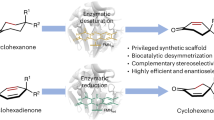
Abstract
Carbonyl desaturation is a fundamental reaction widely practised in organic synthesis. While numerous methods have been developed to expand the scope of this important transformation, most of them necessitate multi-step protocols or suffer from the use of high loadings of metal or strong oxidizing conditions. Moreover, approaches that can achieve precise stereochemical control of the desaturation process are extremely rare. Here we report a biocatalytic platform for desymmetrizing desaturation of cyclohexanones to generate diverse cyclohexenones bearing a remote quaternary stereogenic centre, by reengineering ‘ene’-reductases to efficiently mediate dehydrogenation, the reverse process of their native activity. This ‘ene’-reductase-based desaturation system operates under mild conditions with air as the terminal oxidant, tolerates oxidation-sensitive or metal-incompatible functional groups and, more importantly, exhibits unparalleled stereoselectivity compared with those achieved with small-molecule catalysts. Mechanistic investigations suggest that the reaction proceeded through α-deprotonation followed by a rate-determining β-hydride transfer.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Full experimental details are available in Supplementary Information. Crystallographic data for compound 35 reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition number CCDC 2293360. Copies of the data can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/.
References
Patai, S. & Rappoport, Z. The Chemistry of Enones (John Wiley & Sons, 1989).
Stahl, S. S. & Diao, T. Comprehensive Organic Synthesis 2nd edn (Elsevier, 2014).
Ito, Y., Hirao, T. & Saegusa, T. Synthesis of α,β-unsaturated carbonyl compounds by palladium (II)-catalyzed dehydrosilylation of silyl enol ethers. J. Org. Chem. 43, 1011–1013 (1978).
Back, T. G. Encyclopedia of Inorganic and Bioinorganic Chemistry (John Wiley & Sons, 2011).
Nicolaou, K. C., Zhong, Y. L. & Baran, P. S. A new method saturated alcohols and carbonyl compounds. J. Am. Chem. Soc. 122, 7596–7597 (2000).
Diao, T. & Stahl, S. S. Synthesis of cyclic enones via direct palladium catalyzed aerobic dehydrogenation of ketones. J. Am. Chem. Soc. 133, 14566–14569 (2011).
Chen, Y., Romaire, J. P. & Newhouse, T. R. Synthesis of cyclic enones by allyl-palladium-catalyzed α, β-dehydrogenation of esters and nitriles. J. Am. Chem. Soc. 137, 5875–5878 (2015).
Wang, Z. et al. Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C–H activation. Science 374, 1281–1285 (2021).
Jie, X., Shang, Y., Zhang, X. & Su, W. Cu-catalyzed sequential dehydrogenation-conjugate addition for β-functionalization of saturated ketones: scope and mechanism. J. Am. Chem. Soc. 138, 5623–5633 (2016).
Chen, M., Rago, A. J. & Dong, G. Platinum-catalyzed desaturation of lactams, ketones, and lactones. Angew. Chem. Int. Ed. 57, 16205–16209 (2018).
Wang, Z., He, Z., Zhang, L. & Huang, Y. Iridium-catalyzed aerobic α,β-dehydrogenation of γ,δ-unsaturated amides and acids: activation of both α- and β-C–H bonds through an allyl-iridium intermediates. J. Am. Chem. Soc. 140, 735–740 (2018).
Huang, D., Szewczyk, S. M., Zhang, P. & Newhouse, T. R. Allyl-nickel catalysis enables carbonyl dehydrogenation and oxidative cycloalkenylation of ketones. J. Am. Chem. Soc. 141, 5669–5674 (2019).
Gnaim, S. et al. Electrochemically driven desaturation of carbonyl compounds. Nat. Chem. 13, 367–372 (2021).
Gnaim, S., Vantourout, J. C., Serpier, F., Echeverria, P.-G. & Baran, P. S. Carbonyl desaturation: where does catalysis stand. ACS Catal. 11, 883–892 (2021).
Huang, D. & Newhouse, T. R. Dehydrogenative Pd and Ni catalysis for total synthesis. Acc. Chem. Res. 54, 1118–1130 (2021).
Zhu, L., Zhang, L. & Luo, S. Catalytic desymmetrizing dehydrogenation of 4-substituted cyclohexanones through enamine oxidation. Angew. Chem. Int. Ed. 57, 2253–2258 (2018).
