Abstract
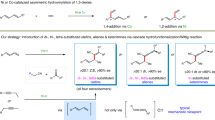
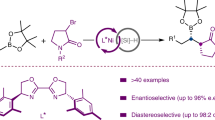
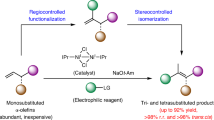
Despite cross-coupling strategies that enable the functionalization of aromatic heterocycles, the enantioselective C(sp3)–H alkylation of readily available saturated hydrocarbons to construct C(sp3)–C(sp3) bonds remains a formidable challenge. Here we describe a nickel-catalysed enantioselective C(sp3)–H alkylation of saturated heterocycles using olefins, providing an efficient strategy for the stereoselective construction of C(sp3)–C(sp3) bonds. Using readily available and stable olefins and simple saturated nitrogen and oxygen heterocycles as prochiral nucleophiles, the coupling reactions proceed under mild conditions and exhibit broad scope and high functional group tolerance. Furthermore, the enantio- and diastereoselective C(sp3)–H alkylation of saturated hydrocarbons with alkenyl boronates has been achieved, enabling the synthesis of versatile alkyl boronates containing 1,2-adjacent C(sp3) stereocentres. Application of this approach to the late-stage modification of natural products and drugs, as well as to the enantioselective synthesis of a range of chiral building blocks and natural products, is demonstrated.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers 2344836 (5) and 2360607 (66). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Newton, C. G., Wang, S., Oliveira, C. C. & Cramer, N. Catalytic enantioselective transformations involving C–H bond cleavage by transition metal complexes. Chem. Rev. 117, 8908–8976 (2017).
He, J., Wasa, M., Chan, K. S. L., Shao, Q. & Yu, J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 117, 8754–8786 (2017).
Zhang, C., Li, Z., Gu, Q. & Liu, X.-Y. Catalytic enantioselective C(sp3)–H functionalization involving radical intermediates. Nat. Commun. 12, 475 (2021).
Golden, D. L., Suh, S.-E. & Stahl, S. S. Radical C(sp3)–H functionalization and cross-coupling reactions. Nat. Rev. Chem. 6, 405–427 (2022).
Rouquet, G. & Chatani, N. Catalytic functionalization of C(sp2)–H and C(sp3)–H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 52, 11726–11743 (2013).
Saint-Denis, T. G., Zhu, R.-Y., Chen, G., Wu, Q.-F. & Yu, J.-Q. Enantioselective C(sp3)–H bond activation by chiral transition metal catalysts. Science 359, eaao4798 (2018).
Davies, H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 3, 347–360 (2019).
Cao, H., Tang, X., Tang, H., Yuan, Y. & Wu, J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem Catal. 1, 523–598 (2021).
Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C–H bonds elaboration. Chem. Rev. 122, 1875–1924 (2022).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Lipp, A., Badir, S. O. & Molander, G. A. Stereoinduction in metallaphotoredox catalysis. Angew. Chem. Int. Ed. 60, 1714–1726 (2021).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Holmberg-Douglas, N. & Nicewicz, D. A. Photoredox-catalyzed C–H functionalization reactions. Chem. Rev. 122, 1925–2016 (2022).
Cheung, K. P. S., Sarkar, S. & Gevorgyan, V. Visible light-induced transition metal catalysis. Chem. Rev. 122, 1543–1625 (2022).
Zhang, Z., Chen, P. & Liu, G. Copper-catalyzed radical relay in C(sp3)–H functionalization. Chem. Soc. Rev. 51, 1640–1658 (2022).
Zhang, J. & Rueping, M. Metallaphotoredox catalysis for sp3 C–H functionalizations through hydrogen atom transfer (HAT). Chem. Soc. Rev. 52, 4099–4120 (2023).
Chen, X. & Kramer, S. Photoinduced transition-metal-catalyzed enantioselective functionalization of non-acidic C(sp3)–H bonds. Chem Catal. 4, 100854 (2024).
