Abstract
Reactive oxygen species (ROS) play a crucial role in lipid peroxidation and the initiation of ferroptosis, markedly affecting chemotherapeutic drug resistance. However, the mechanisms by which ROS function and are sensed remain poorly understood. In this study, we identified O-GlcNAc transferase (OGT), a key enzyme in protein O-GlcNAcylation, as a sensor for ROS during ferroptosis. The ROS-induced oxidation of OGT at C845 in its catalytic domain activates the enzyme. Once activated, OGT O-GlcNAcylates FOXK2, enhancing its interaction with importin α, which facilitates FOXK2’s nuclear translocation and binding to the SLC7A11 promoter region. This, in turn, boosts SLC7A11 transcription, thereby inhibiting ferroptosis. The elevated OGT–FOXK2–SLC7A11 axis contributes to tumorigenesis and resistance to chemoradiotherapy in hepatocellular carcinoma (HCC). Our findings elucidate a ROS-induced oxidation-O-GlcNAcylation cascade that integrates ROS signalling, O-GlcNAcylation, FOXK2-mediated SLC7A11 transcription and resistance to both ferroptosis and chemoradiotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (IDs 4GYW and 7YEA). Previously published ChIP–seq data that were re-analysed here are available under accession codes GSE92123 and GSM1250376. RNA-seq data that support the findings of this study have been deposited in the Sequence Read Archive under accession code SRP534714. Mass spectrometry data have been deposited in ProteomeXchange with accession code PXD064117. Uncropped immunoblot scans and numerical source data supporting the findings of this study are provided as Source Data files. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Li, J. et al. Ferroptosis: past, present and future. Cell Death Dis. 11, 88 (2020).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Su, L.-J. et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 5080843 (2019).
Li, D. & Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 5, 108 (2020).
Glorieux, C., Liu, S., Trachootham, D. & Huang, P. Targeting ROS in cancer: rationale and strategies. Nat. Rev. Drug Discov. https://doi.org/10.1038/s41573-024-00979-4 (2024).
Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 31, 107–125 (2021).
Kinoshita, H. et al. Cystine/glutamic acid transporter is a novel marker for predicting poor survival in patients with hepatocellular carcinoma. Oncol. Rep. 29, 685–689 (2013).
Lo, M., Wang, Y.-Z. & Gout, P. W. The x cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J. Cell. Physiol. 215, 593–602 (2008).
Conrad, M. & Sato, H. The oxidative stress-inducible cystine/glutamate antiporter, system xc-: cystine supplier and beyond. Amino Acids 42, 231–246 (2012).
Sato, H., Tamba, M., Ishii, T. & Bannai, S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 274, 11455–11458 (1999).
Stipanuk, M. H., Dominy, J. E. Jr, Lee, J.-I. & Coloso, R. M. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J. Nutr. 136, 1652S–1659S (2006).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Hassannia, B., Vandenabeele, P. & Vanden Berghe, T. Targeting ferroptosis to iron out cancer. Cancer Cell 35, 830–849 (2019).
Zhang, Y. et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 12, 1589 (2021).
Chen, D. et al. NRF2 is a major target of ARF in p53-independent tumor suppression. Mol. Cell 68, 224–232.e4 (2017).
Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015).
Zhang, Y. et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 20, 1181–1192 (2018).
He, F. et al. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J. Hepatol. 79, 362–377 (2023).
Koppula, P., Zhuang, L. & Gan, B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12, 599–620 (2021).
Yang, X. & Qian, K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 18, 452–465 (2017).
Hanover, J. A., Krause, M. W. & Love, D. C. linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 13, 312–321 (2012).
Hart, G. W., Slawson, C., Ramirez-Correa, G. & Lagerlof, O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 80, 825–858 (2011).
Wells, L. & Hart, G. W. O-GlcNAcylation: a major nutrient/stress sensor that regulates cellular physiology. J. Biol. Chem. 300, 107635 (2024).
Peng, C. et al. Regulation of the Hippo–YAP pathway by glucose sensor O-GlcNAcylation. Mol. Cell 68, 591–604 (2017).
Tan, W. et al. Posttranscriptional regulation of de novo lipogenesis by glucose-induced O-GlcNAcylation. Mol. Cell 81, 1890–1904 (2021).
