Abstract
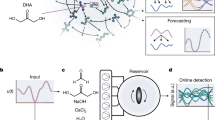
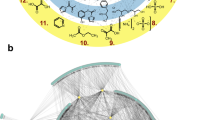
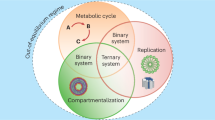
Many open questions about the origins of life are centred on the generation of complex chemical species. Past work has characterized specific chemical reactions that might lead to biological molecules. Here we establish an experimental model of chemical evolution to investigate general processes by which chemical systems continuously change. We used water as a chemical reactant, product and medium. We leveraged oscillating water activity at near-ambient temperatures to cause ratcheting of near-equilibrium reactions in mixtures of organic molecules containing carboxylic acids, amines, thiols and hydroxyl groups. Our system (1) undergoes continuous change with transitions to new chemical spaces while not converging throughout the experiment; (2) demonstrates combinatorial compression with stringent chemical selection; and (3) displays synchronicity of molecular populations. Our results suggest that chemical evolution and selection can be observed in organic mixtures and might ultimately be adapted to produce a broad array of molecules with novel structures and functions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information.
Code availability
Our Python code for calculating continuous chemical change—which corrects the chromatogram baseline, and identifies and integrates peaks—is available at: https://github.com/HUJI-MFP/HPLC_automatic_analysis/blob/main/HPLC_python.py.
References
Bell, E. A., Boehnke, P., Harrison, T. M. & Mao, W. L. Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc. Natl Acad. Sci. USA 112, 14518–14521 (2015).
Orgel, L. E. The origin of life on the earth. Sci. Am. 271, 76–83 (1994).
Trainer, M. G. et al. Organic haze on Titan and the early Earth. Proc. Natl Acad. Sci. USA 103, 18035–18042 (2006).
Reed, N. W., Wing, B. A., Tolbert, M. A. & Browne, E. C. Trace H2S promotes organic aerosol production and organosulfur compound formation in archean analog haze photochemistry experiments. Geophys. Res. Lett. 49, e2021GL097032 (2022).
Zahnle, K. J., Lupu, R., Catling, D. C. & Wogan, N. Creation and evolution of impact-generated reduced atmospheres of early Earth. Planetary Sci. J. 1, 11 (2020).
Hud, N. V., Cafferty, B. J., Krishnamurthy, R. & Williams, L. D. The origin of RNA and ‘my grandfather’s axe’. Chem. Biol. 20, 466–474 (2013).
Brack, A. Selective emergence and survival of early polypeptides in water. Orig. Life Evol. Biosph. 17, 367–379 (1987).
Schneider, C. et al. Noncanonical RNA nucleosides as molecular fossils of an early Earth—generation by prebiotic methylations and carbamoylations. Angew. Chem. Int. Ed. 57, 5943–5946 (2018).
Deamer, D. The role of lipid membranes in life’s origin. Life 7, 5 (2017).
Kim, S. C. et al. A model for the emergence of RNA from a prebiotically plausible mixture of ribonucleotides, arabinonucleotides, and 2′-deoxynucleotides. J. Am. Chem. Soc. 142, 2317–2326 (2020).
Frenkel-Pinter, M. et al. Selective incorporation of proteinaceous over nonproteinaceous cationic amino acids in model prebiotic oligomerization reactions. Proc. Natl Acad. Sci. USA 116, 16338–16346 (2019).
Runnels, C. M. et al. Folding, assembly, and persistence: the essential nature and origins of biopolymers. J. Mol. Evol. 86, 598–610 (2018).
Cleaves II, H. J. The origin of the biologically coded amino acids. J. Theor. Biol. 263, 490–498 (2010).
Vincent, L. et al. Chemical ecosystem selection on mineral surfaces reveals long-term dynamics consistent with the spontaneous emergence of mutual catalysis. Life 9, 80 (2019).
Cafferty, B. J. et al. Robustness, entrainment, and hybridization in dissipative molecular networks, and the origin of life. J. Am. Chem. Soc. 141, 8289–8295 (2019).
Segré, D., Ben-Eli, D. & Lancet, D. Compositional genomes: prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl Acad. Sci. USA 97, 4112–4117 (2000).
Kroiss, D., Ashkenasy, G., Braunschweig, A. B., Tuttle, T. & Ulijn, R. V. Catalyst: can systems chemistry unravel the mysteries of the chemical origins of life? Chem 5, 1917–1920 (2019).
Ashkenasy, G., Hermans, T. M., Otto, S. & Taylor, A. F. Systems chemistry. Chem. Soc. Rev. 46, 2543–2554 (2017).
