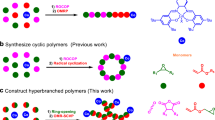
Abstract
Degradation of carbon-backbone polymers, which make up most plastics, remains a formidable challenge owing to strong and inert main-chain C–C bonds. While incorporation of comonomers that generate backbone radicals under certain conditions can induce degradation of the polymer chain, such strategies yield complex oligomer mixtures. Here we report aromatization-driven C–C bond cleavage as a viable and powerful strategy to endow the degradability into carbon backbones using acrylic polymers as a model example. The key to this new strategy is the efficient, living, alternating addition copolymerization of acrylates with simple, commercially available and biorenewable coumarin using a frustrated Lewis pair cooperative catalyst. The resulting acrylic copolymers are strong, transparent thermoplastics with key thermal, optical, mechanical properties comparable or superior to poly(methyl methacrylate). Under strong base, alternating copolymers can completely degrade at room temperature through efficient cleavage of main-chain C–C bonds utilizing aromatization as a thermodynamic driving force, to generate pure, pharmaceutically valuable molecules, thus affording durable, robust yet fully degradable carbon-backbone acrylic polymers.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data that support the findings of this study are available within the article and its Supplementary Information, and/or from the corresponding authors on reasonable request.
References
Industry Agenda. The new plastics economy: rethinking the future of plastics. In The World Economic Forum: Geneva, Switzerland (Ellen MacArthur Foundation, 2016).
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
Hong, M. & Chen, E. Y.-X. Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem. 19, 3692–3706 (2017).
Worch, J. C. & Dove, A. P. 100th anniversary of macromolecular science viewpoint: toward catalytic chemical recycling of waste (and future) plastics. ACS Macro Lett. 9, 1494–1506 (2020).
Pesenti, T. & Nicolas, J. 100th anniversary of macromolecular science viewpoint: degradable polymers from radical ring-opening polymerization: latest advances, new directions, and ongoing challenges. ACS Macro Lett. 9, 1812–1835 (2020).
Tardy, A., Nicolas, J., Gigmes, D., Lefay, C. & Guillaneuf, Y. Radical ring-opening polymerization: scope, limitations, and application to (bio)degradable materials. Chem. Rev. 117, 1319–1406 (2017).
Deng, Y., Mehner, F. & Gaitzsch, J. Current standing on radical ring-opening polymerizations of cyclic ketene acetals as homopolymers and copolymers with one another. Macromol. Rapid Commun. 44, 2200941 (2023).
Smith, R. A., Fu, G., McAteer, O., Xu, M. & Gutekunst, W. R. Radical approach to thioester-containing polymers. J. Am. Chem. Soc. 141, 1446–1451 (2019).
Kiel, G. R. et al. Cleavable comonomers for chemically recyclable polystyrene: a general approach to vinyl polymer circularity. J. Am. Chem. Soc. 144, 12979–12988 (2022).
Do, P. T., Poad, B. L. J. & Frisch, H. Programming photodegradability into vinylic polymers via radical ring-opening polymerization. Angew. Chem. Int. Ed. 62, e202213511 (2023).
Paulusse, J. M. J., Amir, R. J., Evans, R. A. & Hawker, C. J. Free radical polymers with tunable and selective bio- and chemical degradability. J. Am. Chem. Soc. 131, 9805–9812 (2009).
Wang, W. et al. Degradable vinyl random copolymers via photocontrolled radical ring-opening cascade copolymerization. Angew. Chem. Int. Ed. 61, e202113302 (2022).
Watanabe, H. & Kamigaito, H. Direct radical copolymerizations of thioamides to generate vinyl polymers with degradable thioether bonds in the backbones. J. Am. Chem. Soc. 145, 10948–10953 (2023).
Korpusik, A. B. et al. Degradation of polyacrylates by one-pot sequential dehydrodecarboxylation and ozonolysis. J. Am. Chem. Soc. 145, 10480–10485 (2023).
