Abstract
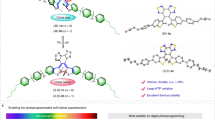
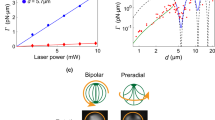
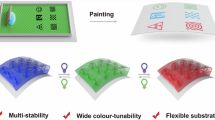
The photomodulation of the helical pitch of cholesteric liquid crystals results in dynamic and coloured canvases that can potentially be used in applications ranging from energy-efficient displays to colour filters, anti-counterfeiting tags and liquid crystal (LC) lasers. Here we report on the analysis of a series of photoswitchable chiral dopants that combine the large geometrical change and bistability of hydrazone switches with the efficient helical pitch induction of the chiral motif, triptycene. We elucidate the effects that conformational flexibility, dispersion forces and π–π interactions have on the chirality transfer ability of the dopant. We then use the irradiation time with visible light (442 nm) combined with a simple digital light processing microscope projection set-up to draw numerous stable multi-coloured images on an LC canvas, showcasing the fine control this dopant yields over the LC assembly.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information. Source data are provided with this paper.
References
Li, Q. Intelligent Stimuli-Responsive Materials: from Well-Defined Nanostructures to Applications (Wiley, 2013).
Goulet‐Hanssens, A., Eisenreich, F. & Hecht, S. Enlightening materials with photoswitches. Adv. Mater. 32, 1905966 (2020).
Wang, Y. & Li, Q. Light‐driven chiral molecular switches or motors in liquid crystals. Adv. Mater. 24, 1926–1945 (2012).
Kim, Y. & Tamaoki, N. Photoresponsive chiral dopants: light-driven helicity manipulation in cholesteric liquid crystals for optical and mechanical functions. ChemPhotoChem. 3, 284–303 (2019).
Zheng, Z. et al.Three-dimensional control of the helical axis of a chiral nematic liquid crystal by light. Nature 531, 352–356 (2016).
Bisoyi, H. K. & Li, Q. Light-driven liquid crystalline materials: from photo-induced phase transitions and property modulations to applications. Chem. Rev. 116, 15089–15166 (2016).
Bisoyi, H. K. & Li, Q. Liquid crystals: versatile self-organized smart soft materials. Chem. Rev. 122, 4887–4926 (2021).
Broer, D. J., Lub, J. & Mol, G. N. Wide-band reflective polarizers from cholesteric polymer networks with a pitch gradient. Nature 378, 467–469 (1995).
Chilaya, G. S. Light-controlled change in the helical pitch and broadband tunable cholesteric liquid-crystal lasers. Crystallogr. Rep. 51, S108–S118 (2006).
Palffy-Muhoray, P. Liquid crystals new designs in cholesteric colour. Nature 391, 745–746 (1998).
Sagisaka, T. & Yokoyama, Y. Reversible control of the pitch of cholesteric liquid crystals by photochromism of chiral fulgide derivatives. Bull. Chem. Soc. Jpn 73, 191–196 (2000).
Janicki, S. Z. & Schuster, G. B. A liquid crystal opto-optical switch: nondestructive information retrieval based on a photochromic fulgide as trigger. J. Am. Chem. Soc. 117, 8524–8527 (1995).
White, T. J. et al. Optically reconfigurable color change in chiral nematic liquid crystals based on indolylfulgide chiral dopants. J. Mater. Chem. 22, 5751–5757 (2012).
Kurosaki, Y., Sagisaka, T., Matsushima, T., Ubukata, T. & Yokoyama, Y. Chiral, thermally irreversible and quasi‐stealth photochromic dopant to control selective reflection wavelength of cholesteric liquid crystal. ChemPhysChem 21, 1375–1383 (2020).
Bosco, A. et al. Photoinduced reorganization of motor-doped chiral liquid crystals: bridging molecular isomerization and texture rotation. J. Am. Chem. Soc. 130, 14615–14624 (2008).
Koumura, N., Zijlstra, R. W., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
van Delden, R. A., Koumura, N., Harada, N. & Feringa, B. L. Unidirectional rotary motion in a liquid crystalline environment: color tuning by a molecular motor. Proc. Natl Acad. Sci. USA 99, 4945–4949 (2002).
Ryabchun, A. et al. Helix inversion controlled by molecular motors in multistate liquid crystals. Adv. Mater. 32, 2004420 (2020).
Hou, J. et al. Photo-responsive helical motion by light-driven molecular motors in a liquid-crystal network. Angew. Chem. Int. Ed. 60, 8251–8257 (2021).
Bandara, H. D. & Burdette, S. C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 41, 1809–1825 (2012).