Guttenberg, N., Virgo, N., Chandru, K., Scharf, C. & Mamajanov, I. Bulk measurements of messy chemistries are needed for a theory of the origins of life. Philos. Trans. R. Soc. A 375, 20160347 (2017).
Wołos, A. et al. Synthetic connectivity, emergence, and self-regeneration in the network of prebiotic chemistry. Science 369, eaaw1955 (2020).
Miao, X. et al. Kinetic selection in the out‐of‐equilibrium autocatalytic reaction networks that produce macrocyclic peptides. Angew. Chem. Int. Ed. 133, 20529–20538 (2021).
Cao, Y. et al. Self‐synthesizing nanorods from dynamic combinatorial libraries against drug resistant cancer. Angew. Chem. Int. Ed. 133, 3099–3107 (2021).
Komáromy, D., Nowak, P. & Otto, S. in Dynamic Covalent Chemistry: Principles, Reactions, and Applications 31–119 (Wiley, 2017).
Chvykov, P. et al. Low rattling: a predictive principle for self-organization in active collectives. Science 371, 90–95 (2021).
Otto, S., Furlan, R. L. & Sanders, J. K. Selection and amplification of hosts from dynamic combinatorial libraries of macrocyclic disulfides. Science 297, 590–593 (2002).
Lam, R. T. et al. Amplification of acetylcholine-binding catenanes from dynamic combinatorial libraries. Science 308, 667–669 (2005).
Liu, B. et al. Complex molecules that fold like proteins can emerge spontaneously. J. Am. Chem. Soc. 141, 1685–1689 (2018).
Carnall, J. M. et al. Mechanosensitive self-replication driven by self-organization. Science 327, 1502–1506 (2010).
Kahana, A. & Lancet, D. Self-reproducing catalytic micelles as nanoscopic protocell precursors. Nat. Rev. Chem. 5, 870–878 (2021).
Mamajanov, I. et al. Ester formation and hydrolysis during wet–dry cycles: generation of far-from-equilibrium polymers in a model prebiotic reaction. Macromolecules 47, 1334–1343 (2014).
Damer, B. & Deamer, D. The hot spring hypothesis for an origin of life. Astrobiology 20, 429–452 (2020).
Foster, K., Hillman, B., Rajaei, V., Seng, K. & Maurer, S. Evolution of realistic organic mixtures for the origins of life through wet–dry cycling. Sci 4, 22 (2022).
Li, Z. et al. The oligomerization of glucose under plausible prebiotic conditions. Orig. Life Evol. Biosph. 49, 225–240 (2019).
Jia, T. Z. et al. Incorporation of basic α-hydroxy acid residues into primitive polyester microdroplets for RNA segregation. Biomacromolecules 22, 1484–1493 (2021).
Jia, T. Z. & Chandru, K. Recent progress in primitive polyester synthesis and membraneless microdroplet assembly. Biophys. Physicobiol. 20, e200012 (2023).
Jia, T. Z. et al. Membraneless polyester microdroplets as primordial compartments at the origins of life. Proc. Natl Acad. Sci. USA 116, 15830–15835 (2019).
Rodriguez-Garcia, M. et al. Formation of oligopeptides in high yield under simple programmable conditions. Nat. Commun. 6, 8385 (2015).
Forsythe, J. G. et al. Ester-mediated amide bond formation driven by wet–dry cycles: a possible path to polypeptides on the prebiotic earth. Angew. Chem. Int. Ed. 54, 9871–9875 (2015).
Yu, S. et al. Elongation of model prebiotic proto-peptides by continuous monomer feeding. Macromolecules 50, 9286–9294 (2017).
Forsythe, J. G. et al. Surveying the sequence diversity of model prebiotic peptides by mass spectrometry. Proc. Natl Acad. Sci. USA 114, E7652–E7659 (2017).
Doran, D., Abul‐Haija, Y. M. & Cronin, L. Emergence of function and selection from recursively programmed polymerisation reactions in mineral environments. Angew. Chem. Int. Ed. 58, 11253–11256 (2019).
Bouza, M. et al. Compositional characterization of complex protopeptide libraries via triboelectric nanogenerator orbitrap mass spectrometry. Rapid Commun. Mass Spectr. 33, 1293–1300 (2019).
Frenkel-Pinter, M. et al. Mutually stabilizing interactions between proto-peptides and RNA. Nat. Commun. 11, 3137 (2020).
Frenkel-Pinter, M., Sargon, A. B., Glass, J. B., Hud, N. V. & Williams, L. D. Transition metals enhance prebiotic depsipeptide oligomerization reactions involving histidine. RSC Adv. 11, 3534–3538 (2021).
