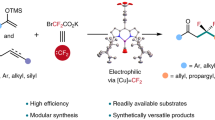
Abstract
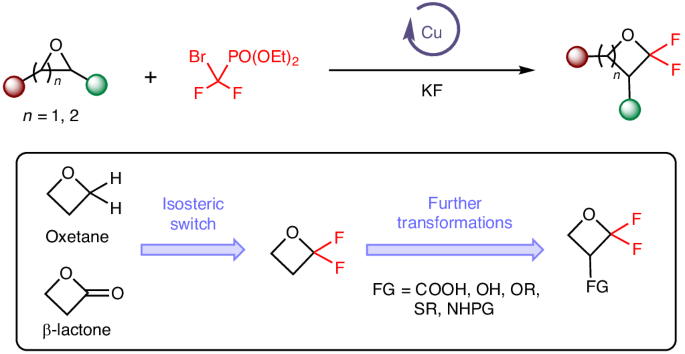
Skeletal editing of heterocyclic building blocks offers an appealing way to expand the accessible chemical space by diversifying molecular scaffolds for drug discovery. Despite the recent boom in this area, catalytic strategies that directly introduce fluorine into the backbone of small-ring heterocycles remain rare owing to the challenges of strain-induced ring cleavage and defluorination. Here we describe a copper-catalysed approach for skeletal expansion of oxygen heterocycles by reaction with a difluorocarbene species generated in situ to induce carbon atom insertion. The α,α-difluoro-oxetane products are potential surrogates of oxetane, β-lactone and carbonyl pharmacophores on the basis of their computed molecular properties and electrostatic potential maps. The utility of this approach is highlighted by synthesis of various drug-like molecules and fluorinated isosteres of biologically active compounds. Experimental and computational investigations provide insight into the mechanism and the unique role of the copper catalyst in promoting both ring-opening and cyclization steps of the reaction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre under reference no. CCDC-2323644 (31). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data are available in the main text or the Supplementary Information.
Change history
28 February 2025
In the second paragraph of the “Mechanistic studies” section, an additional citation to ref. 23 (Zeng, X., Li, Y., Min, Q.-Q., Xue, X.-S. & Zhang, X. Copper-catalysed difluorocarbene transfer enables modular synthesis. Nat. Chem.15, 1064–1073 (2023)) has been added alongside the text “which was first described by Zhang and co-workers”. This correction has been made to the HTML and PDF versions of the article.
References
Hassner, A. Small Ring Heterocycles (Wiley-VCH, 1983).
Rojas, J. J. & Bull, J. A. Oxetanes in drug discovery campaigns. J. Med. Chem. 66, 12697–12709 (2023).
Wuitschik, G. et al. Oxetanes in drug discovery: structural and synthetic insights. J. Med. Chem. 53, 3227–3246 (2010).
Wuitschik, G. et al. Oxetanes as promising modules in drug discovery. Angew. Chem. Int. Ed. 45, 7736–7739 (2006).
Robinson, S. L., Christenson, J. K. & Wackett, L. P. Biosynthesis and chemical diversity of β-lactone natural products. Nat. Prod. Rep. 36, 458–475 (2019).
Baillie, P. A. Targeted covalent inhibitors for drug design. Angew. Chem. Int. Ed. 55, 13408–13421 (2016).
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Meanwell, N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 61, 5822–5880 (2018).
Bauer, M. R. et al. Put a ring on it: application of small aliphatic rings in medicinal chemistry. RSC Med. Chem. 12, 448–471 (2021).
Grygorenko, O. O., Melnykov, K. P., Holovach, S. & Demchuk, O. Fluorinated cycloalkyl building blocks for drug discovery. ChemMedChem 17, e2022003 (2022).
Holovach, S. et al. Effect of gem-difluorination on the key physicochemical properties relevant to medicinal chemistry: the case of functionalized cycloalkanes. Chem. Eur. J. 28, e202200331 (2022).
Ruyet, L. et al. Catalytic ring expanding difluorination: an enantioselective platform to access β,β-difluorinated carbocycles. Angew. Chem. Int. Ed. 63, e202403957 (2024).
