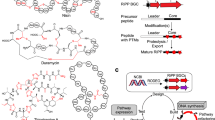
Abstract
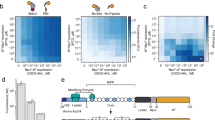
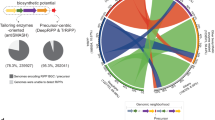
In nature, peptides are enzymatically modified to constrain their structure and introduce functional moieties. De novo peptide structures could be built by combining enzymes from different pathways, but determining the rules of their use is difficult. We present a biophysical model to combine enzymes sourced from bacterial ribosomally synthesized and post-translationally modified peptide (RiPP) gene clusters. Using a pipeline to evaluate more than 1,000 peptides, the model was parameterized under uniform conditions in Escherichia coli for enzymes from different classes (graspetide, spliceotide, pantocin, cyanobactin, glycocin, lasso peptide and lanthipeptide). Synthetic leader peptides with recognition sequences for up to three enzymes were designed to modify core sequences sharing no identity to natural RiPPs. Empirically, RiPPs with the desired modifications constituted 7–67% of the total peptides produced, and 6 of our 8 peptide designs were successfully modified. This work is an example of the design of enzyme-modified peptides and libraries, using a framework that can be expanded to include new enzymes and chemical moieties.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data associated with this Article can be found in the Supplementary Information. All strains and plasmids are available upon request.
Code availability
Code is available on GitHub at https://github.com/VoigtLab/ripp-design (ref. 96). Instructions for its use are provided in Supplementary Note 12.
References
Montalban-Lopez, M. et al. New developments in RiPP discovery, enzymology and engineering. Nat. Prod. Rep. 38, 130–239 (2021).
Wallace, A. K., Chanut, N. & Voigt, C. A. Silica nanostructures produced using diatom peptides with designed post‐translational modifications. Adv. Funct. Mater. 30, 2000849 (2020).
Goto, Y. & Suga, H. Engineering of RiPP pathways for the production of artificial peptides bearing various non-proteinogenic structures. Curr. Opin. Chem. Biol. 46, 82–90 (2018).
Hudson, G. A. & Mitchell, D. A. RiPP antibiotics: biosynthesis and engineering potential. Curr. Opin. Microbiol. 45, 61–69 (2018).
Arnison, P. G. et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30, 108–160 (2013).
Burkhart, B. J. et al. A prevalent peptide-binding domain guides ribosomal natural product biosynthesis. Nat. Chem. Biol. 11, 564–570 (2015).
Oman, T. J. & Van Der Donk, W. A. Follow the leader: the use of leader peptides to guide natural product biosynthesis. Nat. Chem. Biol. 6, 9–18 (2010).
Zhang, Z. et al. Biosynthetic timing and substrate specificity for the thiopeptide thiomuracin. J. Am. Chem. Soc. 138, 15511–15514 (2016).
Crone, W. J. K. et al. Dissecting bottromycin biosynthesis using comparative untargeted metabolomics. Angew. Chem. Int. Ed. 55, 9639–9643 (2016).
Bhushan, A. et al. Genome mining- and synthetic biology-enabled production of hypermodified peptides. Nat. Chem. 11, 931–939 (2019).
Bowers, A. A. et al. Generation of thiocillin ring size variants by prepeptide gene replacement and in vivo processing by Bacillus cereus. J. Am. Chem. Soc. 134, 10313–10316 (2012).
Thibodeaux, C. J., Ha, T. & Van Der Donk, W. A. A price to pay for relaxed substrate specificity: a comparative kinetic analysis of the class II lanthipeptide synthetases ProcM and HalM2. J. Am. Chem. Soc. 136, 17513–17529 (2014).
Li, C., Zhang, F. & Kelly, W. L. Heterologous production of thiostrepton A and biosynthetic engineering of thiostrepton analogs. Mol. Biosyst. 7, 82–90 (2011).
Freeman, M. F. et al. Seven enzymes create extraordinary molecular complexity in an uncultivated bacterium. Nat. Chem. 9, 387–395 (2017).