Bornscheuer, U. T. The fourth wave of biocatalysis is approaching. Phil. Trans. R. Soc. A 376, 20170063 (2018).
Romero, E. O. et al. Enabling broader adoption of biocatalysis in organic chemistry. JACS Au 3, 2073–2085 (2023).
Arnold, F. H. Directed evolution: bringing new chemistry to life. Angew. Chem. Int. Ed. 57, 4143–4148 (2018).
Yang, Y. & Arnold, F. H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 54, 1209–1224 (2021).
Harrison, W., Huang, X. & Zhao, H. Photobiocatalysis for abiological transformations. Acc. Chem. Res. 55, 1087–1096 (2022).
Emmanuel, M. A. et al. Photobiocatalytic strategies for organic synthesis. Chem. Rev. 123, 5459–5520 (2023).
Ghisla, S. & Thorpe, C. Acyl-CoA dehydrogenases—a mechanistic overview. Eur. J. Biochem. 271, 494–508 (2004).
Buist, P. H. Fatty acid desaturases: selecting the dehydrogenation channel. Nat. Prod. Rep. 21, 249–262 (2004).
Cerone, M. & Smith, T. K. Desaturases: structural and mechanistic insights into the biosynthesis of unsaturated fatty acids. IUBMB Life 74, 1036–1051 (2022).
Toogood, H. S. & Scrutton, N. S. Discovery, characterization, engineering, and applications of ene-reductases for industrial biocatalysis. ACS Catal. 8, 3532–3549 (2018).
Winkler, C. K., Faber, K. & Hall, M. Biocatalytic reduction of activated C=C-bonds and beyond: emerging trends. Curr. Opin. Chem. Biol. 43, 97–105 (2018).
Durchschein, K., Hall, M. & Faber, K. Unusual reactions mediated by FMN-dependent ene- and nitro-reductases. Green Chem. 15, 1764–1772 (2013).
Hall, M. Enzymatic strategies for asymmetric synthesis. RSC Chem. Biol. 2, 958–989 (2021).
Roy, T. K., Sreedharan, R., Ghosh, P., Gandhi, T. & Maiti, D. Ene-reductase: a multifaceted biocatalyst in organic synthesis. Chem. Eur. J. 28, e202103949 (2022).
Murthy, Y. V. S. N., Meah, Y. & Massey, V. Conversion of a flavoprotein reductase to a desaturase by manipulation of the flavin redox potential. J. Am. Chem. Soc. 121, 5344–5345 (1999).
Jiang, G. et al. Ene reductase enabled intramolecular β-C–H functionalization of substituted cyclohexanones for efficient synthesis of bridged bicyclic nitrogen scaffolds. Angew. Chem. Int. Ed. 62, e202302125 (2023).
Schittmayer, M. et al. Old yellow enzyme-catalyzed dehydrogenation of saturated ketones. Adv. Synth. Catal. 353, 268–274 (2011).
Rogers, D. W., Zhao, Y., Traetteberg, M., Hulce, M. & Liebman, J. Enthalpies of hydrogenation and formation of enones. resonance energies of 2-cyclopentenone and 2-cyclohexenone. J. Chem. Thermodyn. 30, 1393–1400 (1998).
Vaz, A. D. N., Chakraborty, S. & Massey, V. Old yellow enzyme: aromatization of cyclic enones and the mechanism of a novel dismutation reaction. Biochemistry 34, 4246–4256 (1995).
Stueckler, C., Reiter, T. C., Baudendistel, N. & Faber, K. Nicotinamide-independent asymmetric bioreduction of C=C-bonds via disproportionation of enones catalyzed by enoate reductases. Tetrahedron 66, 663–667 (2010).
Winker, C. K., Clay, D., Entner, M., Plank, M. & Faber, K. NAD(P)H-independent asymmetric C=C bond reduction catalyzed by ene reductases by using artificial co-substrates as the hydrogen donor. Chem. Eur. J. 20, 1403–1409 (2014).
Kelly, P. P. et al. Ene reductase enzymes for the aromatisation of tetralones and cyclohexenones to napththols and phenols. Adv. Synth. Catal. 358, 731–736 (2016).
Nicholls, B. et al. Engineering a non-natural photoenzyme for improved photon efficiency. Angew. Chem. Int. Ed. 61, e202113842 (2022).
