Abstract
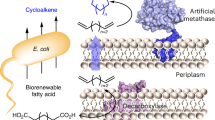
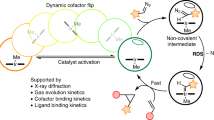
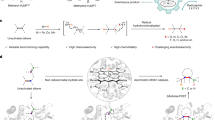
While natural terpenoid cyclases generate complex terpenoid structures via cationic mechanisms, alternative radical cyclization pathways are underexplored. The metal-catalysed H-atom transfer reaction (M-HAT) offers an attractive means for hydrofunctionalizing olefins, providing access to terpenoid-like structures. Artificial metalloenzymes offer a promising strategy for introducing M-HAT reactivity into a protein scaffold. Here we report our efforts towards engineering an artificial radical cyclase (ARCase), resulting from anchoring a biotinylated [Co(Schiff-base)] cofactor within an engineered chimeric streptavidin. After two rounds of directed evolution, a double mutant catalyses a radical cyclization to afford bicyclic products with a cis-5-6-fused ring structure and up to 97% enantiomeric excess. The involvement of a histidine ligation to the Co cofactor is confirmed by crystallography. A time course experiment reveals a cascade reaction catalysed by the ARCase, combining a radical cyclization with a conjugate reduction. The ARCase exhibits tolerance towards variations in the dienone substrate, highlighting its potential to access terpenoid scaffolds.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings in this study are available within this Article and its Supplementary Information and Supplementary Data. Crystallographic data for the ARCase structure of [Co(Biot-en-tBu2-Salphen)] 4 ‧ chSav*_α16 S122V-K131H reported in this article have been deposited at Protein Data Bank under the code 8QEX. Source data are provided with this paper.
References
Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones (Wiley-VCH, 2006).
Le Bideau, F., Kousara, M., Chen, L., Wei, L. & Dumas, F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 117, 6110–6159 (2017).
Christianson, D. W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 117, 11570–11648 (2017).
Chen, K. & Arnold, F. H. Engineering new catalytic activities in enzymes. Nat. Catal. 3, 203–213 (2020).
Emmanuel, M. A. et al. Photobiocatalytic strategies for organic synthesis. Chem. Rev. 123, 5459–5520 (2023).
Zetzsche, L. E. et al. Biocatalytic oxidative cross-coupling reactions for biaryl bond formation. Nature 603, 79–85 (2022).
Ye, Y. X. et al. Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination. Nat. Chem. 15, 206–212 (2023).
Zhou, Q., Chin, M., Fu, Y., Liu, P. & Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 374, 1612–1616 (2021).
Rui, J. Y. et al. Directed evolution of nonheme iron enzymes to access abiological radical-relay C(sp3)–H azidation. Science 376, 869–874 (2022).
Huang, X. Q. et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 584, 69–74 (2020).
Nakano, Y. et al. Photoenzymatic hydrogenation of heteroaromatic olefins using ‘ene’-reductases with photoredox catalysts. Angew. Chem. Int. Ed. 59, 10484–10488 (2020).
Sandoval, B. A. et al. Photoenzymatic reductions enabled by direct excitation of flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc. 143, 1735–1739 (2021).
Crossley, S. W. M., Obradors, C., Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 116, 8912–9000 (2016).
Mukaiyama, T. et al. Oxidation–reduction hydration of olefins with molecular-oxygen and 2-propanol catalyzed by bis(acetylacetonato)cobalt(II). Chem. Lett. 18, 449–452 (1989).
Waser, J. & Carreira, E. M. Convenient synthesis of alkylhydrazides by the cobalt-catalyzed hydrohydrazination reaction of olefins and azodicarboxylates. J. Am. Chem. Soc. 126, 5676–5677 (2004).
Ishikawa, H. et al. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues. J. Am. Chem. Soc. 131, 4904–4916 (2009).
Lo, J. C., Yabe, Y. & Baran, P. S. A practical and catalytic reductive olefin coupling. J. Am. Chem. Soc. 136, 1304–1307 (2014).
Ma, X. S. & Herzon, S. B. Intermolecular hydropyridylation of unactivated alkenes. J. Am. Chem. Soc. 138, 8718–8721 (2016).
Choi, J. W., Tang, L. H. & Norton, J. R. Kinetics of hydrogen atom transfer from (η5-C5H5)Cr(CO)3H to various olefins: influence of olefin structure. J. Am. Chem. Soc. 129, 234–240 (2007).
Kim, D., Rahaman, S. M. W., Mercado, B. Q., Poli, R. & Holland, P. L. Roles of iron complexes in catalytic radical alkene cross-coupling: a computational and mechanistic study. J. Am. Chem. Soc. 141, 7473–7485 (2019).
