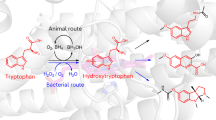
Abstract
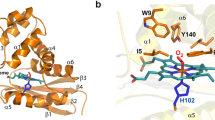
Two of nature’s recurring binding motifs in metalloproteins are the CxxxCxxC motif in radical SAM enzymes and the 2-His-1-carboxylate motif found both in zincins and α-ketoglutarate and non-haem iron enzymes. Here we show the confluence of these two domains in a single post-translational modifying enzyme containing an N-terminal radical S-adenosylmethionine domain fused to a C-terminal 2-His-1-carboxylate (HExxH) domain. The radical SAM domain catalyses three-residue cyclophane formation and is the signature modification of triceptides, a class of ribosomally synthesized and post-translationally modified peptides. The HExxH domain is a defining feature of zinc metalloproteases. Yet the HExxH motif-containing domain studied here catalyses β-hydroxylation and is an α-ketoglutarate non-haem iron enzyme. We determined the crystal structure for this HExxH protein at 2.8 Å, unveiling a distinct structural fold, thus expanding the family of α-ketoglutarate non-haem iron enzymes with a class that we propose to name αKG-HExxH. αKG-HExxH proteins represent a unique family of ribosomally synthesized and post-translationally modified peptide modifying enzymes that can furnish opportunities for genome mining, synthetic biology and enzymology.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The detailed procedures required to duplicate this work are available in Supplementary Information along with full LC–MS and NMR spectra where appropriate. The ChlH atomic coordinates and structure factors are deposited at the Protein Data Bank with ID code 8PP1. Any additional data or unique materials (through a materials transfer agreement) are available from the corresponding authors on reasonable request.
Code availability
The R script used for analysis of αKG-HExxH domain proteins is available at https://github.com/SuzeMa/2022_modHExxH
Change history
17 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41557-025-01803-1
References
Riordan, J. F. The role of metals in enzyme activity. Ann. Clin. Lab. Sci. 7, 119–129 (1977).
Ragsdale, S. W. Metals and their scaffolds to promote difficult enzymatic reactions. Chem. Rev. 106, 3317–3337 (2006).
Nicolet, Y. Structure–function relationships of radical SAM enzymes. Nat. Catal. 3, 337–350 (2020).
Broderick, J. B., Broderick, W. E. & Hoffman, B. M. Radical SAM enzymes: nature’s choice for radical reactions. FEBS Lett. 597, 92–101 (2023).
Imlay, J. A. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 59, 1073–1082 (2006).
Grell, T. A. J., Goldman, P. J. & Drennan, C. L. SPASM and twitch domains in S-adenosylmethionine (SAM) radical enzymes. J. Biol. Chem. 290, 3964–3971 (2015).
Mendauletova, A., Kostenko, A., Lien, Y. & Latham, J. How a subfamily of radical S-adenosylmethionine enzymes became a mainstay of ribosomally synthesized and post-translationally modified peptide discovery. ACS Bio. Med. Chem. Au. 2, 53–59 (2022).
Guo, Q. & Morinaka, B. I. Accessing and exploring the unusual chemistry by radical SAM-RiPP enzymes. Curr. Opin Chem. Biol. 81, 102483 (2024).
Clark, K. A., Bushin, L. B. & Seyedsayamdost, M. R. RaS-RiPPs in Streptococci and the human microbiome. ACS Bio. Med. Chem. Au. 2, 328–339 (2022).
Mahanta, N., Hudson, G. A. & Mitchell, D. A. Radical S-adenosylmethionine enzymes involved in RiPP biosynthesis. Biochemistry 56, 5229–5244 (2017).
Benjdia, A. & Berteau, O. Radical SAM enzymes and ribosomally‐synthesized and post‐translationally modified peptides: a growing importance in the microbiomes. Front. Chem. 9, 678068 (2021).
Hooper, N. M. Families of zinc metalloproteases. FEBS Lett. 354, 1–6 (1994).
