equals -LOG2 P value, spot colour equals -LOG10 GO annotations. d, Retrograde trafficking of α3 integrin. HeLa cells stably expressing GalT–GFP-SNAP were continuously incubated for 4 h at 37 °C with benzylguanine (BG)-labelled anti-α3 integrin antibody (ASC-1) in the presence or absence of 100 ng/ml EGF. The anti-α3 integrin antibody–SNAP conjugate in GFP-trap pulldowns on lysates from the indicated conditions of 4 independent experiments was quantified by western blotting. Note that EGF increases the retrograde trafficking of α3 integrin. Unpaired t-test. e–h, Cell migration on cell-driven matrices. MDA-MB-231 cells were seeded in 1% FCS on cell derived matrices and cell migration was monitored for 16 h at 37 °C in the presence or absence of indicated inhibitors. Velocity and accumulated distance were calculated from 3 independent experiments (≈50 cells analysed per condition), using the manual tracking software ImageJ. One-way ANOVA with Dunnett’s multiple comparison test. For all quantifications in this figure, means ± SEM are shown. In (e–h), means from separate experiments are indicated by solid dots, and measurements of individual cells have different coloured symbols for each experiment. ****p < 0.0001. Source numerical data and unprocessed blots are provided.
Supplementary information
Supplementary Tables 1–6
Table 1: Gal3 and SNA interacting proteins (related to Fig. 2c). MDA-MB-231 cells were subjected to pulldown with cell surface-bound SNA and Gal3, and protein identification by mass spectrometry. This table lists the 93 proteins common to both datasets. Table 2: Proteins that are significantly decreased in SNA pulldowns after incubation with EGF (related to Fig. 2d). Mass spectrometry analysis of pulldowns with SNA that was bound to the surface of MDA-MB-231 cells in the presence or absence of EGF. List of 52 proteins whose binding to SNA was significantly decreased upon incubation with EGF. Table 3: Proteins significantly enriched in Gal3 pulldowns after EGF (related to Fig. 6d). Pulldowns with Gal3 that was bound to the surface of HeLa cells in the presence or absence of EGF. List denotes the 26 proteins whose binding to Gal3 was significantly enhanced after EGF incubation, and proteins uniquely found in Gal3+ EGF condition. Table 4: Retrograde proteome of HeLa cells (related to Fig. 6f). HeLa cells expressing GalT-GFP-SNAP were reacted with NHS-PEG9-BG versus DMSO control. List denotes all proteins that were significantly enriched in pulldowns from NHS-PEG9-BG modified cells. Table 5: EGF-sensitive Gal3 interacting proteins found in the retrograde proteome (related to Fig. 6f). Comparison of proteins that were found enriched in binding to Gal3 upon incubation with EGF and that were present in the retrograde proteome. Table 6: Table of siRNAs, primers, and CRISPR guides that were used in this study.
Supplementary Video 1
TIRF recording of Tf-Acidifluor Orange. MDA-MB-231 cells were incubated at 37 °C with 10 µg/mL of Acidifluor Orange labeled Tf. Cells were washed with imaging buffer (1XMI glucose) and images acquired by TIRF at 37 °C for 3 min with 2 s time intervals in the absence of EGF. Cell boundary is marked from accompanying epifluorescence image.
Supplementary Video 2
TIRF recording of Tf-Acidifluor Orange in the presence of EGF. MDA-MB-231 cells were incubated at 37 °C with 10 µg/mL of Acidifluor Orange labeled Tf. Cells were washed with imaging buffer (1XMI glucose) and images were acquired by TIRF at 37 °C for 3 min with 2 s time intervals in the presence of 100 ng/mL EGF. Cell boundary is marked from accompanying epifluorescence image.
Supplementary Video 3
LLSM recording of β1 integrin endocytosis in the absence of EGF (related to Fig. 5c–e,g). Genome edited SUM159 cells expressing AP2-GFP (green) were incubated for 2 min with 2 μg/mL of anti- β1 integrin-Cy3 (magenta) antibody K20, and transferred to the LLSM for imaging at 27 °C. Full 3D volumes of 60 planes were acquired within 2 s. Colors indicate: In white, β1 integrin uptake tracks that were AP2-negative; in green, β1 integrin uptake tracks that were AP2-positive; in red, non-endocytic β1 integrin tracks.
Supplementary Video 4
LLSM recording of β1 integrin endocytosis in the presence of EGF (related to Fig. 5c–e,g). Genome edited SUM159 expressing AP2-GFP (green) were incubated for 2 min with 2 μg/mL anti-β1 integrin-Cy3 (magenta) antibody K20 in the presence of 100 ng/mL EGF, and transferred to the LLSM for imaging at 27 °C. Full 3D volumes of 60 planes were acquired within 2 s. Colors indicate: In white, β1 integrin uptake tracks that were AP2-negative; in green, β1 integrin uptake tracks that were AP2-positive; in red, non-endocytic β1 integrin tracks.
Supplementary Video 5
LLSM recording of Tf endocytosis in the absence of EGF (related to Fig. 5c–e,g). Genome edited SUM159 expressing AP2-GFP (green) were incubated for 2 min with 1 µg/mL Tf-Cy3 (magenta) and transferred to the LLSM for imaging at 27 °C. Full 3D volumes of 60 planes were acquired within 2 s. Colors indicate: In white, Tf-Cy3 uptake tracks that were AP2-negative; in green, Tf-Cy3 uptake tracks that were AP2-positive; in red, non-endocytic Tf-Cy3 tracks.
Supplementary Video 6
LLSM recording of Tf endocytosis in the presence of EGF (related to Fig. 5c–e,g). Genome edited SUM159 expressing AP2-GFP (green) were incubated for 2 min with 1 µg/mL Tf-Cy3 (magenta) in the presence of 100 ng/mL EGF and transferred to the LLSM for imaging at 27 °C. Full 3D volumes of 60 planes were acquired within 2 s. Colors indicate: In white, Tf-Cy3 uptake tracks that were AP2-negative; in green, Tf-Cy3 uptake tracks that were AP2-positive; in red, non-endocytic Tf-Cy3 tracks.
Supplementary Video 7
2D movie of anti-β1 integrin (K20) antibody endocytosis imaged by LLSM in the presence of EGF (related to Fig. 5c–e,g–i). Same conditions as Extended data Movie 3. This 2D movie shows a side view of 3 cell slices as maximum projection, perpendicular to the detection objective. Representative β1 integrin uptake events are shown: White arrows, AP2-negative; green arrows, AP2-positive. Scale bar: 5 µm.
Supplementary Video 8
Zoom of Movie M7 showing an AP2-negative endocytic event. Single time points from this movie are shown in Fig 5e.
Source data
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Figs. 1–7 and Extended Data Figs. 1–4
Statistical source data.
Source Data Extended Data Figs. 1–4
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
MacDonald, E., Forrester, A., Valades-Cruz, C.A. et al. Growth factor-triggered de-sialylation controls glycolipid-lectin-driven endocytosis. Nat Cell Biol 27, 449–463 (2025). https://doi.org/10.1038/s41556-025-01616-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41556-025-01616-x
This article is cited by
-
Sugar unmasking for trafficking
Nature Cell Biology (2025)
-
Spatial N-glycan rearrangement on α5β1 integrin nucleates galectin-3 oligomers to determine endocytic fate
Nature Communications (2025)