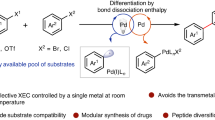
Abstract
Transfer hydrogenation is widely practised across all segments of chemical industry, yet its application to aryl halide reductive cross-coupling is undeveloped because of competing hydrogenolysis. Here, exploiting the distinct reactivity of PdI species, an efficient catalytic system for the reductive cross-coupling of activated aryl bromides with aryl iodides via formate-mediated hydrogen transfer is described. These processes display orthogonality with respect to Suzuki and Buchwald–Hartwig couplings, as pinacol boronates and anilines are tolerated and, owing to the intervention of chelated intermediates, are effective for challenging 2-pyridyl systems. Experimental and computational studies corroborate a unique catalytic cycle for reductive cross-coupling where the PdI precatalyst, [Pd(I)(PtBu3)]2, is converted to the dianionic species, [Pd2I4][NBu4]2, from which aryl halide oxidative addition is more facile. Rapid, reversible Pd-to-Pd transmetallation delivers mixtures of iodide-bridged homo- and hetero-diarylpalladium dimers. The hetero-diarylpalladium dimers are more stable and have lower barriers to reductive elimination, promoting high levels of cross-selectivity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data relating to materials and methods, experimental procedures, mechanistic studies, characterization data for all new compounds (1H NMR, 13C NMR, 19F NMR, 31P NMR, IR and HRMS), computational details and additional computational results are available in the Supplementary Information. Crystallographic data for [Pd2I6][NBu4]2 have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number 2380764. Copies of data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Jana, R., Pathak, T. P. & Sigman, M. S. Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 111, 1417–1492 (2011).
Seechurn, C. C. C. J., Kitching, M. O., Colacot, T. J. & Snieckus, V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 51, 5062–5085 (2012).
Li, H., Seechurn, C. C. C. J. & Colacot, T. J. Development of preformed Pd catalysts for cross-coupling reactions, beyond the 2010 Nobel Prize. ACS Catal. 2, 1147–1164 (2012).
Dumrath, A., Lübbe, C. & Beller, M. in Palladium-Catalyzed Coupling Reactions: Practical Aspects and Future Developments 1st edn (ed. Molnár, Á.) 445–489 (Wiley, 2013).
Biffis, A., Centomo, P., Del Zotto, A. & Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: a critical review. Chem. Rev. 118, 2249–2295 (2018).
Shaughnessy, K. H. Development of palladium precatalysts that efficiently generate LPd(0) active species. Isr. J. Chem. 60, 180–194 (2020).
Firsan, S. J., Sivakumar, V. & Colacot, T. J. Emerging trends in cross-coupling: twelve-electron-based L1Pd(0) catalysts, their mechanism of action, and selected applications. Chem. Rev. 122, 16983–17027 (2022).
Schneider, N., Lowe, D. M., Sayle, R. A., Tarselli, M. A. & Landrum, G. A. Big data from pharmaceutical patents: a computational analysis of medicinal chemists’ bread and butter. J. Med. Chem. 59, 4385–4402 (2016).
Durandetti, M., Gosmini, C. & Périchon, J. Ni-catalyzed activation of α-chloroesters: a simple method for the synthesis of α-arylesters and β-hydroxyesters. Tetrahedron 63, 1146–1153 (2007).
Gosmini, C., Bassene-Ernst, C. & Durandetti, M. Synthesis of functionalized 2-arylpyridines from 2-halopyridines and various aryl halides via a nickel catalysis. Tetrahedron 65, 6141–6146 (2009).
Everson, D. A., Shrestha, R. & Weix, D. J. Nickel-catalyzed reductive cross-coupling of aryl halides with alkyl halides. J. Am. Chem. Soc. 132, 920–921 (2010).
Yu, X., Yang, T., Wang, S., Xu, H. & Gong, H. Nickel-catalyzed reductive cross-coupling of unactivated alkyl halides. Org. Lett. 13, 2138–2141 (2011).
Wang, S., Qian, Q. & Gong, H. Nickel-catalyzed reductive coupling of aryl halides with secondary alkyl bromides and allylic acetate. Org. Lett. 14, 3352–3355 (2012).