Lipscomb, W. N. & Sträter, N. Recent advances in zinc enzymology. Chem. Rev. 96, 2375–2434 (1996).
Spyroulias, G. A., Galanis, A. S., Pairas, G., Manessi-Zoupa, E. & Cordopatis, P. Structural features of angiotensin-I converting enzyme catalytic sites: conformational studies in solution, homology models and comparison with other zinc metallopeptidases. Curr. Top. Med. Chem. 4, 403–429 (2004).
Matthews, B. W., Jansonius, J. N., Colman, P. M., Schoenborn, B. P. & Dupourque, D. Three-dimensional structure of thermolysin. Nat. New Biol. 238, 37–41 (1972).
Gao, S.-S., Naowarojna, N., Cheng, R., Liu, X. & Liu, P. Recent examples of α-ketoglutarate-dependent mononuclear non-haem iron enzymes in natural product biosyntheses. Nat. Prod. Rep. 35, 792–837 (2018).
Loenarz, C. & Schofield, C. J. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat. Chem. Biol. 4, 152–156 (2008).
Hausinger, R. P. FeII/alpha-ketoglutarate-dependent hydroxylases and related enzymes. Crit. Rev. Biochem. Mol. Biol. 39, 21–68 (2004).
Schaeffer, R. D., Kinch, L. N., Liao, Y. & Grishin, N. V. Classification of proteins with shared motifs and internal repeats in the ECOD database. Protein Sci. 25, 1188–1203 (2016).
Aik, W., McDonough, M. A., Thalhammer, A., Chowdhury, R. & Schofield, C. J. Role of the jelly-roll fold in substrate binding by 2-oxoglutarate oxygenases. Curr. Opin. Struct. Biol. 22, 691–700 (2012).
Clifton, I. J. et al. Structural studies on 2-oxoglutarate oxygenases and related double-stranded beta-helix fold proteins. J. Inorg. Biochem. 100, 644–669 (2006).
Martinez, S. & Hausinger, R. P. Catalytic mechanisms of Fe(II)- and 2-oxoglutarate-dependent oxygenases. J. Biol. Chem. 290, 20702–20711 (2015).
Sugiyama, R. et al. The biosynthetic landscape of triceptides reveals radical SAM enzymes that catalyze cyclophane formation on Tyr- and His-containing motifs. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.2c00521 (2022).
Clark, K. A. & Seyedsayamdost, M. R. Bioinformatic atlas of radical SAM enzyme-modified RiPP natural products reveals an isoleucine–tryptophan crosslink. J. Am. Chem. Soc. 144, 17876–17888 (2022).
Freeman, M. F. et al. Metagenome mining reveals polytheonamides as posttranslationally modified ribosomal peptides. Science 338, 387–390 (2012).
Haft, D. H., Selengut, J. D. & White, O. The TIGRFAMs database of protein families. Nucleic Acids Res. 31, 371–373 (2003).
Nguyen, T. Q. N. et al. Post-translational formation of strained cyclophanes in bacteria. Nat. Chem. 12, 1042–1053 (2020).
Phan, C.-S. & Morinaka, B. I. A prevalent group of actinobacterial radical SAM/SPASM maturases involved in triceptide biosynthesis. ACS Chem. Biol. 17, 3284–3289 (2022).
Phan, C.-S. & Morinaka, B. I. Bacterial cyclophane-containing RiPPs from radical SAM enzymes. Nat. Prod. Rep. 41, 708–720 (2024).
Suarez, A. F. L. et al. Functional and promiscuity studies of three-residue cyclophane forming enzymes show nonnative C–C cross-linked products and leader-dependent cyclization. ACS Chem. Biol. 19, 774–783 (2024).
Phan, C.-S. et al. Substrate promiscuity of the triceptide maturase XncB leads to incorporation of various amino acids and detection of oxygenated products. ACS Chem. Biol. 19, 855–860 (2024).