Spring, S. W. et al. Poly(2,3-dihydrofuran): a strong, biorenewable, and degradable thermoplastic synthesized via room temperature cationic polymerization. J. Am. Chem. Soc. 144, 15727–15734 (2022).
Kimura, T., Kuroda, K., Kubota, H. & Ouchi, M. Metal-catalyzed switching degradation of vinyl polymers via introduction of an “in-chain” carbon–halogen bond as the trigger. ACS Macro Lett. 10, 1535–1539 (2021).
Arslan, Z., Kiliclar, H. C. & Yagci, Y. Visible light induced degradation of poly(methyl methacrylate-co-methyl α-chloro acrylate) copolymer at ambient temperature. Macromol. Rapid Commun. 44, 2300066 (2023).
Garrison, J. B., Hughes, R. W. & Sumerlin, B. S. Backbone degradation of polymethacrylates via metal-free ambient-temperature photoinduced single-electron transfer. ACS Macro Lett. 11, 441–446 (2022).
Makino, H., Nishikawa, T. & Ouchi, M. Incorporation of a boryl pendant as the trigger in a methacrylate polymer for backbone degradation. Chem. Commun. 58, 11957–11960 (2022).
Yamamoto, S., Kubo, T. & Satoh, K. Interlocking degradation of vinyl polymers via main-chain C–C bonds scission by introducing pendant-responsive comonomers. J. Polym. Sci. 60, 3435–3446 (2022).
Kimura, T. & Ouchi, M. Photocatalyzed hydrogen atom transfer degradation of vinyl polymers: cleavage of a backbone C–C bond triggered by radical activation of a C–H bond in a pendant. Angew. Chem. Int. Ed. 62, e202305252 (2023).
Čamdžić, L. & Stache, E. E. Controlled radical polymerization of acrylates and isocyanides installs degradable functionality into novel copolymers. J. Am. Chem. Soc. 145, 20311–20318 (2023).
Hong, M. & Chen, E. Y.-X. Future directions for sustainable polymers. Trends Chem. 1, 148–151 (2019).
Haque, F. M. et al. Defining the macromolecules of tomorrow through synergistic sustainable polymer research. Chem. Rev. 122, 6322–6373 (2022).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Jones, G. R. et al. Reversed controlled polymerization (RCP): depolymerization from well-defined polymers to monomers. J. Am. Chem. Soc. 145, 9898–9915 (2023).
Delplace, V. & Nicolas, J. Degradable vinyl polymers for biomedical applications. Nat. Chem. 7, 771–784 (2015).
Korley, L. T. J., Epps, T. H., Helms, B. A. & Ryan, A. J. Toward polymer upcycling—adding value and tackling circularity. Science 373, 66–69 (2021).
Jehanno, C. et al. Critical advances and future opportunities in upcycling commodity polymers. Nature 603, 803–814 (2022).
Okennedy, R. & Thornes, R. D. Coumarins: Biology, Applications and Mode of Action (Wiley, 1997).
Medina, F. G. et al. Coumarin heterocyclic derivatives: chemical synthesis and biological activity. Nat. Prod. Rep. 32, 1472–1507 (2015).
Chun, K. et al. Chromen-based TNF-α converting enzyme (TACE) inhibitors: design, synthesis, and biological evaluation. Bioorg. Med. Chem. 16, 530–535 (2008).
Hou, J. et al. Visible-light-driven alkyne hydro-/carbocarboxylation using CO2 via iridium/cobalt dual catalysis for divergent heterocycle synthesis. J. Am. Chem. Soc. 140, 5257–5263 (2018).
Trenor, S. R., Shultz, A. R., Love, B. J. & Long, T. E. Coumarins in polymers: from light harvesting to photo-cross-linkable tissue scaffolds. Chem. Rev. 104, 3059–3077 (2004).