Qian, Q. et al. Nickel-catalyzed reductive cross-coupling of aryl halides. Synlett 24, 619–624 (2013).
Amatore, M. & Gosmini, C. Efficient cobalt-catalyzed formation of unsymmetrical biaryl compounds and its application in the synthesis of a sartan intermediate. Angew. Chem. Int. Ed. 47, 2089–2092 (2008).
Bégouin, J.-M. & Gosmini, C. Cobalt-catalyzed cross-coupling between in situ prepared arylzinc halides and 2-chloropyrimidine or 2-chloropyrazine. J. Org. Chem. 74, 3221–3224 (2009).
Ackerman, L. K. G., Lovell, M. M. & Weix, D. J. Multimetallic catalysed cross-coupling of aryl bromides with aryl triflates. Nature 524, 454–457 (2015).
Hanna, L. E. & Jarvo, E. R. Selective cross-electrophile coupling by dual catalysis. Angew. Chem. Int. Ed. 54, 15618–15620 (2015).
Komeyama, K., Ohata, R., Kiguchi, S. & Osaka, I. Highly nucleophilic vitamin B12-assisted nickel-catalysed reductive coupling of aryl halides and non-activated alkyl tosylates. Chem. Commun. 53, 6401–6404 (2017).
Gosmini, C. & Moncomble, A. Cobalt-catalyzed cross-coupling reactions of aryl halides. Isr. J. Chem. 50, 568–576 (2010).
Knappke, C. E. I. et al. Reductive cross-coupling reactions between two electrophiles. Chem. Eur. J. 20, 6828–6842 (2014).
Everson, D. A. & Weix, D. J. Cross-electrophile coupling: principles of reactivity and selectivity. J. Org. Chem. 79, 4793–4798 (2014).
Wang, X., Dai, Y. & Gong, H. Nickel-catalyzed reductive couplings. Top. Curr. Chem. 374, 61–89 (2016).
Poremba, K. E., Dibrell, S. E. & Reisman, S. E. Nickel-catalyzed enantioselective reductive cross-coupling reactions. ACS Catal. 10, 8237–8246 (2020).
Charboneau, D. J., Hazari, N., Huang, H., Uehling, M. R. & Zultanski, S. L. Homogeneous organic electron donors in nickel-catalyzed reductive transformations. J. Org. Chem. 87, 7589–7609 (2022).
Anka-Lufford, L. L., Huihui, K. M. M., Gower, N. J., Ackerman, L. K. G. & Weix, D. J. Nickel-catalyzed cross-electrophile coupling with organic reductants in non-amide solvents. Chem. Eur. J. 22, 11564–11567 (2016).
Shu, W. et al. Ni-catalyzed reductive dicarbofunctionalization of nonactivated alkenes: Scope and mechanistic insights. J. Am. Chem. Soc. 141, 13812–13821 (2019).
Charboneau, D. J. et al. Tunable and practical homogeneous organic reductants for cross-electrophile coupling. J. Am. Chem. Soc. 143, 21024–21036 (2021).
Geng, S. et al. Recent progress in transition-metal-catalyzed reductive cross-coupling reactions using diboron reagents as reductants. ACS Catal. 13, 15469–15480 (2023).
Xu, H., Zhao, C., Qian, Q., Deng, W. & Gong, H. Nickel-catalyzed cross-coupling of unactivated alkyl halides using bis(pinacolato)diboron as reductant. Chem. Sci. 4, 4022–4029 (2013).
Liang, Z., Xue, W., Lin, K. & Gong, H. Nickel-catalyzed reductive methylation of alkyl halides and acid chlorides with methyl p-tosylate. Org. Lett. 16, 5620–5623 (2014).
Lu, X. et al. Nickel-catalyzed defluorinative reductive cross-coupling of gem-difluoroalkenes with unactivated secondary and tertiary alkyl halides. J. Am. Chem. Soc. 139, 12632–12637 (2017).
Ishida, N., Masuda, Y., Sun, F., Kamae, Y. & Murakami, M. A strained vicinal diol as a reductant for coupling of organyl halides. Chem. Lett. 48, 1042–1045 (2019).