Purushothaman, M. et al. The triceptide maturase OscB catalyzes uniform cyclophane topology and accepts diverse Gly-rich precursor peptides. ACS Chem. Biol. 19, 1229–1236 (2024).
Ma, S. et al. Post-translational formation of aminomalonate by a promiscuous peptide-modifying radical SAM enzyme. Angew. Chem. Int. Ed. 60, 19957–19964 (2021).
Ma, S. et al. A gene-encoded aldehyde tag repurposed from RiPP cyclophane-forming pathway. Bioorg. Med. Chem. Lett. 101, 129653 (2024).
Bhushan, R. & Bruckner, H. Marfey’s reagent for chiral amino acid analysis: A review. Amino Acids 27, 231–247 (2004).
Doyon, T. J. et al. Scalable and selective β-hydroxy-α-amino acid synthesis catalyzed by promiscuous l-threonine transaldolase ObiH. ChemBioChem 23, e202100577 (2022).
Pan, J. et al. Evidence for modulation of oxygen rebound rate in control of outcome by iron(II)- and 2-oxoglutarate-dependent oxygenases. J. Am. Chem. Soc. 141, 15153–15165 (2019).
Martinez, S. & Hausinger, R. P. Biochemical and spectroscopic characterization of the non-heme Fe(II)- and 2-oxoglutarate-dependent ethylene-forming enzyme from Pseudomonas syringae pv. phaseolicola PK2. Biochemistry 55, 5989–5999 (2016).
Martinez, S. & Hausinger, R. P. Correction to biochemical and spectroscopic characterization of the non-heme Fe(II)- and 2-oxoglutarate-dependent ethylene-forming enzyme from Pseudomonas syringae pv. phaseolicola PK2. Biochemistry 56, 3158 (2017).
Holm, L., Laiho, A., Törönen, P. & Salgado, M. DALI shines a light on remote homologs: one hundred discoveries. Protein Sci. 32, e4519 (2023).
Trame, C. B. et al. New mini-zincin structures provide a minimal scaffold for members of this metallopeptidase superfamily. BMC Bioinf. 15, 1 (2014).
Chwastyk, M., Jaskolski, M. & Cieplak, M. The volume of cavities in proteins and virus capsids. Proteins 84, 1275–1286 (2016).
Chojnowski, G. et al. findMySequence: a neural-network-based approach for identification of unknown proteins in X-ray crystallography and cryo-EM. IUCrJ 9, 86–97 (2022).
Islam, M. S., Leissing, T. M., Chowdhury, R., Hopkinson, R. J. & Schofield, C. J. 2-Oxoglutarate-dependent oxygenases. Annu. Rev. Biochem. 87, 585–620 (2018).
Perez-Riba, A. & Itzhaki, L. S. The tetratricopeptide-repeat motif is a versatile platform that enables diverse modes of molecular recognition. Curr. Opin. Struct. Biol. 54, 43–49 (2019).
Nguyen, H. et al. Characterization of a radical SAM oxygenase for the ether crosslinking in darobactin biosynthesis. J. Am. Chem. Soc. 144 https://doi.org/10.1021/jacs.2c05565 (2022).
Ma, S. et al. Substrate-controlled catalysis in the ether cross-link-forming radical SAM enzymes. J. Am. Chem. Soc. 145, 22945–22953 (2023).
Kaur, G., Burroughs, A. M., Iyer, L. M. & Aravind, L. Highly-regulated, diversifying NTP-dependent biological conflict systems with implications for the emergence of multicellularity. eLife 9, e52696 (2020).
English, A. C., Done, S. H., Caves, L. S., Groom, C. R. & Hubbard, R. E. Locating interaction sites on proteins: the crystal structure of thermolysin soaked in 2% to 100% isopropanol. Proteins 37, 628–640 (1999).