Frenkel-Pinter, M. et al. Thioesters provide a plausible prebiotic path to proto-peptides. Nat. Commun. 13, 2569 (2022).
Lago, I., Black, L., Wilfinger, M. & Maurer, S. E. Synthesis and characterization of amino acid decyl esters as early membranes for the origins of life. Membranes 12, 858 (2022).
Frenkel-Pinter, M., Samanta, M., Ashkenasy, G. & Leman, L. J. Prebiotic peptides: molecular hubs in the origin of life. Chem. Rev. 120, 4707–4765 (2020).
Asche, S., Cooper, G. J. T., Keenan, G., Mathis, C. & Cronin, L. A robotic prebiotic chemist probes long-term reactions of complexifying mixtures. Nat. Commun. 12, 3547 (2021).
Marshall, S. M. et al. Identifying molecules as biosignatures with assembly theory and mass spectrometry. Nat. Commun. 12, 3033 (2021).
Orgel, L. E. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 39, 99–123 (2004).
Colón-Santos, S., Cooper, G. J. & Cronin, L. Taming combinatorial explosion of the formose reaction via recursion within mineral environments. ChemSystemsChem 1, e1900014 (2019).
van Duppen, P., Daines, E., Robinson, W. E. & Huck, W. T. Dynamic environmental conditions affect the composition of a model prebiotic reaction network. J. Am. Chem. Soc. 145, 7559–7568 (2023).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Yu, S. S. et al. Kinetics of prebiotic depsipeptide formation from the ester-amide exchange reaction. Phys. Chem. Chem. Phys. 18, 28441–28450 (2016).
Edri, R., Fisher, S., Menor‐Salvan, C., Williams, L. D. & Frenkel‐Pinter, M. Assembly‐driven protection from hydrolysis as key selective force during chemical evolution. FEBS Lett. 597, 2879–2896 (2023).
Kensil, C. R. & Dennis, E. A. Alkaline hydrolysis of phospholipids in model membranes and the dependence on their state of aggregation. Biochemistry 20, 6079–6085 (1981).
Abkevich, V. I., Gutin, A. M. & Shakhnovich, E. I. How the first biopolymers could have evolved. Proc. Natl Acad. Sci. USA 93, 839–844 (1996).
Prusiner, S. B. Prions. Proc. Natl Acad. Sci. USA 95, 13363–13383 (1998).
Nahvi, A. et al. Genetic control by a metabolite binding mRNA. Chem. Biol. 9, 1043–1049 (2002).
Frenkel-Pinter, M., Rajaei, V., Glass, J. B., Hud, N. V. & Williams, L. D. Water and life: the medium is the message. J. Mol. Evol. 89, 2–11 (2021).
Lowry, T. & Richardson, K. Mechanism and Theory in Organic Chemistry (Harper & Row, 1981).
Jacob, F. Evolution and tinkering. Science 196, 1161–1166 (1977).
Acknowledgements
We thank N. Hud, M.G. Finn, G. Schuster, M. Grover, M. Travisano, D. Lancet and E. Smith for helpful comments. Funding: This research was supported by the National Science Foundation (grant no. 1724274 to L.D.W.), NASA Center for Integration of the Origins of Life (grant no. 80NSSC24K0344), the Azrieli Foundation Early Career Faculty Grant (to M.F.P.), the Israel Science Foundation grant (grant no. 1611/22 to M.F.P.), the Minerva Foundation (to M.F.P.) and FEBS Foundation Excellence Award (to M.F.P.).
Author information
Authors and Affiliations
Contributions
M.F.P. and K.M. performed dry-down and wet–dry cycling experiments. M.P.F., K.M. and V.R. performed chemical analysis. K.M., A.R., J.S.K. and M.F.P. performed analysis of HPLC data. P.C.A. wrote a Python code for automatic analysis of HPLC data. V.R. performed the NMR analysis. A.S.P. and J.T.C. performed analysis of MS data. M.F.P., K.M. and L.D.W. formulated models, and conceived and designed experiments. M.F.P., K.M., V.R., J.C.B., P.C.A. and L.D.W. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks David Baum, Shawn McGlynn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Methods, Supplementary Figs. 1–44 and Table 1.
Supplementary Data 1
Mass spectrometry data from HPLC separations of wet–dry cycling experiments.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matange, K., Rajaei, V., Capera-Aragones, P. et al. Evolution of complex chemical mixtures reveals combinatorial compression and population synchronicity. Nat. Chem. 17, 590–597 (2025). https://doi.org/10.1038/s41557-025-01734-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01734-x