Wang, L., Zhang, Y., Liu, L. & Wang, Y. Palladium-catalyzed homocoupling and cross-croupling reactions of aryl halides in poly(ethylene glycol). J. Org. Chem. 71, 1284–1287 (2006).
Yi, L., Ji, T., Chen, K.-Q., Chen, X.-Y. & Rueping, M. Nickel-catalyzed reductive cross-couplings: new opportunities for carbon-carbon bond formations through photochemistry and electrochemistry. CCS Chem. 4, 9–30 (2022).
Liu, Y., Li, P., Wang, Y. & Qiu, Y. Electroreductive cross-electrophile coupling (eXEC) reactions. Angew. Chem. Int. Ed. 62, e202306679 (2023).
Twilton, J. et al. Quinone-mediated hydrogen anode for non-aqueous reductive electrosynthesis. Nature 623, 71–76 (2023).
Maiti, S. et al. Light-induced Pd catalyst enables C(sp2)–C(sp2) cross-electrophile coupling bypassing the demand for transmetalation. Nat. Catal. 7, 285–294 (2024).
Duan, Z., Li, W. & Lei, A. Nickel-catalyzed reductive cross-coupling of aryl bromides with alkyl bromides: Et3N as the terminal reductant. Org. Lett. 18, 4012–4015 (2016).
Dewanji, A., Bülow, R. F. & Rueping, M. Photoredox/nickel dual-catalyzed reductive cross coupling of aryl halides using an organic reducing agent. Org. Lett. 22, 1611–1617 (2020).
Schwartz, L. A., Spielmann, K., Swyka, R. A., Xiang, M. & Krische, M. J. Formate-mediated cross-electrophile reductive coupling of aryl iodides and bromopyridines. Isr. J. Chem. 61, 298–301 (2021).
Mukhopadhyay, S., Rothenberg, G., Qafisheh, N. & Sasson, Y. Supported phase-transfer catalysts as selective agents in biphenyl synthesis from haloaryls. Tetrahedron Lett. 42, 6117–6119 (2001).
Ngai, M.-Y., Kong, J.-R. & Krische, M. J. Hydrogen-mediated C–C bond formation: a broad new concept in catalytic C–C coupling. J. Org. Chem. 72, 1063–1072 (2007).
Nguyen, K. D. et al. Metal-catalyzed reductive coupling of olefin-derived nucleophiles: reinventing carbonyl addition. Science 354, aah5133 (2016).
Santana, C. G. & Krische, M. J. From hydrogenation to transfer hydrogenation to hydrogen auto-transfer in enantioselective metal-catalyzed carbonyl reductive coupling: past, present and future. ACS Catal. 11, 5572–5585 (2021).
Swyka, R. A., Zhang, W., Richardson, J., Ruble, J. C. & Krische, M. J. Rhodium-catalyzed aldehyde arylation via formate-mediated transfer hydrogenation: beyond metallic reductants in Grignard/Nozaki–Hiyama–Kishi-type addition. J. Am. Chem. Soc. 141, 1828–1832 (2019).
Swyka, R. A. et al. Conversion of aldehydes to branched or linear ketones via regiodivergent rhodium-catalyzed vinyl bromide reductive coupling-redox isomerization mediated by formate. J. Am. Chem. Soc. 141, 6864–6868 (2019).
Shuler, W. G., Swyka, R. A., Schempp, T. T., Spinello, B. J. & Krische, M. J. Vinyl triflate-aldehyde reductive coupling-redox isomerization mediated by formate: rhodium-catalyzed ketone synthesis in the absence of stoichiometric metals. Chem. Eur. J. 25, 12517–12520 (2019).
Chang, Y.-H., Shen, W., Shezaf, J. Z., Ortiz, E. & Krische, M. J. Palladium(I)-iodide-catalyzed deoxygenative Heck reaction of vinyl triflates: A formate-mediated cross-electrophile reductive coupling with cine-substitution. J. Am. Chem. Soc. 145, 22890–22895 (2023).
Santana, C. G., Teoh, Y. S., Evarts, M. M., Shezaf, J. Z. & Krische, M. J. Formate-mediated reductive cross-coupling of vinyl halides and aryl iodides: cine-substitution via palladium(I) catalysis. Org. Lett. 26, 7055–7059 (2024).