O’Brien, J. R., Schuller, D. J., Yang, V. S., Dillard, B. D. & Lanzilotta, W. N. Substrate-induced conformational changes in Escherichia coli taurine/alpha-ketoglutarate dioxygenase and insight into the oligomeric structure. Biochemistry 42, 5547–5554 (2003).
Acknowledgements
This work was supported by grants from the National Key Research and Development Program (2018Y F A0900402) to Q.Z., the Innovative research team of high-level local universities in Shanghai and a key laboratory programme of the Education Commission of Shanghai Municipality (ZDSYS14005) to Q.Z., the West Light Foundation of The Chinese Academy of Sciences (xbzg-zdsys-202105) to Q.Z., The National Natural Science Foundation of China (21921003 and U22A20451 to Q.Z. and 223B2701 to S.M.), The Japan Society for Promotion of Science to Y.M. and R.S., The Naito Foundation to Y.M., The Human Frontier Science Program to R.S. and the Ministry of Education Singapore (A-0004623-00-00, A-0008495-00-00 and A-8000449-00-00) to B.I.M. E.D.L.M. and P.A. thank J.C. Fontecilla-Camps for helpful discussions. P.A. appreciates the help from the staff of the computing facility provided by the Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA/DRF/GIPSI), Saclay and CCRT, Bruyères-le-Châtel. Part of this work was supported by the French National Research Agency (ANR-20-CE44-0005) and used the platforms of the Grenoble Instruct-ERIC center (ISBG; UMS 3518 CNRS-CEA-UGA-EMBL) within the Grenoble Partnership for Structural Biology, which is supported by FRISBI (ANR-10-INBS-05-02) and GRAL and financed within the Université Grenoble Alpes graduate school (Ecoles Universitaires de Recherche) CBH-EUR-GS (ANR-17-EURE-0003). The authors thank S. Matsunaga (The University of Tokyo) for providing theonellamides as a standard of l-erythro β-OH-Asp. We acknowledge the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities and we would like to thank Christoph Mueller-Dieckmann and Philippe Carpentier for assistance in using beamline ID30B.
Author information
Authors and Affiliations
Contributions
B.I.M, Q.Z. and Y.N. designed the research. Y.M., Y.W.T., R.S. and G.G. performed functional and biochemical studies for MscBH, ChlBH and SamBH. S.M., H.L., H.C. and X.J. performed the functional and biochemical studies for SjiBH and SgaBH. E.D.L.M. and A.U. performed crystallography studies for ChlH. P.A. performed in silico modelling. Y.M., S.M., E.D.L.M., Y.N., Q.Z. and B.I.M. wrote the manuscript. All authors analysed the data, discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Peer review
Peer review information
Nature Chemistry thanks Gong-Li Tang and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The hidden sequence space of αKG-HExxH family in Nature.
a, Summary of the protein families that are fused to αKG-HExxH family protein, including standalone αKG-HExxH proteins, and αKG-HExxH proteins that are fused with an rSAM, tetratricopeptide repeat (TPR) or other protein families. b, Unrooted maximum-likelihood phylogenetic tree of the αKG-HExxH domains excised from their native protein sequence. The phylum information and the presence (+) or absence (−) of the HExxH and PWRxxxRP motif are annotated on the tree.
Supplementary information
Supplementary Information
Materials and equipment, and Supplementary methods, Figs. 1–63 and Tables 1–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morishita, Y., Ma, S., De La Mora, E. et al. Fused radical SAM and αKG-HExxH domain proteins contain a distinct structural fold and catalyse cyclophane formation and β-hydroxylation. Nat. Chem. 16, 1882–1893 (2024). https://doi.org/10.1038/s41557-024-01596-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-024-01596-9
This article is cited by
-
Photobiocatalytic radical repositioning for enantioselective acylation of remote C–C/C–H bonds
Nature Catalysis (2025)
-
Aromatic side-chain crosslinking in RiPP biosynthesis
Nature Chemical Biology (2025)
-
Azetidine amino acid biosynthesis by non-haem iron-dependent enzymes
Nature Chemistry (2025)