Chen, L. et al. Direct stimulation of de novo nucleotide synthesis by O-GlcNAcylation. Nat. Chem. Biol. 20, 19–29 (2024).
Ferrer, C. M., Sodi, V. L. & Reginato, M. J. O-GlcNAcylation in cancer biology: linking metabolism and signaling. J. Mol. Biol. 428, 3282–3294 (2016).
Zhang, D., Qi, Y., Inuzuka, H., Liu, J. & Wei, W. O-GlcNAcylation in tumorigenesis and its implications for cancer therapy. J. Biol. Chem. 300, 107709 (2024).
Tang, J. et al. The deubiquitinase EIF3H promotes hepatocellular carcinoma progression by stabilizing OGT and inhibiting ferroptosis. Cell Commun. Signal. 21, 198 (2023).
Yu, F. et al. Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis. Cell Discov. 8, 40 (2022).
Zhang, C.-C. et al. O-GlcNAcylation mediates H2O2-induced apoptosis through regulation of STAT3 and FOXO1. Acta Pharmacol. Sin. 45, 714–727 (2024).
Martinez, M. et al. Quantitative proteomics reveals that the OGT interactome is remodeled in response to oxidative stress. Mol. Cell. Proteomics 20, 100069 (2021).
Chen, Y. et al. CHK2–FOXK axis promotes transcriptional control of autophagy programs. Sci. Adv. 6, eaax5819 (2020).
Bowman, C. J., Ayer, D. E. & Dynlacht, B. D. Foxk proteins repress the initiation of starvation-induced atrophy and autophagy programs. Nat. Cell Biol. 16, 1202–1214 (2014).
Sakaguchi, M. et al. FoxK1 and FoxK2 in insulin regulation of cellular and mitochondrial metabolism. Nat. Commun. 10, 1582 (2019).
Sukonina, V. et al. FOXK1 and FOXK2 regulate aerobic glycolysis. Nature 566, 279–283 (2019).
Li, Y. et al. FOXK2 affects cancer cell response to chemotherapy by promoting nucleotide de novo synthesis. Drug Resist. Updat. 67, 100926 (2023).
Seong, C., Kim, H. J., Byun, J.-S., Kim, Y. & Kim, D.-Y. FoxO1 controls redox regulation and cellular physiology of BV-2 microglial cells. Inflammation 46, 752–762 (2023).
Zhong, S. et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis. Redox Biol. 63, 102760 (2023).
Yu, Y. et al. FOXK2 amplification promotes breast cancer development and chemoresistance. Cancer Lett. 597, 217074 (2024).
Miyamoto, Y., Yamada, K. & Yoneda, Y. Importin α: a key molecule in nuclear transport and non-transport functions. J. Biochem. 160, 69–75 (2016).
Shan, L. et al. FOXK2 elicits massive transcription repression and suppresses the hypoxic response and breast cancer carcinogenesis. Cancer Cell 30, 708–722 (2016).
Lei, G., Zhuang, L. & Gan, B. The roles of ferroptosis in cancer: tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell 42, 513–534 (2024).
Cui, Q. et al. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updat. 41, 1–25 (2018).
Friedmann Angeli, J. P., Krysko, D. V. & Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 19, 405–414 (2019).
Stockwell, B. R., Jiang, X. & Gu, W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 30, 478–490 (2020).
Gorrini, C., Harris, I. S. & Mak, T. W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12, 931–947 (2013).
Wang, Y. et al. O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc. Natl Acad. Sci. USA 114, 13732–13737 (2017).
Chu, Y.-D., Fan, T.-C., Lai, M.-W. & Yeh, C.-T. GALNT14-mediated O-glycosylation on PHB2 serine-161 enhances cell growth, migration and drug resistance by activating IGF1R cascade in hepatoma cells. Cell Death Dis. 13, 956 (2022).
Lee, H. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22, 225–234 (2020).
You, M. et al. Signaling pathways in cancer metabolism: mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 8, 196 (2023).
Han, C. et al. O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat. Commun. 8, 1491 (2017).
Yi, W. et al. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 337, 975–980 (2012).
Rao, X. et al. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat. Commun. 6, 8468 (2015).
Li, X. et al. Targeting SIRT3 sensitizes glioblastoma to ferroptosis by promoting mitophagy and inhibiting SLC7A11. Cell Death Dis. 15, 168 (2024).
Song, X. et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Xc– activity. Curr. Biol. 28, 2388–2399.e5 (2018).