Billingsley, K. L. & Buchwald, S. L. A general and efficient method for the Suzuki–Miyaura coupling of 2-pyridyl nucleophiles. Angew. Chem. Int. Ed. 47, 4695–4698 (2008).
Dick, G. R., Woerly, E. M. & Burke, M. D. A general solution for the 2-pyridyl problem. Angew. Chem. Int. Ed. 51, 2667–2672 (2012).
Cox, P. A., Leach, A. G., Campbell, A. D. & Lloyd-Jones, G. C. Protodeboronation of heteroaromatic, vinyl, and cyclopropyl boronic acids: pH-rate profiles, autocatalysis, and disproportionation. J. Am. Chem. Soc. 138, 9145–9157 (2016).
Cook, X. A. F., de Gombert, A., McKnight, J., Pantaine, L. R. E. & Willis, M. C. The 2-pyridyl problem: challenging nucleophiles in cross-coupling arylations. Angew. Chem. Int. Ed. 60, 11068–11091 (2021).
Wang, D., Izawa, Y. & Stahl, S. S. Pd-catalyzed aerobic oxidative coupling of arenes: evidence for transmetalation between two Pd(II)-aryl intermediates. J. Am. Chem. Soc. 136, 9914–9917 (2014).
Pérez-Iglesias, M., Lozano-Lavilla, O. & Casares, J. A. [Cu(C6Cl2F3)(tht)]4: an extremely efficient catalyst for the aryl scrambling between palladium complexes. Organometallics 38, 739–742 (2019).
Lin, Z., Oliveira, J. C. A., Scheremetjew, A. & Ackermann, L. Palladium-catalyzed electrooxidative double C–H arylation. J. Am. Chem. Soc. 146, 228–239 (2024).
Pinder, A. R. The hydrogenolysis of organic halides. Synthesis 1980, 425–452 (1980).
Urbano, F. J. & Marinas, J. M. Hydrogenolysis of organohalogen compounds over palladium supported catalysts. J. Mol. Catal. A 173, 329–345 (2001).
Alonso, F., Beletskaya, I. P. & Yus, M. Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 102, 4009–4092 (2002).
Schroeter, F., Soellner, J. & Strassner, T. Cross-coupling catalysis by an anionic palladium complex. ACS Catal. 7, 3004–3009 (2017).
Hruszkewycz, D. P., Balcells, D., Guard, L. M., Hazari, N. & Tilset, M. Insight into the efficiency of cinnamyl-supported precatalysts for the Suzuki–Miyaura reaction: observation of Pd(I) dimers with bridging allyl ligands during catalysis. J. Am. Chem. Soc. 136, 7300–7316 (2014).
Fricke, C., Sperger, T., Mendel, M. & Schoenebeck, F. Catalysis with palladium(I) dimers. Angew. Chem. Int. Ed. 60, 3355–3366 (2021).
Bonney, K. J., Proutiere, F. & Schoenebeck, F. Dinuclear Pd(I) complexes—solely precatalysts? Demonstration of direct reactivity of a Pd(I) dimer with an aryl iodide. Chem. Sci. 4, 4434–4439 (2013).
Maitlis, P. M., Haynes, A., James, B. R., Catellani, M. & Chiusoli, G. P. Iodide effects in transition metal catalyzed reactions. Dalton Trans. 2004, 3409–3419 (2004).
Fagnou, K. & Lautens, M. Halide effects in transition metal catalysis. Angew. Chem. Int. Ed. 41, 26–47 (2002).
Uehling, M. R., King, R. P., Krska, S. W., Cernak, T. & Buchwald, S. L. Pharmaceutical diversification via palladium oxidative addition complexes. Science 363, 405–408 (2019).
Milner, P. J. et al. Investigating the dearomative rearrangement of biaryl phosphine-ligated Pd(II) complexes. J. Am. Chem. Soc. 134, 19922–19934 (2012).
Vicente, J., Arcas, A., Juliá-Hernández, F. & Bautista, D. Synthesis of a palladium(IV) complex by oxidative addition of an aryl halide to palladium(II) and its use as precatalyst in a C–C coupling reaction. Angew. Chem. Int. Ed. 50, 6896–6899 (2011).