Morinaka, B. I. et al. Radical S-adenosyl methionine epimerases: regioselective introduction of diverse D-amino acid patterns into peptide natural products. Angew. Chem. Int. Ed. 53, 8503–8507 (2014).
Van Der Velden, N. S. et al. Autocatalytic backbone N-methylation in a family of ribosomal peptide natural products. Nat. Chem. Biol. 13, 833–835 (2017).
Ruffner, D. E., Schmidt, E. W. & Heemstra, J. R. Assessing the combinatorial potential of the RiPP cyanobactin tru pathway. ACS Synth. Biol. 4, 482–492 (2015).
Si, Y. et al. Cell-free biosynthesis to evaluate lasso peptide formation and enzyme-substrate tolerance. J. Am. Chem. Soc. 143, 5917–5927 (2021).
Mordhorst, S. et al. Posttranslationally acting arginases provide a ribosomal route to non‐proteinogenic ornithine residues in diverse peptide sequences. Angew. Chem. Int. Ed. 59, 21442–21447 (2020).
Hao, Y. et al. Molecular basis for the broad substrate selectivity of a peptide prenyltransferase. Proc. Natl Acad. Sci. USA 113, 14037–14042 (2016).
Kupke, T. et al. Oxidative decarboxylation of peptides catalyzed by flavoprotein EpiD. J. Biol. Chem. 270, 11282–11289 (1995).
Zhang, Q. & Van Der Donk, W. A. Catalytic promiscuity of a bacterial α-N-methyltransferase. FEBS Lett. 586, 3391–3397 (2012).
Lohans, C. T., Li, J. L. & Vederas, J. C. Structure and biosynthesis of carnolysin, a homologue of enterococcal cytolysin with d-amino acids. J. Am. Chem. Soc. 136, 13150–13153 (2014).
Fleming, S. R. et al. Exploring the post-translational enzymology of PaaA by mRNA display. J. Am. Chem. Soc. 142, 5024–5028 (2020).
Fuchs, S. W. et al. A lanthipeptide-like N-terminal leader region guides peptide epimerization by radical SAM epimerases: implications for RiPP evolution. Angew. Chem. Int. Ed. 55, 12330–12333 (2016).
Hegemann, J. D. et al. Assessing the flexibility of the prochlorosin 2.8 scaffold for bioengineering applications. ACS Synth. Biol. 8, 1204–1214 (2019).
Cotter, P. D. et al. Complete alanine scanning of the two‐component lantibiotic lacticin 3147: generating a blueprint for rational drug design. Mol. Microbiol. 62, 735–747 (2006).
Young, T. S. Biosynthesis and directed evolution of unnatural peptides and proteins. Rev. Cell Biol. Mol. Med. 1, 3 (2015).
Young, T. S., Dorrestein, P. C. & Walsh, C. T. Codon randomization for rapid exploration of chemical space in thiopeptide antibiotic variants. Chem. Biol. 19, 1600–1610 (2012).
Vinogradov, A. A. et al. Minimal lactazole scaffold for in vitro thiopeptide bioengineering. Nat. Commun. 11, 2272 (2020).
Schmitt, S. et al. Analysis of modular bioengineered antimicrobial lanthipeptides at nanoliter scale. Nat. Chem. Biol. 15, 437–443 (2019).
Pan, S. J. & Link, A. J. Sequence diversity in the lasso peptide framework: discovery of functional microcin J25 variants with multiple amino acid substitutions. J. Am. Chem. Soc. 133, 5016–5023 (2011).
Tran, H. L. et al. Structure-activity relationship and molecular mechanics reveal the importance of ring entropy in the biosynthesis and activity of a natural product. J. Am. Chem. Soc. 139, 2541–2544 (2017).
Fleming, S. R. et al. Flexizyme-enabled benchtop biosynthesis of thiopeptides. J. Am. Chem. Soc. 141, 758–762 (2019).