Wang, X. et al. O-GlcNAcylation of ZEB1 facilitated mesenchymal pancreatic cancer cell ferroptosis. Int. J. Biol. Sci. 18, 4135–4150 (2022).
Chen, Y. et al. O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell. Signal. 63, 109384 (2019).
Zhu, G. et al. O-GlcNAcylation enhances sensitivity to RSL3-induced ferroptosis via the YAP/TFRC pathway in liver cancer. Cell Death Discov. 7, 83 (2021).
Yang, Z. et al. OGT/HIF-2α axis promotes the progression of clear cell renal cell carcinoma and regulates its sensitivity to ferroptosis. iScience 26, 108148 (2023).
Li, D., Song, C., Zhang, J. & Zhao, X. ROS and iron homeostasis dependent ferroptosis play a vital role in 5-fluorouracil induced cardiotoxicity in vitro and in vivo. Toxicology 468, 153113 (2022).
Castro-Mondragon, J. A. et al. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 50, D165–D173 (2022).
Zhang, J. et al. Systematic identification of anticancer drug targets reveals a nucleus-to-mitochondria ROS-sensing pathway. Cell 186, 2361–2379.e25 (2023).
Wu, C. et al. Coupling suspension trapping–based sample preparation and data-independent acquisition mass spectrometry for sensitive exosomal proteomic analysis. Anal. Bioanal. Chem. 414, 2585–2595 (2022).
Wu, C. et al. Design and preparation of novel nitro-oxide-grafted nanospheres with enhanced hydrogen bonding interaction for O-GlcNAc analysis. ACS Appl. Mater. Interfaces 14, 47482–47490 (2022).
Acknowledgements
This research was financially supported by the National Institutes of Health R01-NS121243 to H.P. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We also acknowledge the support from the Mass Spectrometry and Analytical Pharmacology Shared Resource, Histopathology and Tissue Shared Resource, and Flow Cytometry and Cell Sorting Shared Resource (P30-CA051008). Instrumentation used in this study included the BD LSRFortessa and S10OD016213.
Author information
Authors and Affiliations
Contributions
H.Z., J.M., C.H., X.L., S.Z., Z.J., S.M., A.D. and M.C. performed the experiments; H.Z. performed most of the cell and animal experiments and data analysis. J.M., X.L., S.M. and A.D. contributed to the clinical sample collection, pathology and data preparation. LC–MS/MS analysis and data interpretation were carried out by C.H. H.Z. and J.M. Y.P., C.P., P.L., H.M., Y.X., G.M.K., H.Z., J.M., J.L., S.H. and H.P. joined discussions and designed the research. H.Z., J.M. and H.P. wrote the paper. S.H. and H.P. supervised and administrated the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Sheng-hong Chen, Haitao Wen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
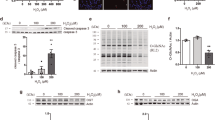
Extended Data Fig. 1 OGT is oxidized and activated by ROS produced during ferroptosis, related to Fig. 1.
a-d, PLC/PRF/5 cells were treated with erastin for the indicated time (a, b) and concentrations (c, d). Immunoblot analyses were conducted with indicated antibodies(a, c) and quantified by ImageJ (b, d) (n = 3 experiments). e. f, Huh7 cells were treated with the indicated concentrations of RSL3. Immunoblot analyses were conducted with indicated antibodies and quantified by ImageJ (n = 3 experiments). g, MS analysis was performed to identify the oxidation sites on OGT. HA-OGT was purified from HEK293T cells following H2O2 treatment and then analysed by mass spectrometry. h, Alignments of the oxidation motif in OGT across human and other species. i-k, Huh7 cells were treated with erastin (10 μM) for 24 h (i), RSL3 (500 nM) for 24 h (j), and H2O2 (100 μM) for 1 h (k). The intracellular ROS level was detected by flow cytometry analysis. n = 3 independent experiments. l. m, Huh7 cells were treated with the indicated concentrations of H2O2. Immunoblot analyses were conducted with indicated antibodies (l) and quantified by ImageJ (m) (n = 3 experiments). n, Endogenous OGT in Huh7 cells was depleted using shRNA, then rescued with either OGT-WT or OGT-C845S. Immunoblot analyses were conducted with indicated antibodies. b,d,f,i,j,k,m Data are presented as the mean ± SEM; two-tailed Student’s t-test.