Zeng, Y. & Xia, Y. Rhodium-catalyzed regio- and diastereoselective [3 + 2] cycloaddition of gem-difluorinated cyclopropanes with internal olefins. Angew. Chem. Int. Ed. 62, e202307129 (2023).
Patani, G. A. & LaVoie, E. J. Bioisosterism: a rational approach in drug design. Chem. Rev. 96, 3147–3176 (1996).
Sap, J. B. I. et al. Late-stage difluoromethylation: concepts, developments and perspective. Chem. Soc. Rev. 50, 8214–8247 (2021).
Conn, P. J. et al. Bicyclic triazole and pyrazole lactams as allosteric modulators of mglur5 receptors. International patent WO2012083224A1 (2012).
Ikeda, S. & Sonoi, T. Process for producing 2,2,3,3-tetrafluorooxetane. International patent WO2005080365A1 (2005).
Liang, T., Neumann, C. N. & Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 52, 8214–8264 (2013).
Porco, J. A. & Schreiber, S. L. The Paterno-Büchi reaction. Compr. Org. Synth. 5, 151–192 (1991).
Bull, J. A., Croft, R. A., Davis, O. A., Doran, R. & Morgan, K. F. Oxetanes: recent advances in synthesis, reactivity, and medicinal chemistry. Chem. Rev. 116, 12150–12233 (2016).
Okuma, K., Tanaka, Y., Kaji, S. & Ohta, H. Reaction of dimethyloxosulfonium methylide with epoxides. preparation of oxetanes. J. Org. Chem. 48, 5133–5134 (1983).
Takahira, H. et al. Electrochemical C(sp3)–H fluorination. Synlett 30, 1178–1182 (2019).
Zeng, X., Li, Y., Min, Q.-Q., Xue, X.-S. & Zhang, X. Copper-catalysed difluorocarbene transfer enables modular synthesis. Nat. Chem. 15, 1064–1073 (2023).
Padwa, A. & Hornbuckle, S. F. Ylide formation from the reaction of carbenes and carbenoids with heteroatom lone pairs. Chem. Rev. 91, 263–309 (1991).
Jurczyk, J. et al. Single-atom logic for heterocycle editing. Nat. Synth. 1, 352–364 (2022).
Reisenbauer, J. C., Green, O., Franchino, A., Finkelstein, P. & Morandi, B. Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 377, 1104–1109 (2022).
Kamitani, M. et al. Single–carbon atom transfer to α, β-unsaturated amides from N-heterocyclic carbenes. Science 379, 484–488 (2023).
Wang, H. et al. Dearomative ring expansion of thiophenes by bicyclobutane insertion. Science 381, 75–81 (2023).
Pearson, T. J. et al. Aromatic nitrogen scanning by ipso-selective nitrene internalization. Science 381, 1474–1479 (2023).
Woo, J., Stein, C., Christian, A. H. & Levin, M. D. Carbon-to-nitrogen single-atom transmutation of azaarenes. Nature 623, 77–82 (2023).
Empel, C., Jana, S. & Koenigs, R. M. Advances in [1,2]-sigmatropic rearrangements of onium ylides via carbene transfer reactions. Synthesis 53, 4567–4587 (2021).
Parker, R. E. & Isaacs, N. S. Mechanisms of epoxide reactions. Chem. Rev. 59, 737–799 (1959).
Mack, D. J., Batory, L. A. & Njardarson, J. T. Intermolecular oxonium ylide mediated synthesis of medium-sized oxacycles. Org. Lett. 14, 378–381 (2012).
Stevens, T. et al. On the mechanism of the Stevens rearrangement. Synthesis 52, 21–26 (2020).
Hayashi, H. et al. In silico reaction screening with difluorocarbene for N-difluoroalkylative dearomatization of pyridines. Nat. Synth. 1, 804–814 (2022).
Su, J., Ma, X., Ou, Z. & Song, Q. Deconstructive functionalizations of unstrained carbon-nitrogen cleavage enabled by difluorocarbene. ACS Cent. Sci. 6, 1819–1826 (2020).
