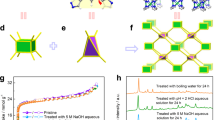
Abstract
The removal of SO2 from flue gas remains a challenge. Adsorption-based separation of SO2 using porous materials has been proposed as a more energy-efficient and cost-effective alternative to more traditional methods such as cryogenic distillations. Here we report a flexible hydrogen-bonded organic framework (HOF-NKU-1) that enables the sieving of SO2 through the guest-adaptive response and shape-memory effect of the material. HOF-NKU-1 exhibits a high selectivity of 7,331 for the separation of SO2/CO2 and a high SO2 storage density of 3.27 g cm−3 within the pore space at ambient conditions. The hydrophobic nature of HOF-NKU-1 enables high dynamic SO2 uptake and SO2 recovery, even in conditions of 95% humidity. The SO2/CO2 separation mechanism is studied through combinatorial gas sorption isotherms, breakthrough experiments and single-crystal diffraction studies, paving the way for the development of multifunctional shape-memory porous materials in the future.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the main text and the supplementary information or are available from the corresponding authors upon reasonable request. Crystallographic data for the structures in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2368931 (HOF-NKU-1), 2368932 (SO2@HOF-NKU-1), 2220262 (CO2@HOF-NKU-1) and 2368933 (SO2@HOF-NKU-1-removal). Copies of the data can be obtained free of charge from https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Keith, D. W. Why capture CO2 from the atmosphere? Science 325, 1654–1655 (2009).
Smith, G. L. et al. Reversible coordinative binding and separation of sulfur dioxide in a robust metal–organic framework with open copper sites. Nat. Mater. 18, 1358–1365 (2019).
Yang, S. et al. Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host. Nat. Chem. 4, 887–894 (2012).
Li, W. et al. Adsorption of sulfur dioxide in Cu(II)–carboxylate framework materials: the role of ligand functionalization and open metal sites. J. Am. Chem. Soc. 144, 13196–13204 (2022).
Xu, W. et al. Fabrication of pillar-cage fluorinated anion pillared metal-organic frameworks via a pillar embedding strategy and efficient separation of SO2 through multi-site trapping. Angew. Chem. Int. Ed. 62, e202312029 (2023).
Cui, X. et al. Ultrahigh and selective SO2 uptake in inorganic anion-pillared hybrid porous materials. Adv. Mater. 29, 1606929 (2017).
Han, Z. et al. A {Ni12}-wheel-based metal–organic framework for coordinative binding of sulphur dioxide and nitrogen dioxide. Angew. Chem. Int. Ed. 61, e202115585 (2022).
Bandosz, T. in Activated Carbon Surfaces in Environmental Remediation (ed. Bandosz, T. J.) 231–292 (Elsevier, 2006).
Yang, R. T., Hernández-Maldonado, A. J. & Yang, F. H. Desulfurization of transportation fuels with zeolites under ambient conditions. Science 301, 79–81 (2003).
Li, J. et al. Structural and dynamic analysis of sulphur dioxide adsorption in a series of zirconium-based metal–organic frameworks. Angew. Chem. Int. Ed. 61, e202207259 (2022).
Lin, J. Y. S. Molecular sieves for gas separation. Science 353, 121–122 (2016).
Lin, R.-B. et al. Molecular sieving of ethylene from ethane using a rigid metal–organic framework. Nat. Mater. 17, 1128–1133 (2018).
Yang, Y. et al. Ethylene/ethane separation in a stable hydrogen-bonded organic framework through a gating mechanism. Nat. Chem. 13, 933–939 (2021).
Deng, H. et al. Large-pore apertures in a series of metal–organic frameworks. Science 336, 1018–1023 (2012).
Gu, C. et al. Design and control of gas diffusion process in a nanoporous soft crystal. Science 363, 387–391 (2019).
Feng, P., Bu, X. & Stucky, G. D. Hydrothermal syntheses and structural characterization of zeolite analogue compounds based on cobalt phosphate. Nature 388, 735–741 (1997).
Xie, F. et al. Complete separation of benzene-cyclohexene-cyclohexane mixtures via temperature-dependent molecular sieving by a flexible chain-like coordination polymer. Nat. Commun. 15, 2240 (2024).
Zeng, H. et al. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures. Nature 595, 542–548 (2021).
He, C.-T. et al. Hyperfine adjustment of flexible pore-surface pockets enables smart recognition of gas size and quadrupole moment. Chem. Sci. 8, 7560–7565 (2017).
Chen, K. J. et al. Tuning pore size in square‐lattice coordination networks for size‐selective sieving of CO2. Angew. Chem. Int. Ed. 128, 10424–10428 (2016).
Su, S. et al. Metal–organic frameworks with a bioinspired porous polymer coating for sieving separation. J. Am. Chem. Soc. 145, 13195–13203 (2023).
Jaramillo, D. E. et al. Selective nitrogen adsorption via backbonding in a metal-organic framework with exposed vanadium sites. Nat. Mater. 19, 517–521 (2020).
Liu, G. et al. Mixed matrix formulations with MOF molecular sieving for key energy-intensive separations. Nat. Mater. 17, 283–289 (2018).
Liao, P.-Q., Huang, N.-Y., Zhang, W.-X., Zhang, J.-P. & Chen, X.-M. Controlling guest conformation for efficient purification of butadiene. Science 356, 1193–1196 (2017).
Meng, Q.-W. et al. Guanidinium-based covalent organic framework membrane for single-acid recovery. Sci. Adv. 9, eadh0207 (2023).
Carter, J. H. et al. Exceptional adsorption and binding of sulfur dioxide in a robust zirconium-based metal–organic framework. J. Am. Chem. Soc. 140, 15564–15567 (2018).