Zhang, W. et al. Enantioselective cyanation of benzylic C–H bonds via copper-catalyzed radical relay. Science 353, 1014–1018 (2016).
Li, J. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019).
Zhang, Z., Zhang, X. & Nagib, D. A. Chiral piperidines from acyclic amines via enantioselective, radical-mediated δ C–H cyanation. Chem 5, 3127–3134 (2019).
Ahneman, D. T. & Doyle, A. G. C–H functionalization of amines with aryl halides by nickel-photoredox catalysis. Chem. Sci. 7, 7002–7006 (2016).
Heitz, D. R., Tellis, J. C. & Molander, G. A. Photochemical nickel-catalyzed C–H arylation: synthetic scope and mechanistic investigations. J. Am. Chem. Soc. 138, 12715–12718 (2016).
Perry, I. B. et al. Direct arylation of strong aliphatic C–H bonds. Nature 560, 70–75 (2018).
Shen, Y., Gu, Y. & Martin, R. sp3 C–H arylation and alkylation enabled by the synergy of triplet excited ketones and nickel catalysts. J. Am. Chem. Soc. 140, 12200–12209 (2018).
Cheng, X., Lu, H. & Lu, Z. Enantioselective benzylic C–H arylation via photoredox and nickel dual catalysis. Nat. Commun. 10, 3549 (2019).
Zhang, W., Wu, L., Chen, P. & Liu, G. Enantioselective arylation of benzylic C–H bonds by copper-catalyzed radical relay. Angew. Chem. Int. Ed. 58, 6425–6429 (2019).
Rand, A. X. et al. Dual catalytic platform for enabling sp3 α C–H arylation and alkylation of benzamides. ACS Catal. 10, 4671–4676 (2020).
Sakurai, S., Matsumoto, A., Kano, T. & Maruoka, K. Cu-catalyzed enantioselective alkylarylation of vinylarenes enabled by chiral binaphthyl-BOX hybrid ligands. J. Am. Chem. Soc. 142, 19017–19022 (2020).
Shu, X., Zhong, D., Lin, Y., Qin, X. & Huo, H. Modular access to chiral α-(hetero)aryl amines via Ni/photoredox catalyzed enantioselective cross-coupling. J. Am. Chem. Soc. 144, 8797–8806 (2022).
Xu, S. et al. Enantioselective C(sp3)–H functionalization of oxacycles via photo-HAT/nickel dual catalysis. J. Am. Chem. Soc. 145, 5231–5241 (2023).
Xu, S. et al. Stereoselective and site-divergent synthesis of C-glycosides. Nat. Chem. 16, 2054–2065 (2024).
Xu, J., Li, Z., Xu, Y., Shu, X. & Huo, H. Stereodivergent synthesis of both Z- and E-alkenes by photoinduced, Ni-catalyzed enantioselective C(sp3)–H alkenylation. ACS Catal. 11, 13567–13574 (2021).
Cheng, X., Li, T., Liu, Y. & Lu, Z. Stereo- and enantioselective benzylic C–H alkenylation via photoredox/nickel dual catalysis. ACS Catal. 11, 11059–11065 (2021).
Fu, L., Zhang, Z., Chen, P., Lin, Z. & Liu, G. Enantioselective copper-catalyzed alkynylation of benzylic C–H bonds via radical relay. J. Am. Chem. Soc. 142, 12493–12500 (2020).
Liu, L. et al. Copper-catalyzed intermolecular enantioselective radical oxidative C(sp3)–H/C(sp)–H cross-coupling with rationally designed oxazoline-derived N,N,P(O)-ligands. Angew. Chem. Int. Ed. 60, 26710–26717 (2021).
Shu, X., Huan, L., Huang, Q. & Huo, H. Direct enantioselective C(sp3)–H acylation for the synthesis of α-amino ketones. J. Am. Chem. Soc. 142, 19058–19064 (2020).
Cuesta-Galisteo, S., Schörgenhumer, J., Hervieu, C. & Nevado, C. Dual nickel/photoredox-catalyzed asymmetric carbamoylation of benzylic C(sp3)–H bonds. Angew. Chem. Int. Ed. 63, e202313717 (2024).