Irie, M., Fukaminato, T., Matsuda, K. & Kobatake, S. Photochromism of diarylethene molecules and crystals: memories, switches, and actuators. Chem. Rev. 114, 12174–12277 (2014).
Li, Y., Urbas, A. & Li, Q. Reversible light-directed red, green, and blue reflection with thermal stability enabled by a self-organized helical superstructure. J. Am. Chem. Soc. 134, 9573–9576 (2012).
Li, Y., Xue, C., Wang, M., Urbas, A. & Li, Q. Photodynamic chiral molecular switches with thermal stability: from reflection wavelength tuning to handedness inversion of self‐organized helical superstructures. Angew. Chem. Int. Ed. 52, 13703–13707 (2013).
Li, Y., Wang, M., Wang, H., Urbas, A. & Li, Q. Rationally designed axially chiral diarylethene switches with high helical twisting power. Chem. Eur. J. 20, 16286–16292 (2014).
Denekamp, C. & Feringa, B. L. Optically active diarylethenes for multimode photoswitching between liquid–crystalline phases. Adv. Mater. 10, 1080–1082 (1998).
Uchida, K., Kawai, Y., Shimizu, Y., Vill, V. & Irie, M. An optically active diarylethene having cholesterol units: a dopant for photoswitching of liquid crystal phases. Chem. Lett. 29, 654–655 (2000).
Yamaguchi, T., Inagawa, T., Nakazumi, H., Irie, S. & Irie, M. Photoswitching of helical twisting power of a chiral diarylethene dopant: pitch change in a chiral nematic liquid crystal. Chem. Mater. 12, 869–871 (2000).
van Leeuwen, T. et al. Photoinduced pitch changes in chiral nematic liquid crystals formed by doping with chiral diarylethene. J. Mater. Chem. 21, 3142–3246 (2011).
Li, Y., Urbas, A. & Li, Q. Synthesis and characterization of light-driven dithienylcyclopentene switches with axial chirality. J. Org. Chem. 76, 7148–7156 (2011).
Zheng, Z. et al. Digital photoprogramming of liquid-crystal superstructures featuring intrinsic chiral photoswitches. Nat. Photonics 16, 226–234 (2022).
Shao, B. & Aprahamian, I. Hydrazones as new molecular tools. Chem 6, 2162–2173 (2020).
Qian, H., Pramanik, S. & Aprahamian, I. Photochromic hydrazone switches with extremely long thermal half-lives. J. Am. Chem. Soc. 139, 9140–9143 (2017).
Shao, B., Qian, H., Li, Q. & Aprahamian, I. Structure property analysis of the solution and solid-state properties of bistable photochromic hydrazones. J. Am. Chem. Soc. 141, 8364–8371 (2019).
Moran, M. J., Magrini, M., Walba, D. M. & Aprahamian, I. Driving a liquid crystal phase transition using a photochromic hydrazone. J. Am. Chem. Soc. 140, 13623–13627 (2018).
Shin, S. et al. Tuning helical twisting power of isosorbide-based chiral dopants by chemical modifications. Mol. Cryst. Liq. Cryst. 534, 19–31 (2011).
Khan, M. N. & Wirth, T. Chiral triptycenes: concepts, progress and prospects. Chem. Eur. J. 27, 7059–7068 (2021).
Norvez, S. Liquid crystalline triptycene derivatives. J. Org. Chem. 58, 2414–2418 (1993).
Norvez, S. & Simon, J. Epitaxygens: mesomorphic properties of triptycene derivatives. Liq. Cryst. 14, 1389–1395 (1993).
Long, T. M. & Swager, T. M. Triptycene-containing bis(phenylethynyl)benzene nematic liquid crystals. J. Mater. Chem. 12, 3407–3412 (2002).
Lohr, A. & Swager, T. M. Stabilization of the nematic mesophase by a homogeneously dissolved conjugated polymer. J. Mater. Chem. 20, 8107–8111 (2010).
Kelly, T. R., Silva, R. A., De Silva, H., Jasmin, S. & Zhao, Y. A rationally designed prototype of a molecular motor. J. Am. Chem. Soc. 122, 6935–6949 (2000).
Ube, H., Yasuda, Y., Sato, H. & Shionoya, M. Metal-centred azaphosphatriptycene gear with a photo-and thermally driven mechanical switching function based on coordination isomerism. Nat. Commun. 8, 4296 (2017).
Long, T. M. & Swager, T. M. Minimization of free volume: alignment of triptycenes in liquid crystals and stretched polymers. Adv. Mater. 13, 601–604 (2001).