Korneli, M. et al. Promiscuous installation of d-amino acids in gene-encoded peptides. ACS Synth. Biol. 10, 236–242 (2021).
Burkhart, B. J. et al. Chimeric leader peptides for the generation of non-natural hybrid RiPP products. ACS Central Sci. 3, 629–638 (2017).
Huang, C.-F. & Mrksich, M. Profiling protein tyrosine phosphatase specificity with self-assembled monolayers for matrix-assisted laser desorption/ionization mass spectrometry and peptide arrays. ACS Comb. Sci. 21, 760–769 (2019).
Sardar, D. et al. Recognition sequences and substrate evolution in cyanobactin biosynthesis. ACS Synth. Biol. 4, 167–176 (2015).
Sardar, D., Lin, Z. & Schmidt, E. W. Modularity of RiPP enzymes enables designed synthesis of decorated peptides. Chem. Biol. 22, 907–916 (2015).
Van Heel, A. J. et al. Designing and producing modified, new-to-nature peptides with antimicrobial activity by use of a combination of various lantibiotic modification enzymes. ACS Synth. Biol. 2, 397–404 (2013).
Ghodge, S. V. et al. Post-translational Claisen condensation and decarboxylation en route to the bicyclic core of Pantocin A. J. Am. Chem. Soc. 138, 5487–5490 (2016).
Morinaka, B. I. et al. Natural noncanonical protein splicing yields products with diverse β-amino acid residues. Science 359, 779–782 (2018).
Su, Y. et al. Discovery and characterization of a novel C-terminal peptide carboxyl methyltransferase in a lassomycin-like lasso peptide biosynthetic pathway. Appl. Microbiol. Biotechnol. 103, 2649–2664 (2019).
Zhu, S. et al. Dual substrate-controlled kinase activity leads to polyphosphorylated lasso peptides. FEBS Lett. 590, 3323–3334 (2016).
Zhu, S. et al. Insights into the unique phosphorylation of the lasso peptide paeninodin. J. Biol. Chem. 291, 13662–13678 (2016).
Meyer, A. J. et al. Escherichia coli ‘Marionette’ strains with 12 highly optimized small-molecule sensors. Nat. Chem. Biol. 15, 196–204 (2019).
Glassey, E. et al. Functional expression of diverse post-translational peptide-modifying enzymes in Escherichia coli under uniform expression and purification conditions. PLoS ONE 17, e0266488 (2022).
Clifton, K. P. et al. The genetic insulator RiboJ increases expression of insulated genes. J. Biol. Eng. 12, 23 (2018).
Salis, H. M., Mirsky, E. A. & Voigt, C. A. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 27, 946–950 (2009).
Walsh, C. T., Malcolmson, S. J. & Young, T. S. Three ring posttranslational circuses: insertion of oxazoles, thiazoles and pyridines into protein-derived frameworks. ACS Chem. Biol. 7, 429–442 (2012).
Michael, J. P. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 24, 191–222 (2007).
Roh, H. et al. A topologically distinct modified peptide with multiple bicyclic core motifs expands the diversity of microviridin‐like peptides. ChemBioChem 20, 1051–1059 (2019).
Zhang, Y. et al. A distributive peptide cyclase processes multiple microviridin core peptides within a single polypeptide substrate. Nat. Commun. 9, 1780 (2018).
Wells, J. A. Additivity of mutational effects in proteins. Biochemistry 29, 8509–8517 (1990).
Carneiro, M. & Hartl, D. L. Colloquium papers: adaptive landscapes and protein evolution. Proc. Natl Acad. Sci. USA 107, 1747–1751 (2010).
Koch, P. et al. Optimization of the antimicrobial peptide Bac7 by deep mutational scanning. BMC Biol. 20, 114 (2022).
Tracewell, C. A. & Arnold, F. H. Directed enzyme evolution: climbing fitness peaks one amino acid at a time. Curr. Opin. Chem. Biol. 13, 3–9 (2009).
Roy, R. S. et al. Role of the microcin B17 propeptide in substrate recognition: solution structure and mutational analysis of McbA1-26. Chem. Biol. 5, 217–228 (1998).