Shu, X., Zhong, D., Huang, Q., Huan, L. & Huo, H. Site- and enantioselective cross-coupling of saturated N-heterocycles with carboxylic acids by cooperative Ni/photoredox catalysis. Nat. Commun. 14, 125 (2023).
Xu, P., Fan, W., Chen, P. & Liu, G. Enantioselective radical trifluoromethylation of benzylic C–H bonds via cooperative photoredox and copper catalysis. J. Am. Chem. Soc. 144, 13468–13474 (2022).
Murphy, J. J., Bastida, D., Paria, S., Fagnoni, M. & Melchiorre, P. Asymmetric catalytic formation of quaternary carbons by iminium ion trapping of radicals. Nature 532, 218–222 (2016).
Li, Y., Lei, M. & Gong, L. Photocatalytic regio- and stereoselective C(sp3)–H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat. Catal. 2, 1016–1026 (2019).
Fan, L., Liu, R., Ruan, X., Wang, P. & Gong, L.-Z. Asymmetric 1,2-oxidative alkylation of conjugated dienes via aliphatic C–H bond activation. Nat. Synth. 1, 946–955 (2022).
Tahara, Y., Michino, M., Ito, M., Kanyivab, K. S. & Shibata, T. Enantioselective sp3 C–H alkylation of γ-butyrolactam by a chiral Ir(I) catalyst for the synthesis of 4-substituted γ-amino acids. Chem. Commun. 51, 16660–16663 (2015).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Cordier, C. J., Lundgren, R. J. & Fu, G. C. Enantioconvergent cross-couplings of racemic alkylmetal reagents with unactivated secondary alkyl electrophiles: catalytic asymmetric Negishi α‑alkylations of N‑Boc-pyrrolidine. J. Am. Chem. Soc. 135, 10946–10949 (2013).
Mu, X., Shibata, Y., Makida, Y. & Fu, G. C. Control of vicinal stereocenters through nickel-catalyzed alkyl–alkyl cross-coupling. Angew. Chem. Int. Ed. 56, 5821–5824 (2017).
Vasilopoulos, A., Krska, S. W. & Stahl, S. S. C(sp3)–H methylation enabled by peroxide photosensitization and Ni-mediated radical coupling. Science 372, 398–403 (2021).
Gong, Y., Su, L., Zhu, Z., Ye, Y. & Gong, H. Nickel-catalyzed thermal redox functionalization of C(sp3)–H bonds with carbon electrophiles. Angew. Chem. Int. Ed. 61, e202201662 (2022).
Cheng, L., Liu, J., Chen, Y. & Gong, H. Nickel-catalysed hydrodimerization of unactivated terminal alkenes. Nat. Synth. 2, 364–372 (2023).
Chen, M. et al. Oxidative cross dehydrogenative coupling of N‑heterocycles with aldehydes through C(sp3)–H functionalization. J. Am. Chem. Soc. 145, 20176–20181 (2023).
Breitenfeld, J., Scopelliti, R. & Hu, X. Synthesis, reactivity, and catalytic application of a nickel pincer hydride complex. Organometallics 31, 2128–2136 (2012).
Lu, X. et al. Practical carbon–carbon bond formation from olefins through nickel-catalyzed reductive olefin hydrocarbonation. Nat. Commun. 7, 11129 (2016).
He, Y. L., Cai, Y. L. & Zhu, S. L. Mild and regioselective benzylic C–H functionalization: Ni-catalyzed reductive arylation of remote and proximal olefins. J. Am. Chem. Soc. 139, 1061–1064 (2017).
Zhou, F., Zhu, J., Zhang, Y. & Zhu, S. NiH-catalyzed reductive relay hydroalkylation: a strategy for the remote C(sp3)–H alkylation of alkenes. Angew. Chem. Int. Ed. 57, 4058–4062 (2018).
Wang, Z. B., Yin, H. L. & Fu, G. C. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 563, 379–383 (2018).