Cao, D. et al. Coumarin-based small-molecule fluorescent chemosensors. Chem. Rev. 119, 10403–10519 (2019).
Hong, M., Chen, J. & Chen, E. Y.-X. Polymerization of polar monomers mediated by main-group Lewis acid–base pairs. Chem. Rev. 118, 10551–10616 (2018).
Hong, M. in Frustrated Lewis Pairs. Molecular Catalysis Vol. 2 (eds Slootweg, J. C. & Jupp, A. R.) 283–317 (Springer, 2021).
McGraw, M. L. & Chen, E. Y.-X. Lewis pair polymerization: perspective on a ten-year journey. Macromolecules 53, 6102–6122 (2020).
Zhao, W., He, J. & Zhang, Y. Lewis pairs polymerization of polar vinyl monomers. Sci. Bull. 64, 1830–1840 (2019).
Zhao, W., He, J. & Zhang, Y. Research progress in the living/controlled polymerization of (meth)acrylate monomers by Lewis pair. Sci. China Chem. 66, 2256–2266 (2023).
Knaus, M. G. M., Giuman, M. M., Pöthig, A. & Rieger, B. End of frustration: catalytic precision polymerization with highly interacting Lewis pairs. J. Am. Chem. Soc. 138, 7776–7781 (2016).
Zhou, Y., Jiang, S. & Xu, X. Isospecific polymerization of methyl methacrylate by intramolecular rare-earth metal based Lewis pairs. Chin. J. Chem. 39, 149–156 (2021).
Welch, G. C., Juan, R. R. S., Masuda, J. D. & Stephan, D. W. Reversible, metal-free hydrogen activation. Science 314, 1124–1126 (2006).
Stephan, D. W. The broadening reach of frustrated Lewis pair chemistry. Science 354, aaf7229 (2016).
Kamber, N. E. et al. Organocatalytic ring-opening polymerization. Chem. Rev. 107, 5813–5840 (2007).
Ottou, W. N., Sardon, H., Mecerreyes, D., Vignolle, J. & Taton, D. Update and challenges in organo-mediated polymerization reactions. Prog. Polym. Sci. 56, 64–115 (2016).
Xu, T. & Chen, E. Y.-X. Probing site cooperativity of frustrated phosphine/borane Lewis pairs by a polymerization study. J. Am. Chem. Soc. 136, 1774–1777 (2014).
Song, Y., He, J., Zhang, Y., Gilsdorf, R. A. & Chen, E. Y.-X. Recyclable cyclic bio-based acrylic polymer via pairwise monomer enchainment by a trifunctional Lewis pair. Nat. Chem. 15, 366–376 (2023).
Zhang, Z.-H., Wang, X., Wang, X.-J., Li, Y. & Hong, M. Tris(2,4-difluorophenyl)borane/triisobutylphosphine Lewis pair: a thermostable and air/moisture-tolerant organic catalyst for the living polymerization of acrylates. Macromolecules 54, 8495–8502 (2021).
Welch, G. C. & Stephan, D. W. Facile heterolytic cleavage of dihydrogen by phosphines and boranes. J. Am. Chem. Soc. 129, 1880–1881 (2007).
Zhang, Y., Miyake, G. M. & Chen, E. Y.-X. Alane-based classical and frustrated Lewis pairs in polymer synthesis: rapid polymerization of MMA and naturally renewable methylene butyrolactones into high-molecular-weight polymers. Angew. Chem. Int. Ed. 49, 10158–10162 (2010).
Jia, Y.-B. et al. Mechanistic aspects of initiation and deactivation in N-heterocyclic olefin mediated polymerization of acrylates with alane as activator. Macromolecules 47, 1966–1972 (2014).
Jia, Y.-B. et al. Controlled divinyl monomer polymerization mediated by Lewis pairs: a powerful synthetic strategy for functional polymers. ACS Macro Lett. 3, 896–899 (2014).