Shevick, S. L. et al. Catalytic hydrogen atom transfer to alkenes: a roadmap for metal hydrides and radicals. Chem. Sci. 11, 12401–12422 (2020).
Discolo, C. A., Touney, E. E. & Pronin, S. V. Catalytic asymmetric radical–polar crossover hydroalkoxylation. J. Am. Chem. Soc. 141, 17527–17532 (2019).
Ebisawa, K. et al. Catalyst- and silane-controlled enantioselective hydrofunctionalization of alkenes by cobalt-catalyzed hydrogen atom transfer and radical–polar crossover. J. Am. Chem. Soc. 142, 13481–13490 (2020).
Qin, T. et al. Cobalt-catalyzed radical hydroamination of alkenes with N-fluorobenzenesulfonimides. Angew. Chem. Int. Ed. 60, 25949–25957 (2021).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Stappen, C. V. et al. Designing artificial metalloenzymes by tuning of the environment beyond the primary coordination sphere. Chem. Rev. 122, 11974–12045 (2022).
Wilson, M. E. & Whitesides, G. M. Conversion of a protein to a homogeneous asymmetric hydrogenation catalyst by site-specific modification with a diphosphinerhodium(I) moiety. J. Am. Chem. Soc. 100, 306–307 (1978).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Studer, S. et al. Evolution of a highly active and enantiospecific metalloenzyme from short peptides. Science 362, 1285–1288 (2018).
Natoli, S. N. & Hartwig, J. F. Noble-metal substitution in hemoproteins: an emerging strategy for abiological catalysis. Acc. Chem. Res. 52, 326–335 (2019).
Mirts, E. N., Petrik, I. D., Hosseinzadeh, P., Nilges, M. J. & Lu, Y. A designed heme-[4Fe-4S] metalloenzyme catalyzes sulfite reduction like the native enzyme. Science 361, 1098–1101 (2018).
Oohora, K., Onoda, A. & Hayashi, T. Hemoproteins reconstituted with artificial metal complexes as biohybrid catalysts. Acc. Chem. Res. 52, 945–954 (2019).
Song, W. J. & Tezcan, F. A. A designed supramolecular protein assembly with in vivo enzymatic activity. Science 346, 1525–1528 (2014).
Roelfes, G. LmrR: a privileged scaffold for artificial metalloenzymes. Acc. Chem. Res. 52, 545–556 (2019).
Heinisch, T. & Ward, T. R. Artificial metalloenzymes based on the biotin–streptavidin technology: challenges and opportunities. Acc. Chem. Res. 49, 1711–1721 (2016).
Shu, T. & Cossy, J. Asymmetric desymmetrization of alkene-, alkyne- and allene-tethered cyclohexadienones using transition metal catalysis. Chem. Soc. Rev. 50, 658–666 (2021).
Giese, B. Formation of CC bonds by addition of free radicals to alkenes. Angew. Chem. Int. Ed. 22, 753–764 (1983).
Salahi, F., Yao, C., Norton, J. R. & Snyder, S. A. The synthesis of diverse terpene architectures from phenols. Nat. Synth. 1, 313–321 (2022).
Christoffel, F. et al. Design and evolution of chimeric streptavidin for protein-enabled dual gold catalysis. Nat. Catal. 4, 643–653 (2021).
Pellizzoni, M. M. et al. Chimeric streptavidins as host proteins for artificial metalloenzymes. ACS Catal. 8, 1476–1484 (2018).
Grimm, A. R. et al. Cavity size engineering of a β-barrel protein generates efficient biohybrid catalysts for olefin metathesis. ACS Catal. 8, 3358–3364 (2018).
Eiben, C. B. et al. Increased diels-alderase activity through backbone remodeling guided by Foldit players. Nat. Biotechnol. 30, 190–192 (2012).
Demarteau, J., Debuigne, A. & Detrembleur, C. Organocobalt complexes as sources of carbon-centered radicals for organic and polymer chemistries. Chem. Rev. 119, 6906–6955 (2019).
Dodani, S. C. et al. Discovery of a regioselectivity switch in nitrating P450s guided by molecular dynamics simulations and Markov models. Nat. Chem. 8, 419–425 (2016).
Loving, G. & Imperiali, B. Thiol-reactive derivatives of the solvatochromic 4-N,N-dimethylamino-1,8-naphthalimide fluorophore: a highly sensitive toolset for the detection of biomolecular interactions. Bioconjug. Chem. 20, 2133–2141 (2009).