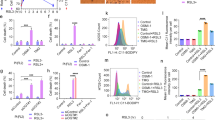
Extended Data Fig. 2 OGT regulates ferroptosis by controlling the transcription of SLC7A11, related to Fig. 2.
a-c, The protein levels of SLC7A11 were analysed in PLC/PRF/5 cells following OGT knockdown (a), treatment with OSMI-1 for 24 h as indicated (b), and overexpression OGT (c). Immunoblotting was performed using the specified antibodies (n = 3 experiments). d. e, Endogenous OGT was depleted in PLC/PRF/5 (d) and Huh7 (e) cells using shRNA, and cystine uptake levels were subsequently measured. n = 3 independent experiments. f. g, Endogenous OGT was depleted in PLC/PRF/5 (f) and Huh7 (g) cells using shRNA, and cellular GSH levels were subsequently measured. n = 3 independent experiments. h, Endogenous OGT was depleted in Huh7 cells, treated with the indicated reagents, and lipid peroxidation levels were assessed by flow cytometry. n = 3 independent experiments. i. j, Endogenous OGT was depleted in PLC/PRF/5 (i) and Huh7 (j) cells using shRNA. The cells were treated with increasing concentrations of erastin for 24 h, and cell viability was subsequently measured. Data are presented as mean ± SEM. Two-way ANOVA was used. n = 3 independent experiments. k-m, OGT was stably depleted in PLC/PRF/5 cells by shRNA and stably transfected with SLC7A11. SLC7A11 and OGT protein levels in the indicated cells were determined by Immunoblotting (k). Cellular Cystine uptake (l). and GSH levels (m) in the indicated cells were examined. n = 3 independent repeats. d,e,f,g,h,l,m, Data are presented as the mean ± SEM; two-tailed Student’s t-test; ns, not significant.
Extended Data Fig. 3 SLC7A11 is the target gene of FOXK2, related to Fig. 3.
a, Volcano plot of differentially expressed genes in FOXK2 KO versus control samples, identified by whole-transcriptome RNA-seq in Huh7 cells. Upregulated or downregulated genes were filtered by fold change > 1.0 and P < 0.05. Statistical analysis was performed using the DESeq2 software. b, Heatmap of significantly altered genes in control versus FOXK2 KO Huh7 cells. c, FOXK2 and O-GlcNAc ChIP–seq occupancy profiles at the SLC7A11 locus were visualized using IGV software. Data are from GEO with accession numbers indicated in parentheses. d, f, g, Endogenous FOXK2 in Huh7 (d), PLC/PRF/5 (f) and Hep3B (g) was depleted by shRNA, and mRNA levels (left panel) and protein levels (right panel) of SLC7A11 were determined by qRT-PCR and immunoblotting, respectively. The independent experiments were repeated three times with similar results. e, Endogenous FOXK2 in Huh7 cells was depleted by shRNA, with erasrin (10 μM) treatment for 24 h. MRNA levels (left panel) and protein levels (right panel) of SLC7A11 were determined by qRT-PCR and immunoblotting, respectively. n = 3 independent experiments. h, Endogenous FOXK1 in Huh7 cells was depleted by shRNA, and SLC7A11 protein level was determined by immunoblotting (n = 3 experiments). i, The JASPAR website predicted FOXK2 binding sites with the SLC7A11 promoter sequence. j, HEK293T cells were subjected to ChIP assays with an anti-FOXK2-specific antibody, and the amount of precipitated DNA was quantitated by qRT-PCR. k, HEK293T cells were transfected with the indicated plasmids. The cells were lysed and then incubated with either biotin-labelled oligonucleotides or an excess of unlabelled oligonucleotides for pulldown assays. The bound proteins were pulled down and analysed by immunoblotting (n = 3 experiments). l. m, HEK293T cells were transfected with the indicated plasmids (l) and shRNA (m). The SLC7A11 promoter activity was measured using dual-luciferase reporter assay. n. o, Endogenous FOXK2 in Huh7 (n) and PLC/PRF/5 (o) cells was depleted by shRNA. Cystine uptake levels were examined. p, Endogenous FOXK2 in Huh7 cells was depleted by shRNA. Lipid peroxidation level was examined. q, Endogenous FOXK2 was depleted in Huh7 cells using shRNA. The cells were then exposed to the indicated concentrations of erastin for 24 h, followed by cell viability assay. Data are presented as mean ± SEM. Two-way ANOVA was used. For j,l,m,n,o,p,q, n = 3 independent experiments. d,e,f,g,j,l,m,n,o,p Data are presented as the mean ± SEM; two-tailed Student’s t-test.