Long, T. M. & Swager, T. M. Using ‘internal free volume’ to increase chromophore alignment. J. Am. Chem. Soc. 124, 3826–3827 (2002).
Zhu, Z. & Swager, T. M. Conjugated polymer liquid crystal solutions: control of conformation and alignment. J. Am. Chem. Soc. 124, 9670–9671 (2002).
Ohira, A. & Swager, T. M. Ordering of poly(p-phenylene ethynylene)s in liquid crystals. Macromolecules 40, 19–25 (2007).
Yang, S. et al. Multistage reversible Tg photomodulation and hardening of hydrazone-containing polymers. J. Am. Chem. Soc. 143, 16348–16353 (2021).
Haris, U., Plank, J. T., Li, B., Page, Z. A. & Lippert, A. R. Visible light chemical micropatterning using a digital light processing fluorescence microscope. ACS Cent. Sci. 8, 67–76 (2021).
Gu, C., Jia, A.-B., Zhang, Y.-M. & Zhang, S. X.-A. Emerging electrochromic materials and devices for future displays. Chem. Rev. 122, 14679–14721 (2022).
Chen, Z. & Swager, T. M. Synthesis and characterization of poly (2,6-triptycene). Macromolecules 41, 6880–6885 (2008).
Zhang, Q. P. et al. Triptycene‐based chiral porous polyimides for enantioselective membrane separation. Angew. Chem. Int. Ed. 133, 12891–12895 (2021).
Ryabchun, A., Li, Q., Lancia, F., Aprahamian, I. & Katsonis, N. Shape-persistent actuators from hydrazone photoswitches. J. Am. Chem. Soc. 141, 1196–1200 (2019).
Narazaki, Y., Nishikawa, H., Higuchi, H., Okumura, Y. & Kikuchi, H. Substituent effects of bridged binaphthyl-type chiral dopants on the helical twisting power in dopant-induced chiral liquid crystals. RSC Adv. 8, 971–979 (2018).
Eelkema, R. & Feringa, B. L. Amplification of chirality in liquid crystals. Org. Biomol. Chem. 4, 3729–3745 (2006).
Pieraccini, S., Masiero, S., Ferrarini, A. & Spada, G. P. Chirality transfer across length-scales in nematic liquid crystals: fundamentals and applications. Chem. Soc. Rev. 40, 258–271 (2011).
Katsonis, N., Lacaze, E. & Ferrarini, A. Controlling chirality with helix inversion in cholesteric liquid crystals. J. Mater. Chem. 22, 7088–7097 (2012).
Wang, D., Park, S.-Y. & Kang, I.-K. Liquid crystals: emerging materials for use in real-time detection applications. J. Mater. Chem. C 3, 9038–9047 (2015).
White, T. J., McConney, M. E. & Bunning, T. J. Dynamic color in stimuli-responsive cholesteric liquid crystals. J. Mater. Chem. C 20, 9832–9847 (2010).
Acknowledgements
I.A. is thankful for the generous support from National Science Foundation (NSF) Division of Materials Research (DMR) (2104464). A.R.L. gratefully acknowledges support from the Welch Research Foundation (N-2038-202004010). We thank L.D. Custis for experimental support. I.A. is thankful to T. Swager (Massachusetts Institute of Technology) for suggesting the use of triptycene as the chiral motif in LC dopants at a Telluride workshop in 2016, and T. Ikai (Nagoya University) for supplying racemic 2,6-diaminotriptycene at a very early stage of this research.
Author information
Authors and Affiliations
Contributions
I.B., J.T.P., D.H. and B.B. planned and carried out the experimental work and characterization studies. I.A. and A.R.L. directed the research. All authors contributed to the analysis of the results and the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.R.L. declares a financial stake in BioLum Sciences, LLC. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Masoud Kazem-Rostami, Quan Li and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–99, Tables 1–15, Scheme 1, Discussion and experimental procedures.
Source data
Source Data Fig. 1
Circular dichroism data underlying Fig. 1b,c.
Source Data Fig. 2
Transmittance data underlying Fig. 2b.
Source Data Fig. 3
Transmittance data underlying Fig. 3a.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bala, I., Plank, J.T., Balamut, B. et al. Multi-stage and multi-colour liquid crystal reflections using a chiral triptycene photoswitchable dopant. Nat. Chem. 16, 2084–2090 (2024). https://doi.org/10.1038/s41557-024-01648-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-024-01648-0
This article is cited by
-
Hydrazone-based photocages for precise sub-organelle visualization and drug release
Communications Chemistry (2025)
-
Dynamic Structural Colors in Helical Superstructures: from Supramolecules to Polymers
Chinese Journal of Polymer Science (2025)