Chekan, J. R., Ongpipattanakul, C. & Nair, S. K. Steric complementarity directs sequence promiscuous leader binding in RiPP biosynthesis. Proc. Natl Acad. Sci. USA 116, 24049–24055 (2019).
Bobeica, S. C. et al. Insights into AMS/PCAT transporters from biochemical and structural characterization of a double Glycine motif protease. eLife 8, e42305 (2019).
Koehnke, J. et al. Structural analysis of leader peptide binding enables leader-free cyanobactin processing. Nat. Chem. Biol. 11, 558–563 (2015).
Huang, W. et al. Amino acid sequence determinants of β-lactamase structure and activity. J. Mol. Biol. 258, 688–703 (1996).
Gavrish, E. et al. Lassomycin, a ribosomally synthesized cyclic peptide, kills Mycobacterium tuberculosis by targeting the ATP-dependent protease ClpC1P1P2. Chem. Biol. 21, 509–518 (2014).
Lipinski, C. A. et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001).
Palm, K. et al. Evaluation of dynamic polar molecular surface area as predictor of drug absorption: comparison with other computational and experimental predictors. J. Med. Chem. 41, 5382–5392 (1998).
Kaunietis, A. et al. Heterologous biosynthesis and characterization of a glycocin from a thermophilic bacterium. Nat. Commun. 10, 1115 (2019).
Butler, M. S. et al. Glycopeptide antibiotics: back to the future. J. Antibiotics 67, 631–644 (2014).
Moradi, S. V. et al. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 7, 2492–2500 (2016).
Schnell, N. et al. Analysis of genes involved in the biosynthesis of lantibiotic epidermin. Eur. J. Biochem. 204, 57–68 (1992).
Schnell, N. et al. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature 333, 276–278 (1988).
Sit, C. S., Yoganathan, S. & Vederas, J. C. Biosynthesis of aminovinyl-cysteine-containing peptides and its application in the production of potential drug candidates. Acc. Chem. Res. 44, 261–268 (2011).
Morrison, C. Constrained peptides’ time to shine? Nat. Rev. Drug Discov. 17, 531–533 (2018).
Mandal, P. K. et al. Potent and selective phosphopeptide mimetic prodrugs targeted to the Src Homology 2 (SH2) domain of signal transducer and activator of transcription 3. J. Med. Chem. 54, 3549–3563 (2011).
Kapust, R. B. et al. The P1′ specificity of tobacco etch virus protease. Biochem. Biophys. Res. Commun. 294, 949–955 (2002).
Eng, C. H. et al. ClusterCAD: a computational platform for type I modular polyketide synthase design. Nucleic Acids Res. 46, D509–D515 (2018).
Knappe, T. A. et al. Introducing lasso peptides as molecular scaffolds for drug design: engineering of an integrin antagonist. Angew. Chem. Int. Ed. 50, 8714–8717 (2011).
Yang, X. et al. A lanthipeptide library used to identify a protein–protein interaction inhibitor. Nat. Chem. Biol. 14, 375–380 (2018).
King, A. M. et al. Selection for constrained peptides that bind to the SARS-CoV-2 spike protein. Nat. Commun. 12, 6343 (2021).
Rahman, I. R. et al. Substrate recognition by the Class II lanthipeptide synthetase HalM2. ACS Chem. Biol. 15, 1473–1486 (2020).
Goto, Y. et al. One-pot synthesis of azoline-containing peptides in a cell-free translation system integrated with a posttranslational cyclodehydratase. Chem. Biol. 21, 766–774 (2014).
Tianero, M. D. et al. Metabolic model for diversity-generating biosynthesis. Proc. Natl Acad. Sci. USA 113, 1772–1777 (2016).
Gu, W. & Schmidt, E. W. Three principles of diversity-generating biosynthesis. Acc. Chem. Res. 50, 2569–2576 (2017).