Extended Data Fig. 4 OGT interacts with and O-GlcNAcylates FOXK2, related to Fig. 3.
a, Huh7 cells were transfected with either siCon or si-FOXO1 (left panel) and FOXO3 (right panel). Knockdown efficiency was evaluated by qRT-PCR. n = 3 independent experiments. b. HEK293T cells were transfected with the indicated plasmids, and the interaction between OGT and FOXK2 was examined by Co-IP and immunoblotting using the indicated antibodies (n = 3 experiments). c, GST, GST-FOXK2 full-length (FL), and truncated mutants (M1 and M2) proteins were expressed and purified from E. coli BL21 cells. The interaction between GST–FOXK2 or M1, M2 and OGT were examined by GST pulldown assay (n = 3 experiments). Upper panel: Schematic diagrams illustrating FOXK2 truncated mutants are presented. d, HEK293T cells were transfected with plasmids encoding full-length (FL) or truncated forms of OGT (M1 and M2). Co-IP was performed to map the region of OGT involved in binding to FOXK2 (n = 3 experiments). Upper panel: Schematic diagrams illustrating the truncated OGT mutants are presented. e, Huh7 cells were transfected with Flag-FOXK2 and treated with 30 μM OSMI-1 for 24 h. FOXK2 O-GlcNAcylation was analysed by IP and immunoblotting using the indicated antibodies (n = 3 experiments). f. g, Huh7 (f) and PLC/PRF/5 (g) cells were transfected with indicated siRNAs. Immunoblotting was conducted with indicated antibodies(n = 3 experiments). h, Huh7 cells were transfected with indicated plasmids, and the O-GlcNAcylation of FOXK2 was examined by IP and immunoblotting with indicated antibodies (n = 3 experiments). i, Huh7 cells were stably transfected with OGT shRNA and/or FOXK2 shRNA, and cell death following indicated treatment was evaluated. n = 3 independent repeats. a,i, Data are presented as the mean ± SEM; two-tailed Student’s t-test; ns, not significant.
Extended Data Fig. 5 OGT O-GlcNAcylates FOXK2 at S424, related to Fig. 4.
a, MS analysis of FOXK2 O-GlcNAcylation sites. FOXK2 was purified from HEK293T cells and analysed by MS to identify the O-GlcNAcylation sites. b, Huh7 cells transfected with HA–FOXK2 WT or S424A were treated with DMSO or 10μm Thiamet-G for 24 h. FOXK2 O-GlcNAcylation was analysed by IP and immunoblotting (n = 3 experiments). c, HEK293T cells were transfected with either HA–FOXK2 WT or S424A. The interaction between OGT and FOXK2 was analysed by Co-IP (n = 3 experiments). d, Alignments of the OGT consensus O-GlcNAcylation motif in FOXK2 from human and other species. e, FOXK2 KO PLC/PRF/5 cells were reintroduced with either FOXK2 WT or S424A. SLC7A11 mRNA levels in the indicated PLC/PRF/5 cells were measured by RT–PCR. n = 3 independent repeats. f. g, FOXK2 KO PLC/PRF/5 cells were reintroduced with either FOXK2 WT or S424A. Cystine uptake (f) and GSH levels (g) in the indicated PLC/PRF/5 cells were measured. n = 3 independent repeats. h, PLC/PRF/5 cells were depleted of FOXK2 using CRISPR-Cas9 and then stably reconstituted with either WT or S424A mutant FOXK2. The cells were then treated with increasing concentrations of erastin for 24 h, and cell viability was evaluated. Data are presented as the mean ± SEM. Two-way ANOVA was used. n = 3 independent repeats. e,f,g, Data are presented as the mean ± SEM; two-tailed Student’s t-test; ns, not significant.