Wang, Q. et al. Living polymerization of conjugated polar alkenes catalyzed by N-heterocyclic olefin-based frustrated Lewis pairs. ACS Catal. 8, 3571–3578 (2018).
Zhang, P., Zhou, H. & Lu, X.-B. Living and chemoselective (co)polymerization of polar divinyl monomers mediated by bulky Lewis pairs. Macromolecules 52, 4520–4525 (2019).
Wang, X.-J. & Hong, M. Lewis-pair-mediated selective dimerization and polymerization of lignocellulose-based β-angelica lactone into biofuel and acrylic bioplastic. Angew. Chem. Int. Ed. 59, 2664–2668 (2020).
Zhao, W. et al. Lewis-pair-catalyzed regioselective polymerization of (E,E)-alkyl sorbates for the synthesis of (AB)n sequenced polymers. Angew. Chem. Int. Ed. 60, 24306–24311 (2021).
Reilly, L. T. et al. Compounded interplay of kinetic and thermodynamic control over comonomer sequences by Lewis pair polymerization. J. Am. Chem. Soc. 144, 23572–23584 (2022).
Wu, H., Ma, G. & Xia, Y. Experimental study of tensile properties of PMMA at intermediate strain rate. Mater. Lett. 58, 3681–3685 (2004).
Castro, L. et al. Are solvent and dispersion effects crucial in olefin polymerization DFT calculations? Some insights from propylene coordination and insertion reactions with group 3 and 4 metallocenes. ACS Catal. 5, 416–425 (2015).
Acknowledgements
This work was supported by the National Key R&D Program of China (grant number 2021YFA1501700), the National Natural Science Foundation of China (grant numbers 22471286 and 52203020), the Science and Technology Commission of Shanghai Municipality (grant numbers 22ZR1481900, 23XD1424600 and 22YF1458100), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant number XDB0610000) and the CAS Project for Young Scientists in Basic Research (YSBR-094). Y.S. is thankful for the financial support from the Youth Innovation Promotion Association CAS (2022253). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the paper. We thank F. Xu at the Institute of Process Engineering, Chinese Academy of Sciences, for the assistance with the measurement of optical property.
Author information
Authors and Affiliations
Contributions
Z.-H.Z., Y.S. and M.H. conceived the idea and designed the experiments. Z.-H.Z. performed the polymerization experiments. Y.S. performed the degradation experiments. Z.-H.Z. and Y.L. conducted the physical property measurements. T.R. and L.M. performed the theoretical calculations. All authors participated in the data analyses and discussions. M.H. directed the project and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
M.H., Z.-H.Z. and Y.S. are inventors on a Chinese patent application (number 2024104783860) submitted by the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, that covers the synthesis of acrylate/CM random and alternating copolymers by Lewis pair catalyst and their degradations enabled by ACBC.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Overlay of 13C NMR spectra (CDCl3, RT) of polyacrylates and copolymers with different CM incorporations.
Long A-A and short A-A: long and short continuous acrylate sequences, respectively; A-CM: acrylate-CM alternating sequence.
Extended Data Fig. 2 Illustrative scheme of synthesis and full degradation of poly(MA-alt-CM)-based thermoset.
[BDA]0:[MA]0:[CM]0:[B(2,4-F2Ph)3]0:[PtBu3]0 = 5:200:525:2:1. In contrast to uncrosslinked analogue (Tg = 158.0 °C, Td = 274 °C), thermoset exhibited comparable Tg value (157.2 °C) and slightly lower thermal stability (Td = 251 °C).
Supplementary information
Supplementary Information
Full descriptions of the methods, computational details and optimized geometries, and Supplementary Tables 1–6 and Figs. 1–68.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, ZH., Sun, Y., Rajeshkumar, T. et al. Vinyl polymers with fully degradable carbon backbones enabled by aromatization-driven C–C bond cleavage. Nat. Chem. 17, 746–755 (2025). https://doi.org/10.1038/s41557-025-01751-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01751-w