Dang, Y. et al. The mechanism of a ligand-promoted C(sp3)–H activation and arylation reaction via palladium catalysis: theoretical demonstration of a Pd(II)/Pd(IV) redox manifold. J. Am. Chem. Soc. 137, 2006–2014 (2015).
Whitehurst, W. G., Blackwell, J. H., Hermann, G. N. & Gaunt, M. J. Carboxylate-assisted oxidative addition to aminoalkyl Pd(II) complexes: C(sp3)-H arylation of alkylamines by distinct Pd(II)/Pd(IV) pathway. Angew. Chem. Int. Ed. 58, 9054–9059 (2019).
Manna, K. & Jana, R. Palladium-catalyzed cross-electrophile coupling between aryl diazonium salt and aryl iodide/diaryliodonium salt in H2O–EtOH. Org. Lett. 25, 341–346 (2023).
Roy, A. H. & Hartwig, J. F. Directly observed reductive elimination of aryl halides from monomeric arylpalladium(II) halide complexes. J. Am. Chem. Soc. 125, 13944–13945 (2003).
Carrow, B. P. & Hartwig, J. F. Ligandless, anionic, arylpalladium halide intermediates in the Heck reaction. J. Am. Chem. Soc. 132, 79–81 (2010).
Galardon, E. et al. Profound steric control of reactivity in aryl halide addition to bisphosphane palladium(0) complexes. Angew. Chem. Int. Ed. 41, 1760–1763 (2002).
Shekhar, S. & Hartwig, J. F. Distinct electronic effects on reductive eliminations of symmetrical and unsymmetrical bis-aryl platinum complexes. J. Am. Chem. Soc. 126, 13016–13027 (2004).
Acknowledgements
The Robert A. Welch Foundation (F-0038) and the National Institutes of Health (NIH)-National Institute of General Medical Sciences (NIGMS) (RO1-GM069445 and R35 GM128779) are acknowledged for partial support of this research. Genentech is acknowledged for summer predoctoral internship support (Y.C.). N.S.T. is supported in part by the NIH-NIGMS (R35GM-133566). Instrumentation for the University of Minnesota (UMN) Chemistry NMR facility was supported from a grant through the NIH (S10OD011952) and the UMN Department of Chemistry Mass Spectrometry Laboratory is supported by the Office of the Vice President for Research, College of Science and Engineering and the Department of Chemistry at UMN, as well as the National Science Foundation (NSF) (CHE-1336940). X-ray diffraction experiments were performed by A. Lovstedt and G. Murphy with a diffractometer purchased through a grant from NSF/MRI (1229400) and the UMN. DFT calculations were carried out at the University of Pittsburgh Center for Research Computing and the Advanced Cyberinfrastructure Co-ordination Ecosystem: Services and Support (ACCESS) programme, supported by NSF award numbers OAC-2117681, OAC-1928147 and OAC-1928224. We thank M. Huestis, M. Ashley and C. Stivala for helpful scientific discussions.
Author information
Authors and Affiliations
Contributions
Y.C., Y.-H.C., Z.J.D. and S.L. designed the project under the guidance of N.A.W. and M.J.K. Y.C., Y.-H.C., Z.H.S., Z.J.D. and S.L. carried out experimental work and mechanistic studies notwithstanding Supplementary Information section XI. K.P.Q. performed calculations under the guidance of P.L. N.S.T. carried out mechanistic studies described in Supplementary Information section XI under the guidance of J.M.H. N.N. performed the HTE experiments. P.L. and M.J.K. composed the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Corinne Gosmini, Kevin Shaughnessy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, detailed experimental procedures, optimization, characterization and computational data.
Supplementary Data 1
Crystallographic data for compound [Pd2I6][NBu4]2; CCDC reference 2380764.
Supplementary Data 2
Cartesian coordinates raw file for all computations.
Supplementary Data 3
Checkcif file for [Pd2I6][NBu4]2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, Y., Chang, YH., Quirion, K.P. et al. Aryl halide cross-coupling via formate-mediated transfer hydrogenation. Nat. Chem. 17, 710–718 (2025). https://doi.org/10.1038/s41557-024-01729-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-024-01729-0