Christoffers, J. & Baro, A. Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis (Wiley, 2005).
Reetz, M. T. & Carballeira, J. D. Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nat. Protoc. 2, 891–903 (2007).
Krstenansky, J. L. Mesembrin alkaloids: review of their occurrence, chemistry, and pharmacology. J. Ethnopharmacol. 195, 10–19 (2017).
Fringuelli, F., Pizzo, F., Taticchi, A., Halls, T. D. J. & Wenkert, E. Diels–Alder reactions of cycloalkenones. 1. Preparation and structure of the adducts. J. Org. Chem. 47, 5056–5065 (1982).
Schanze, K., Mattox, F. & Whitten, D. G. Solvent effects upon the thermal cis–trans isomerization and charge-transfer absorption of 4-(diethylamino)-4′-nitroazobenzene. J. Org. Chem. 48, 2808–2813 (1983).
Lonsdale, R. & Reetz, M. T. Reduction of α,β-unsaturated ketones by old yellow enzymes: mechanistic insights from quantum mechanics/molecular mechanics calculations. J. Am. Chem. Soc. 137, 14733–14742 (2015).
Lai, M.-T., Li, D., Oh, E. & Liu, H.-W. Inactivation of medium-chain acyl-CoA dehydrogenase by a metabolite of hypoglycin: characterization of the major turnover product and evidence suggesting an alternative flavin modification pathway. J. Am. Chem. Soc. 115, 1619–1628 (1993).
Li, D. et al. Spiropentylacetyl-CoA, a mechanism-based inactivator of acyl-CoA dehydrogenases. J. Am. Chem. Soc. 120, 2008–2017 (1998).
Kohli, R. M. & Massey, V. The oxidative half-reaction of old yellow enzyme. The role of tyrosine 196. J. Biol. Chem. 273, 32763–32770 (1998).
Acknowledgements
This work was supported by the National Key R&D Program of China (no. 2022YFA1505600 to Y.Y.). We thank the Zhejiang Provincial Key Laboratory Construction Project and Research Center for Industries of the Future at Westlake University and the Zhejiang Provincial National Science Foundation of China (XHD24B0101 to Y.Y.) for partially supporting this work. We thank Westlake University Instrumentation and Service Center for Molecular Sciences for the facility support and technical assistance. We thank X. Lu, Z. Chen and D. Gu from Westlake University Instrumentation and Service Center for Molecular Sciences for the assistance with the stopped-flow experiment. We thank X. Cui and B. Zhang from CAP for Solar Fuels at Westlake University for the assistance in measuring the oxygen concentrations. We also thank X. Huang (Johns Hopkins University) for the suggestions on the manuscript and T. Hyster (Princeton University), Y. Yang (UCSB), H. Fu (CAMS and PUMS) and P. Hu (Westlake University) for helpful discussions.
Author information
Authors and Affiliations
Contributions
Y.Y. conceived and directed the project. H.W. performed the protein engineering, substrate scope evaluation and mechanistic study. B.G., H.C., S.C. and X.M. synthesized the substrates and analysed spectroscopy data. Y.C. conducted the deuterium-labelling analysis. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Scott France, Maciej Szaleniec and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary protocols, discussions, figures, NMR spectra and HPLC traces.
Supplementary Data 1
Raw data on the Clark experiment to study the kinetics of substrate desaturation.
Supplementary Data 2
Raw data on the stopped-flow experiment to study the kinetics of substrate reduction.
Supplementary Data 3
Raw data on the stopped-flow experiment to study the kinetics of enzyme regeneration by oxygen.
Supplementary Data 4
Primers used in the directed evolution.
Supplementary Data 5
Crystallographic data for compound 35, CCDC reference 2293360.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Gao, B., Cheng, H. et al. Unmasking the reverse catalytic activity of ‘ene’-reductases for asymmetric carbonyl desaturation. Nat. Chem. 17, 74–82 (2025). https://doi.org/10.1038/s41557-024-01671-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-024-01671-1
This article is cited by
-
Stereoselective cyano translocation reaction enabled by photoenzymatic catalysis
Nature Communications (2026)
-
Molecular-dynamics-simulation-guided directed evolution of flavoenzymes for atroposelective desaturation
Nature Synthesis (2025)
-
Pushing the boundaries of biocatalysis
Nature Catalysis (2025)