Extended Data Fig. 6 An elevated OGT–FOXK2-SLC7A11 axis contributes to chemotherapy resistance in HCC, related to Fig. 7.
a, FOXK2 KO Huh7 cells were reintroduced with either WT or FOXK2 S424A mutant. The cells were treated with increasing concentrations of 5-FU for 72 h, and cell viability was measured. n = 3 independent replicates. b, The indicated Huh7 cells (from a) were seeded and treated with 4 Gy irradiation. Colony-formation assays were conducted. n = 3 independent repeats. c. d, Huh7, Huh7 5-FUR, and Huh7 IRR cells were treated with increasing concentrations of 5-FU (c) or varying doses of irradiation (d) for 72 h, and cell viability was assessed. The IC50 values are indicated. n = 3 independent replicates. e, Intracellular ROS levels in HepG2 and 5-FU-resistant HepG2 (HepG2 5-FUR) cells were measured by flow cytometry. n = 3 independent experiments. f, The protein levels of SLC7A11, along with total O-GlcNAcylation, were determined in HepG2 5-FUR cells by immunoblotting with the specified antibodies (n = 3 experiments). g, FOXK2 O-GlcNAcylation in HepG2 5-FUR cells was analysed by the chemoenzymatic labelling method and determined by immunoblotting. h, SLC7A11 mRNA level in HepG2 5-FUR cells was determined by qPCR. n = 3 independent repeats. i, HepG2 and HepG2 5-FUR cells were treated with increasing concentrations of 5-FU for 72 h, and cell viability was assessed. The IC50 values are indicated. n = 3 independent replicates. j, Intracellular ROS levels in PLC/PRF/5 and 5-FU-resistant PLC/PRF/5 (PLC/PRF/5 5-FUR) cells were measured by flow cytometry. n = 3 independent experiments. k, The protein levels of SLC7A11 and total O-GlcNAcylation were determined in PLC/PRF/5 5-FUR cells by immunoblotting (n = 3 experiments). l, Schematic model illustrating a ROS-induced oxidation-O-GlcNAcylation cascade specific to ferroptosis, which contributes to drug resistance in HCC. Created in BioRender. Zhang, H. (2025) https://BioRender.com/ky9usx1. a,c,d,i, Data are presented as mean ± SEM, with statistical analysis performed using two-way ANOVA. b,e,h,j, Data are presented as the mean ± SEM; two-tailed Student’s t-test; ns, not significant.
Supplementary information
Supplementary Information
Sample of FACS gating strategies for DCFH-DA (cytoplasmic ROS) and C11-BODIPY (lipid ROS) staining.
Supplementary Tables 1–3
Supplementary Tables 1–3.
Source data
Source Data Fig. 1
Unprocessed western blots and/or gels of Fig. 1.
Source Data Fig. 2
Unprocessed western blots and/or gels of Fig. 2.
Source Data Fig. 3
Unprocessed western blots and/or gels of Fig. 3.
Source Data Fig. 4
Unprocessed western blots and/or gels of Fig. 4.
Source Data Fig. 5
Unprocessed western blots and/or gels of Fig. 5.
Source Data Fig. 6
Unprocessed western blots and/or gels of Fig. 6.
Source Data Fig. 7
Unprocessed western blots and/or gels of Fig. 7.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels of Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels of Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels of Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Unprocessed western blots and/or gels of Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Unprocessed western blots and/or gels of Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels of Extended Data Fig. 6.
Source Data Table 1
Statistical source data of Figs. 1–7 and Extended Data Figs. 1–6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Ma, J., Hou, C. et al. A ROS-mediated oxidation-O-GlcNAcylation cascade governs ferroptosis. Nat Cell Biol 27, 1288–1300 (2025). https://doi.org/10.1038/s41556-025-01722-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41556-025-01722-w
This article is cited by
-
Mechanistic insights into EOC-PMCs-Induced macrophage polarization via the Galnt15-PPARγ pathway
Journal of Ovarian Research (2026)
-
A machine learning-based predictive model for radiosensitivity in nasopharyngeal carcinoma utilizing serum proteomics
Journal of Translational Medicine (2026)
-
pCancer and FOXK2 (Forkhead Box K2): Oncogenic and Tumor-Suppressive Roles of FOXK2 in Cancer
Biochemical Genetics (2026)
-
Molecular regulatory network of glycolytic reprogramming in hepatocellular carcinoma and its clinical implications
Biological Procedures Online (2025)
-
O-GlcNAcylation in novel regulated cell death: ferroptosis, pyroptosis, and necroptosis
Cell Death Discovery (2025)