Zhou, F., Zhang, Y., Xu, X. & Zhu, S. NiH-catalyzed remote asymmetric hydroalkylation of alkenes with racemic α-bromo amides. Angew. Chem. Int. Ed. 58, 1754–1758 (2019).
He, S. et al. Nickel-catalyzed enantioconvergent reductive hydroalkylation of olefins with α-heteroatom phosphorus or sulfur alkyl electrophiles. J. Am. Chem. Soc. 142, 214–221 (2020).
Yang, Z. & Fu, G. C. Convergent catalytic asymmetric synthesis of esters of chiral dialkyl carbinols. J. Am. Chem. Soc. 142, 5870–5875 (2020).
Qian, D., Bera, S. & Hu, X. Chiral alkyl amine synthesis via catalytic enantioselective hydroalkylation of enecarbamates. J. Am. Chem. Soc. 143, 1959–1967 (2021).
Wang, J. W. et al. Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines. Nat. Commun. 12, 1313 (2021).
Wang, S. et al. Enantioselective access to chiral aliphatic amines and alcohols via Ni-catalyzed hydroalkylations. Nat. Commun. 12, 2771 (2021).
Cuesta-Galisteo, S., Schorgenhumer, J., Wei, X., Merino, E. & Nevado, C. Nickel-catalyzed asymmetric synthesis of α-arylbenzamides. Angew. Chem. Int. Ed. 60, 1605–1609 (2021).
Sun, S.-Z. et al. Enantioselective deaminative alkylation of amino acid derivatives with unactivated olefins. J. Am. Chem. Soc. 144, 1130–1137 (2022).
Wang, X.-X., Lu, X., Li, Y., Wang, J.-W. & Fu, Y. Recent advances in nickel-catalyzed reductive hydroalkylation and hydroarylation of electronically unbiased alkenes. Sci. China Chem. 63, 1586–1600 (2020).
Zhang, Z., Bera, S., Fan, C. & Hu, X. Streamlined alkylation via nickel-hydride-catalyzed hydrocarbonation of alkenes. J. Am. Chem. Soc. 144, 7015–7029 (2022).
Wang, Y., He, Y. & Zhu, S. NiH-catalyzed functionalization of remote and proximal olefins: new reactions and innovative strategies. Acc. Chem. Res. 55, 3519–3536 (2022).
Yang, H. & Ye, Y. Recent progress in NiH-catalyzed linear or branch hydrofunctionalization of terminal or internal alkenes. Top. Curr. Chem. 381, 23 (2023).
Mao, R., Bera, S., Turla, A. C. & Hu, X. Copper-catalyzed intermolecular functionalization of unactivated C(sp3)–H bonds and aliphatic carboxylic acids. J. Am. Chem. Soc. 143, 14667–14675 (2021).
Huo, H., Gorsline, B. J. & Fu, G. C. Catalyst-controlled doubly enantioconvergent coupling of racemic alkyl nucleophiles and electrophiles. Science 367, 559–564 (2020).
Bera, S. & Hu, X. Nickel-catalyzed regioselective hydroalkylation and hydroarylation of alkenyl boronic esters. Angew. Chem. Int. Ed. 58, 13854–13859 (2019).
Zhang, Y., Han, B. & Zhu, S. Rapid access to highly functionalized alkyl boronates by NiH-catalyzed remote hydroarylation of boron-containing alkenes. Angew. Chem. Int. Ed. 58, 13860–13864 (2019).
Bera, S., Mao, R. & Hu, X. Enantioselective C(sp3)–C(sp3) cross-coupling of non-activated alkyl electrophiles via nickel hydride catalysis. Nat. Chem. 13, 270–277 (2021).
Bera, S., Fan, C. & Hu, X. Enantio- and diastereoselective construction of vicinal C(sp3) centres via nickel-catalysed hydroalkylation of alkenes. Nat. Catal. 5, 1180–1187 (2022).