Savage, M. et al. Selective adsorption of sulfur dioxide in a robust metal–organic framework material. Adv. Mater. 28, 8705–8711 (2016).
He, Y., Xiang, S. & Chen, B. A Microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature. J. Am. Chem. Soc. 133, 14570–14573 (2011).
Li, P. et al. A rod-packing microporous hydrogen-bonded organic framework for highly selective separation of C2H2/CO2 at room temperature. Angew. Chem. Int. Ed. 54, 574–577 (2015).
Wang, H. et al. A flexible microporous hydrogen-bonded organic framework for gas sorption and separation. J. Am. Chem. Soc. 137, 9963–9970 (2015).
Karmakar, A. et al. Hydrogen-bonded organic frameworks (HOFs): a new class of porous crystalline proton-conducting materials. Angew. Chem. Int. Ed. 55, 10667–10671 (2016).
Wang, B. et al. Microporous hydrogen-bonded organic framework for highly efficient turn-up fluorescent sensing of aniline. J. Am. Chem. Soc. 142, 12478–12485 (2020).
Huang, Q. et al. An exceptionally flexible hydrogen-bonded organic framework with large-scale void regulation and adaptive guest accommodation abilities. Nat. Commun. 10, 3074 (2019).
Liu, B.-T. et al. Ionic hydrogen-bonded organic frameworks for ion-responsive antimicrobial membranes. Adv. Mater. 34, 2202280 (2022).
Yu, D. et al. Hydrogen-bonded organic framework (HOF)-based single-neural stem cell encapsulation and transplantation to remodel impaired neural networks. Angew. Chem. Int. Ed. 61, e202201485 (2022).
Lin, R.-B. et al. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 48, 1362–1389 (2019).
Sakata, Y. et al. Shape-memory nanopores induced in coordination frameworks by crystal downsizing. Science 339, 193–196 (2013).
Li, L. et al. Emission-tunable soft porous organic crystal based on squaraine for single-crystal analysis of guest-induced gate-opening transformation. J. Am. Chem. Soc. 143, 3856–3864 (2021).
Shivanna, M., Yang, Q.-Y., Bajpai, A., Patyk-Kazmierczak, E. & Zaworotko, M. J. A dynamic and multi-responsive porous flexible metal–organic material. Nat. Commun. 9, 3080 (2018).
Chen, S. et al. Precise construction of nitrogen-enriched porous ionic polymers as highly efficient sulfur dioxide adsorbents. Small 20, 2400746 (2024).
Jia, J. et al. Porous organic polymers for efficient and selective SO2 capture from CO2-rich flue gas. Angew. Chem. Int. Ed. 63, e202318844 (2024).
Chen, F. et al. Deep desulfurization with record SO2 adsorption on the metal–organic frameworks. J. Am. Chem. Soc. 143, 9040–9047 (2021).
Xing, S. et al. Capture and separation of SO2 traces in metal–organic frameworks via pre‐synthetic pore environment tailoring by methyl groups. Angew. Chem. Int. Ed. 60, 17998–18005 (2021).
Gong, W. et al. Modulator-dependent dynamics synergistically enabled record SO2 uptake in Zr(IV) metal–organic frameworks based on pyrene-cored molecular quadripod ligand. J. Am. Chem. Soc. 145, 26890–26899 (2023).
Bien, C. E. et al. Bioinspired metal–organic framework for trace CO2 capture. J. Am. Chem. Soc. 140, 12662–12666 (2018).
Acknowledgements
This work was supported by the National Key R&D Program of China (grant no. 2022YFA1503301 to B.L.), the National Natural Science Foundation of China (grant no. 22471131 to B.L., 22035003 to X.-H.B. and W2431013 to B.C.), the Fundamental Research Funds for the Central Universities (Nankai University) to B.L., China Postdoctoral Science Foundation (grant no. 2022TQ0162) to L.L. and Haihe Laboratory of Sustainable Chemical Transformations (grant no. YYJC202101) to B.L. and X.-H.B.
Author information
Authors and Affiliations
Contributions
L.L., B.L., B.C. and X.-H.B. conceived the research idea and designed the experiments. L.L. synthesized the materials, grew the crystals and prepared the adsorbents. L.L., X.Z., L.Z., Z.Z. and X.L. performed the characterization. X.L. and T.H. performed the theoretical calculations. L.L., B.L., B.C. and X.-H.B. analysed the data and wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A patent that covers the synthesis of the hydrogen-bonded organic framework (HOF-NKU-1) and its use in FGD experiments has been filed by Nankai University with X.-H.B., L.L. and B.L. listed as inventors (Chinese National Invention Patent, application no. 202411558505.X, status of application: under review). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Wei Gong, Jiangtao Jia and Jin Shang Yes for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–45 and Tables 1–5.
Supplementary Data 1
Crystallographic data for HOF-NKU-1 (CCDC 2368931).
Supplementary Data 2
Crystallographic data for SO2@HOF-NKU-1 (CCDC 2368932).
Supplementary Data 3
Crystallographic data for SO2@HOF-NKU-1-removal (CCDC 2368933).
Supplementary Data 4
Crystallographic data for CO2@HOF-NKU-1 (CCDC 2220262).
Source data
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Zhang, X., Lian, X. et al. Flue gas desulfurization and SO2 recovery within a flexible hydrogen-bonded organic framework. Nat. Chem. 17, 727–733 (2025). https://doi.org/10.1038/s41557-025-01744-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01744-9
This article is cited by
-
Exploring the synergistic control paths of carbon and pollutant emissions from technological abatement perspective: A case study of the Guangdong-Hong Kong-Macau Greater Bay Area
Environment, Development and Sustainability (2026)