Kim, Y., Heo, J., Kim, D., Chang, S. & Seo, S. Ring-opening functionalizations of unstrained cyclic amines enabled by difluorocarbene transfer. Nat. Commun. 11, 4761 (2020).
Zhang, R., Li, Q., Xie, Q., Ni, C. & Hu, J. Difluorocarbene-induced ring-opening difluoromethylation-halogenation of cyclic (thio)ethers with TMSCF2X (X=Br, Cl). Chem. Eur. J. 27, 17773–17779 (2021).
Mao, T. et al. N-Difluoromethylation of imidazoles and pyrazoles using BrCF2PO(OEt)2 under mild condition. Tetrahedron Lett. 59, 2752–2754 (2018).
Ghigo, G., Cagnina, S., Maranzana, A. & Tonachini, G. The mechanism of the Stevens and Sommelet-Hauser rearrangements. A theoretical study. J. Org. Chem. 75, 3608–3617 (2010).
Franzen, V. Untersuchungen über carbene, XII1) Bestimmung der Lebensdauer des difluorcarbens. Chem. Ber. 95, 1964–1970 (1962). .
Ruah, S. S., Miller, M. T., Bear, B., McCartney, J. & Grootenhuis, P. D. J. Modulators of ATP-binding cassette transporters. International patent WO2007087066A2 (2007).
Bouaboula, M. et al. Substituted 6,7-dihydro-5H-benzo[7]annulene compounds, processes for their preparation and therapeutic uses thereof. US patent US9714221B1 (2017).
Gersch, M. et al. The mechanism of caseinolytic protease (ClpP) inhibition. Angew. Chem. Int. Ed. 52, 3009–3014 (2013).
Furuya, T. et al. Discovery of potent allosteric DRP1 inhibitors by disrupting protein-protein interaction with MiD49. ACS Med. Chem. Lett. 14, 1095–1099 (2023).
Wise, D. E. et al. Photoinduced oxygen transfer using nitroarenes for the anaerobic cleavage of alkenes. J. Am. Chem. Soc. 144, 15437–15442 (2022).
Chen, S. et al. Pyrrolopyrazine kinase inhibitors. International patent WO2013030138A1 (2013).
Acknowledgements
This research was supported by the Ministry of Education of Singapore Academic Research Fund Tier 1: A-8001693-00-00 (M.J.K.) and the National Science Foundation (NSF): CHE-2247505 (P.L.). F.Z. acknowledges support from the postdoctoral programme of the International Training Plan for Young Talents of Guangdong Province. DFT calculations were carried out at the University of Pittsburgh Center for Research Computing and the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) program, supported by NSF award numbers: OAC-2117681, OAC-1928147 and OAC-1928224 (P.L.). G. K. Tan assisted with X-ray crystallographic measurements.
Author information
Authors and Affiliations
Contributions
T.-D.T. and F.Z. contributed equally to this work. M.J.K. and T.-D.T. conceived the work. T.-D.T., F.Z., Y.-Q.W. and X.L. conducted the optimization, reaction scope and mechanistic studies. K.P.Q. conducted the DFT calculations. D.Z.W.N. conducted the physicochemical property and metabolic stability studies. M.J.K., P.L. and E.C.Y.C. directed the research. M.J.K. wrote the manuscript, with revisions provided by the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Oleksandr Grygorenko and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Tables 1–7, experimental data, synthesis and characterization data, DFT calculation data, X-ray crystallographic data, NMR spectra and references.
Supplementary Data 1
Crystallographic data for compound 31; CCDC reference 2323644.
Supplementary Data 2
Cartesian coordinates of DFT-optimized structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, TD., Zhou, F., Quirion, K.P. et al. Catalytic difluorocarbene insertion enables access to fluorinated oxetane isosteres. Nat. Chem. 17, 719–726 (2025). https://doi.org/10.1038/s41557-024-01730-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-024-01730-7
This article is cited by
-
Multi-component reactions via copper(I) difluorocarbene as carbonyl source for constructing α—aminoamide derivatives
Nature Communications (2025)