Pezzetta, C., Bonifazi, D. & Davidson, R. W. M. Enantioselective synthesis of N-benzylic heterocycles: a nickel and photoredox dual catalysis approach. Org. Lett. 21, 8957–8961 (2019).
Maddocks, C. J., Ermanis, K. & Clarke, P. A. Asymmetric “clip-cycle” synthesis of pyrrolidines and spiropyrrolidines. Org. Lett. 22, 8116–8121 (2020).
Zhang, S., Chen, X., Zhang, J., Wang, W. & Duan, W. An enantioselective formal synthesis of (+)-(R)-α-lipoic acid by an l-proline-catalyzed aldol reaction. Synthesis 3, 383–386 (2008).
Yadav, J. S., Sridhar Reddy, M. & Prasad, A. R. Stereoselective syntheses of (–)-tetrahydrolipstatin via Prins cyclisations. Tetrahedron Lett. 47, 4995–4998 (2006).
Sudau, A., Munch, W., Bats, J.-W. & Nubbemyer, U. Total synthesis of (–)-pumiliotoxin 251D. Eur. J. Org. Chem. 2002, 3315–3325 (2002).
Becker, D. P. et al. Pyrrolizidine esters and amides as 5-HT4 receptor agonists and antagonists. J. Med. Chem. 49, 1125–1139 (2006).
Du, X. & Huang, Z. Advances in base-metal-catalyzed alkene hydrosilylation. ACS Catal. 7, 1227–1243 (2017).
Wang, K., Ding, Z., Zhou, Z. & Kong, W. Ni-catalyzed enantioselective reductive diarylation of activated alkenes by domino cyclization/cross-coupling. J. Am. Chem. Soc. 140, 12364–12368 (2018).
Maity, B. et al. Mechanistic insight into the photoredox-nickel-HAT triple catalyzed arylation and alkylation of α‑amino Csp3–H bonds. J. Am. Chem. Soc. 142, 16942–16952 (2020).
Acknowledgements
This project was supported by the National Natural Science Foundation of China (22171215 to W.K. and 22301225 to Y.P.), the Cultivation Program of Wuhan Institute of Photochemistry and Technology (GHY2023KF007 to W.K.), Hubei Provincial Outstanding Youth Fund (2022CFA092 to W.K.), Hubei Provincial Natural Science Foundation (2023AFB034 to Y.P.), GuangDong Basic and Applied Basic Research Foundation (2023A1515110137 to Z.Z. and 2022A1515110113 to Y.P.) and the 75th China Postdoctoral Science Foundation (2024M752461 to Z.Z.). We thank the Core Facility of Wuhan University for X-ray single-crystal diffraction analysis.
Author information
Authors and Affiliations
Contributions
W.K. conceived and directed this project. Z.Z., Y.K., Y.P., R.M., F.H., X.W. and S.X. conducted the experimental investigations. Z.Z., Y.K. and Y.P. analysed and interpreted the experimental data. W.K. wrote the manuscript with feedback from the other authors. Z.Z., Y.P. and Y.K. prepared the Supplementary Information. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Xi Lu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–14, Figs. 1–194, experimental procedures, product characterization and X-Ray crystallographic analysis.
Supplementary Data 1
Crystallographic data for compound 5; CCDC 2344836.
Supplementary Data 2
Crystallographic data for compound 66; CCDC 2360607.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Ke, Y., Miao, R. et al. Direct stereoselective C(sp3)–H alkylation of saturated heterocycles using olefins. Nat. Chem. 17, 344–355 (2025). https://doi.org/10.1038/s41557-025-01747-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01747-6
This article is cited by
-
Hydrogen atom transfer-enabled hydroacylation of alkenes with acyl chlorides
Science China Chemistry (2026)
-
Atom-economic enantioselective photoenzymatic radical hydroalkylation via single-electron oxidation of carbanions
Nature Catalysis (2025)
-
Stereoselective alkylation of saturated heterocycles
Nature Chemistry (2025)
-
Stereoselective C(sp3)—H alkylation of saturated heterocycles with olefins
Science China Chemistry (2025)