Keith, J. M., Larrow, J. F. & Jacobsen, E. N. Practical considerations in kinetic resolution reactions. Adv. Synth. Catal. 343, 5–26 (2001).
Rodriguez, A. D., Ramirez, C. & Shi, Y. P. The cumbiasins, structurally novel diterpenes possessing intricate carbocyclic skeletons from the West Indian sea whip Pseudopterogorgia elisabethae (Bayer). J. Org. Chem. 65, 6682–6687 (2000).
Kamo, S. et al. Synthetic and biological studies of juglorubin and related naphthoquinones. J. Org. Chem. 84, 13957–13966 (2019).
Lu, Z. et al. Total synthesis of aplysiasecosterol A. J. Am. Chem. Soc. 140, 9211–9218 (2018).
Lu, H. J. & Zhang, X. P. Catalytic C–H functionalization by metalloporphyrins: recent developments and future directions. Chem. Soc. Rev. 40, 1899–1909 (2011).
Lang, K., Hu, Y., Cindy Lee, W.-C. & Zhang, X. P. Combined radical and ionic approach for the enantioselective synthesis of β-functionalized amines from alcohols. Nat. Synth. 1, 548–557 (2022).
Vlasie, M., Chowdhury, S. & Banerjee, R. Importance of the histidine ligand to coenzyme B in the reaction catalyzed by methylmalonyl-CoA mutase. J. Biol. Chem. 277, 18523–18527 (2002).
Acknowledgements
T.R.W. thanks the NCCR Catalysis (grant number 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation. Additional funding was provided by the NCCR Molecular Systems Engineering (grant number 200021_178760). X.Z. thanks the SIOC postdoc fellowship for the financial support. R.T. thanks the Naito Foundation for financial support. We acknowledge the Paul Scherrer Institut, Villigen, Switzerland, for provision of synchrotron radiation beamtime at beamline X06SA of the Swiss Light Source.
Author information
Authors and Affiliations
Contributions
T.R.W., X.Z. and D.C. conceived and designed the study. X.Z. contributed to the synthesis of the substrates, products and complexes. D.C. contributed to the mutagenesis and protein expression and protein purification and protein mass. D.C. and X.Z. performed the catalytic and time course experiment, designed the screening workflow and recorded the data. T.R.W., X.Z. and D.C. analysed the data. R.P.J., T.M. and D.C. contributed to the crystallization and crystallographic structural analysis. A.A.V., B.E.C., A.S. and T.R.W. contributed to the collaboration on the computational design of chimeric streptavidin. R.T. contributed to the molecular modelling and QM/MM calculation. Z.Z. offered the molecular biology instructions. D.A.G. offered synthetic suggestions. A.L. provided critical insight regarding the M-HAT reaction. A.S. established the Chim_Sav library workflow and associated molecular biology methods. T.R.W., X.Z. and D.C. wrote the paper, which was further supplemented through contributions from R.P.J., R.T. and A.A.V. All authors have given approval to the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Jean-Pierre Mahy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Asymmetric radical cyclization of dienone 1 and kinetic resolution of the cyclized enone 2 to the corresponding ketone 8 at 10 °C.
a) [Co(Biot-en-tBu2-Salphen)] 4 · chSav*_α16 S122V-K131H catalyzed cascade reaction consisting of a radical cyclization followed by a conjugate reduction. b) Time-course monitoring at 10 °C of ARCase progress, product-distribution (top) and enantioselectivity (bottom).
Supplementary information
Supplementary Information
Supplementary Tables 1–20 and Figs. 1–86.
Supplementary Data 1
Coordinates for the ARCase [Co(Biot-en-tBu2-Salphen)] 4 ‧ Sav*_α16 S122V-K131H.
Source data
Source Data Table 1
Source data for Table 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 5
Source data for Fig. 5.
Source Data Extended Data Fig./Table 1
Source data for Extended Data Fig. 1 in the main text.
Source Data Extended Data Fig./Table 2
Source data for Extended Data Table 1 in the main text.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, D., Zhang, X., Vorobieva, A.A. et al. An evolved artificial radical cyclase enables the construction of bicyclic terpenoid scaffolds via an H-atom transfer pathway. Nat. Chem. 16, 1656–1664 (2024). https://doi.org/10.1038/s41557-024-01562-5
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-024-01562-5
This article is cited by
-
Repurposing haemoproteins for asymmetric metal-catalysed H atom transfer
Nature (2025)
-
A cytokine-based designer enzyme with an abiological multinuclear metal center exhibits intrinsic and extrinsic catalysis
Nature Communications (2025)
-
Stereoconvergent reduction of alkenes using a repurposed iron-based dioxygenase
Nature Synthesis (2025)