Bennallack, P. R. & Griffitts, J. S. Elucidating and engineering thiopeptide biosynthesis. World J. Microbiol. Biotechnol. 33, 119 (2017).
Vagstad, A. L. et al. Introduction of d‐amino acids in minimalistic peptide substrates by an S‐adenosyl‐L‐methionine radical epimerase. Angew. Chem. Int. Ed. 58, 2246–2250 (2019).
Sarkar, S., Gu, W. & Schmidt, E. W. Expanding the chemical space of synthetic cyclic peptides using a promiscuous macrocyclase from prenylagaramide biosynthesis. ACS Catal. 10, 7146–7153 (2020).
Ortega, M. A. et al. Substrate specificity of the lanthipeptide peptidase ElxP and the oxidoreductase ElxO. ACS Chem. Biol. 9, 1718–1725 (2014).
Cebrián, R. et al. Design and expression of specific hybrid lantibiotics active against pathogenic Clostridium spp. Front. Microbiol. 10, 2154 (2019).
Liu, R. et al. A cell‐free platform based on nisin biosynthesis for discovering novel lanthipeptides and guiding their overproduction in vivo. Adv. Sci. 7, 2001616 (2020).
Kloosterman, A. M. et al. RRE-Finder: a genome-mining tool for class-independent RiPP discovery. mSystems 5, e00267-20 (2020).
Skinnider, M. A. et al. Genomic charting of ribosomally synthesized natural product chemical space facilitates targeted mining. Proc. Natl Acad. Sci. USA 113, E6343–E6351 (2016).
Kloosterman, A. M. et al. Expansion of RiPP biosynthetic space through integration of pan-genomics and machine learning uncovers a novel class of lanthipeptides. PLoS Biol. 18, e3001026 (2020).
Kloosterman, A. M., Medema, M. H. & Van Wezel, G. P. Omics-based strategies to discover novel classes of RiPP natural products. Curr. Opin. Biotechnol. 69, 60–67 (2021).
Blin, K. et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47, W81–W87 (2019).
Jin, M. et al. Structural and functional analysis of pantocin A: an antibiotic from Pantoea agglomerans discovered by heterologous expression of cloned genes. Angew. Chem. Int. Ed. 42, 2898–2901 (2003).
Goodman, K. J. & Brenna, J. T. Curve fitting for restoration of accuracy for overlapping peaks in gas chromatography/combustion isotope ratio mass spectrometry. Anal. Chem. 66, 1294–1301 (1994).
Glassey, E., Zhang, Z., King, A. M., Niquille, D. L. & Voigt, C. A. ripp-design. GitHub https://github.com/VoigtLab/ripp-design
Acknowledgements
This research was funded by the US Defense Advanced Research Projects Agency’s Living Foundries programme award (HR0011-12-C-0067), the US Defense Advanced Research Project Agency’s 1KM programme award (HR0011-15-C-0084) and a research grant from Novartis Institutes for Biomedical Research (NIMBR), a research grant from DSM Research, and a research grant from the US Office of Naval Research (N00014-18-1-2632). Research was additionally sponsored by the Army Research Office and was accomplished under cooperative agreement no. W911NF-22-2-0246. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Office or the US Government. The US Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation herein. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
E.G. and C.A.V. conceived the study and designed the experiments. E.G. wrote the software. Z.Z., A.M.K., D.L.N. and E.G. performed MS/MS and analysed the data. Z.Z. performed NMR and analysed the data. E.G. and Z.Z. performed all other experiments and analysed the data. E.G., Z.Z. and C.A.V. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–13, Figs. 1–45 and Tables 1–6.
Supplementary Table 1
Source data for Figs. 1 and 2 and for Supplementary notes 3–11.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Glassey, E., Zhang, Z., King, A.M. et al. De novo design of ribosomally synthesized and post-translationally modified peptides. Nat. Chem. 17, 233–245 (2025). https://doi.org/10.1038/s41557-024-01685-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-024-01685-9
This article is cited by
-
LassoESM a tailored language model for enhanced lasso peptide property prediction
Nature Communications (2025)